Supplemental Digital Content is available in the text
Keywords: cost-effectiveness, economic evaluation, HIV, HIV diagnosis, mathematical models, prevention, testing
Abstract
Background:
Increased rates of testing, with early antiretroviral therapy (ART) initiation, represent a key potential HIV-prevention approach. Currently, in MSM in the United Kingdom, it is estimated that 36% are diagnosed by 1 year from infection, and the ART initiation threshold is at CD4+ cell count 350/μl. We investigated what would be required to reduce HIV incidence in MSM to below 1 per 1000 person-years (i.e. <535 new infections per year) by 2030, and whether this is likely to be cost-effective.
Methods:
A dynamic, individual-based simulation model was calibrated to multiple data sources on HIV in MSM in the United Kingdom. Outcomes were projected according to future alternative HIV testing and ART initiation scenarios to 2030, considering also potential changes in levels of condomless sex.
Results:
For ART use to result in an incidence of close to 1/1000 person-years requires the proportion of all HIV-positive MSM with viral suppression to increase from below 60% currently to 90%, assuming no rise in levels of condomless sex. Substantial increases in HIV testing, such that over 90% of men are diagnosed within a year of infection, would increase the proportion of HIV-positive men with viral suppression to 80%, and it would be 90%, if ART is initiated at diagnosis. The scenarios required for such a policy to be cost-effective are presented.
Conclusion:
This analysis provides targets for the proportion of all HIV-positive MSM with viral suppression required to achieve substantial reductions in HIV incidence.
Introduction
HIV is endemic in MSM in many settings [1]. There is widespread interest in the potential effect of antiretroviral therapy (ART) in controlling HIV epidemics [2], but there are a number of areas, including the United Kingdom, in which ART use is high among MSM and yet incidence has not declined [3–7], and its potential impact in epidemics in MSM has been questioned [5,8].
Multiple factors will influence the impact of ART on HIV incidence. First, how low the transmission rate is from a person on ART with a suppressed plasma viral load to an HIV-negative partner. Until recently, there was almost no direct evidence for what this risk is for anal sex, but interim results from the Partners of people on ART - a New Evaluation of the Risks (PARTNR) study estimate the risk as zero, even with other sexually transmitted infections (STIs) reported, although with a wide confidence interval (CI) [9]. Second, it is important to consider overall trends in condomless sex and, in particular, whether therapeutic success of ART will lead to further increases in condomless sex as fear of having HIV is diminished. The fact that a high proportion of new infections come from men who are not yet diagnosed, and in many cases, men who have only just been infected, is a third consideration [7,10,11]. This might seem to limit the influence ART can have in reducing new HIV infections in MSM. We should consider how high the rate of HIV testing is, and how rapidly those who are infected become diagnosed and linked to care. Then, there is the threshold for initiation of ART from an individual health perspective. This was uncertain for a long period and guidelines differed in their recommendations [12–14]. In the United Kingdom, the lower limit threshold for ART initiation has been CD4+ cell count of 350/μl [13], lower than in other guidelines [12]. Just recently, however, the Strategic Timing of Antiretroviral Treatment (http://insight.ccbr.umn.edu/start/) and Temprano [15] trials have indicated that initiation of ART is beneficial to health even in people with high CD4+ cell count above 500/μl. Also, there is offer and uptake of ‘treatment as prevention’ (TasP), defined in a community statement as when a person is offered ART, at a time when it is not unequivocally needed for the person's health, in order to reduce the risk that they transmit HIV [16]. Further, we have to consider not just whether people initiate and remain on ART, but their levels of adherence and viral suppression [5,17]. The development of drug resistance and onward transmission of resistance, and its potential to undermine benefits of ART, should also be taken into account.
To inform policy aimed at reducing HIV incidence, a cost-effectiveness analysis based around a model calibrated to multiple data sources, which take all these factors above into account, is required. Here, we report on such analyses in the UK context and address what it would take, in terms of testing rates and ART initiation and coverage, to achieve a decline in incidence from the current level of approximately 6 per 1000 person-years, to below 1 per 1000 person-years among MSM (i.e. 535 new infections in an adult HIV-negative MSM population of approximate size 535 000), which would be much closer to the level experienced in the heterosexual population, and evaluate whether the required investment to achieve this reduction is justified, given its potential use elsewhere in the healthcare system.
Methods
Simulation of HIV among the MSM population in the United Kingdom
We use a dynamic, individual-level, simulation model (HIV Synthesis MSM transmission model) to recreate the lifetime HIV risks and, for those acquiring HIV, HIV progression and treatment outcomes of the MSM population in the United Kingdom, since 1980. The model and this calibration approach have been described previously in detail [7], and are now updated to include observed data to 2012 [18] (see Supplementary material). In brief, we take into account age, condomless sex with primary (long-term) and short-term (e.g. casual) partners, presence of other STIs, HIV testing patterns, and then in those infected with HIV, viral load, CD4+ cell count, use of specific antiretroviral drugs, adherence, presence of specific resistance mutations, risk of AIDS and death, including death from non-AIDS conditions. We consider a closed population and thus conceive of in and out-migration as approximately balancing. The parameter values determining sexual behaviour, the transmission rate, testing patterns and the extent to which HIV diagnosis leads to a reduction in condomless sex are varied with each model simulation run by sampling from distributions. The resulting outputs from the model are compared with various data sources – in all we ran the model over 100 000 times and selected 101 sets of parameter values that are able to reproduce an epidemic close to that observed. These 101 sets of parameter values that lead to outputs that fit closely to observed data are re-used when projecting forward.
Future scenarios compared and economic analysis
Having reconstructed the epidemic to date, we projected future outcomes from 2015 to 2030. This was done first assuming that there was no change in HIV-testing patterns or in the initiation criteria for ART (CD4+ cell count 350/μl). Main outcomes considered were (in MSM aged 15–65 years) the proportion of MSM with HIV who have viral load suppressed (<500 copies/ml), number of incident cases of HIV per year, prevalence of HIV, number of men on ART, total quality-adjusted life-years (QALYs) lived and the costs of care (both these latter were discounted at 3.5% per year) [19]. We then considered outcomes under situations in which, from 2015, rates of testing were increased (by pre-specified amounts referred to as ‘test rate +’ and ‘test rate ++’, which correspond to increases from 19% of all MSM tested in the past year to 38 and 65%, with testing being strongly related to recent condomless sex). Further, ART initiation policy was to change so that ART was initiated immediately at diagnosis.
We initially did this assuming no change in levels of condomless sex or in the levels of linkage and retention in healthcare and adherence to antiretroviral drugs, but then considered the effects of increases in condomless sex, and decreases in the level of linkage and retention in care and adherence to antiretroviral drugs. The incremental cost and incremental change in QALYs compared with the current scenario were plotted for all other scenarios, and suitable incremental cost-effectiveness ratios (ICERs) were calculated, along with a cost-effectiveness acceptability curve. We first considered a situation in which drug prices remain at the current full list price, and another in which price discounts and use of generic drugs are included (with an assumed linear decline in drug prices from a current price in 2015 of 30% below the full list price to 80% below the current full list price). Our analysis takes a health-systems perspective. Supplementary Table 2 indicates the unit costs associated with various aspects of HIV testing and care.
Results
The simulated outputs from the model generally fit closely to a range of observed data up to 2012, as illustrated in Suppl Fig. 2. In 2015 – the time at which changes in scenario are considered – it is estimated that there are 53 500 MSM living with HIV (with around 3500 new infections in 2015, incidence rate 6 per 1000 person-years), of which 40 200 (75%) diagnosed and 33 200 (62%) on ART (of 34 700 who ever started ART, illustrating the high levels of retention). Of those on ART, 92% have viral load below 500 copies/ml. Overall, 58% of MSM living with HIV in the United Kingdom are estimated to be on ART, with a viral load below 500 copies/ml.
The potential increases in HIV testing considered after 2015 are illustrated in Fig. 1a. Test rates + and ++, respectively, entail an increase from around 110 000 tests per year to 215 000 and 370 000 (Suppl Fig. 3). Currently (lightest blue line), around 36% of men are diagnosed by 1 year and 70% by 5 years from infection. In the test rate + scenario, these percentages are increased to 65 and 85%, respectively, and in the test rate ++ scenario to 90 and 93%. In Fig. 1b, we indicate the percentage of MSM who have started ART by time from infection, and here (in pink/claret) we also consider an ART initiation strategy in which ART is started at diagnosis, in addition to the existing strategy of starting at a CD4+ cell count of 350/μl. Currently, less than 20% of men start ART by 1 year from infection, whereas in the test rate ++ and ART at diagnosis scenario, that would be 90%. Fig. 1c shows the predicted proportion of all HIV-positive MSM with viral load below 500 copies/ml by year, according to the six testing rate and ART initiation scenarios. An increase in testing rate to test rate ++, even with no change in ART initiation strategy, would correspond to an increase in this percentage from around 60 to 80% by 2030. If ART is initiated at diagnosis, this percentage increases to close to 90%.
Fig. 1.
Potential changes in HIV testing and ART initiation considered after 2015 and effect on proportion with viral suppression.
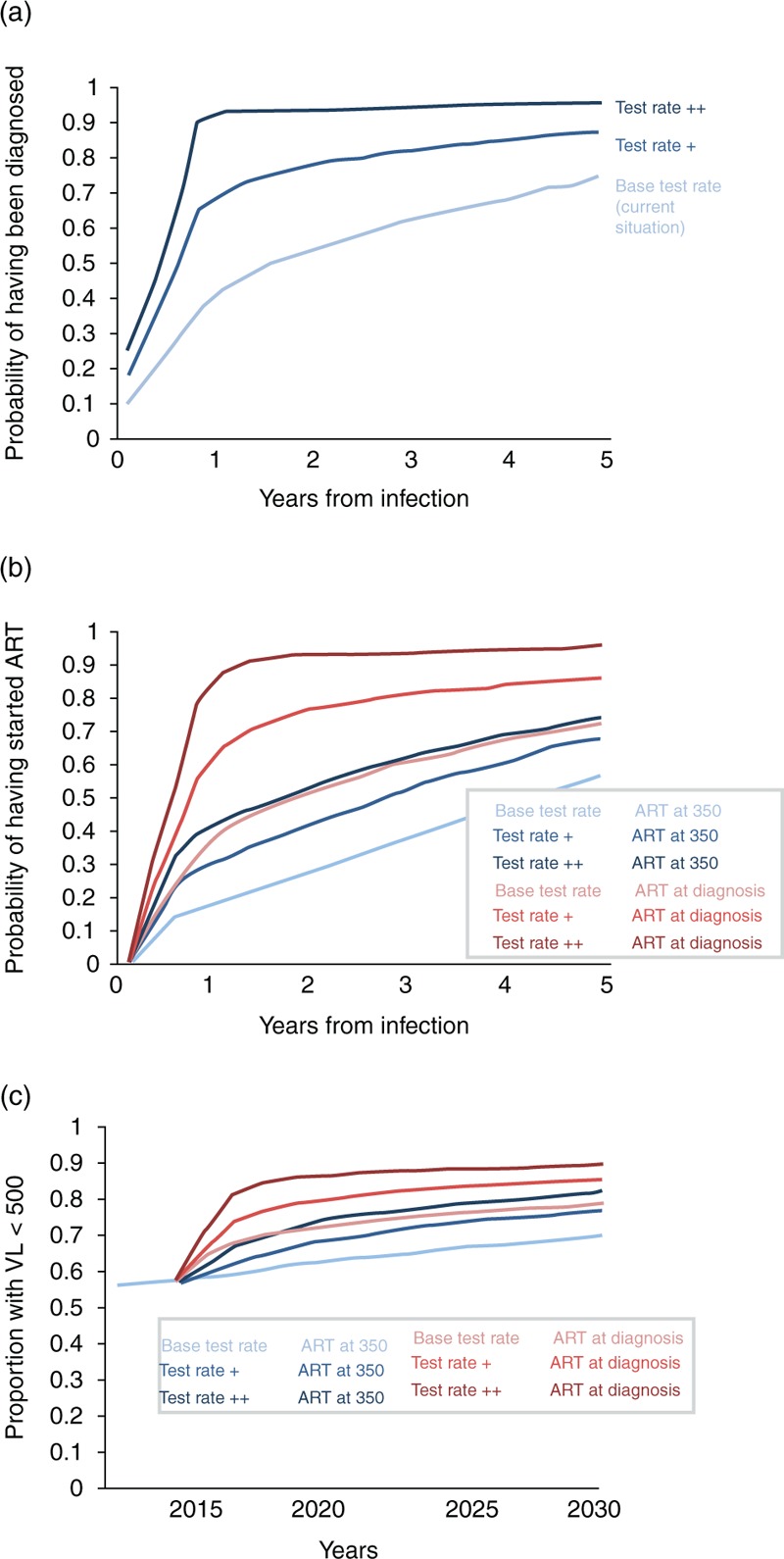
(a) Kaplan–Meier plot of time from infection to diagnosis, according to the HIV test rate. For men infected after 2015. (b) Kaplan–Meier plot of time from infection to ART initiation, according to the HIV test rate and ART initiation strategy. For men infected after 2015. (c) Proportion of all HIV-positive men who have viral load below 500 copies/ml according to calendar year.
The predicted impact of the alternative scenarios on HIV incidence and prevalence is shown in Fig. 2. There is a marked reduction in incidence associated with the increases in testing, even in the absence of a change in ART initiation strategy, a 54% decrease in the test rate ++ scenario in 2030, to a rate of 2.5 per 1000 person-years, for example. However, combining these marked increases in testing with ART initiation at diagnosis results in substantially greater changes – a 79% reduction in 2030, to 675 new infections per year (1.2 per 1000 person-years). This latter scenario is the only one in which the number of men living with HIV is predicted to be less in 2030 than 2015 (50 000, compared with 82 000 with continuation of the current scenario), and represents a situation in which each person with HIV on average gives rise to 0.014 new infections per year – well below one in a sexual lifetime. The predicted number of men on ART increases rapidly with the test rate ++/ART at diagnosis scenario, but reaches a plateau within 5 years and then starts to decline (Suppl Fig. 4). By 2030, this is the scenario with the lowest number of men on ART.
Fig. 2.
Projected HIV incidence and number of men living with HIV.
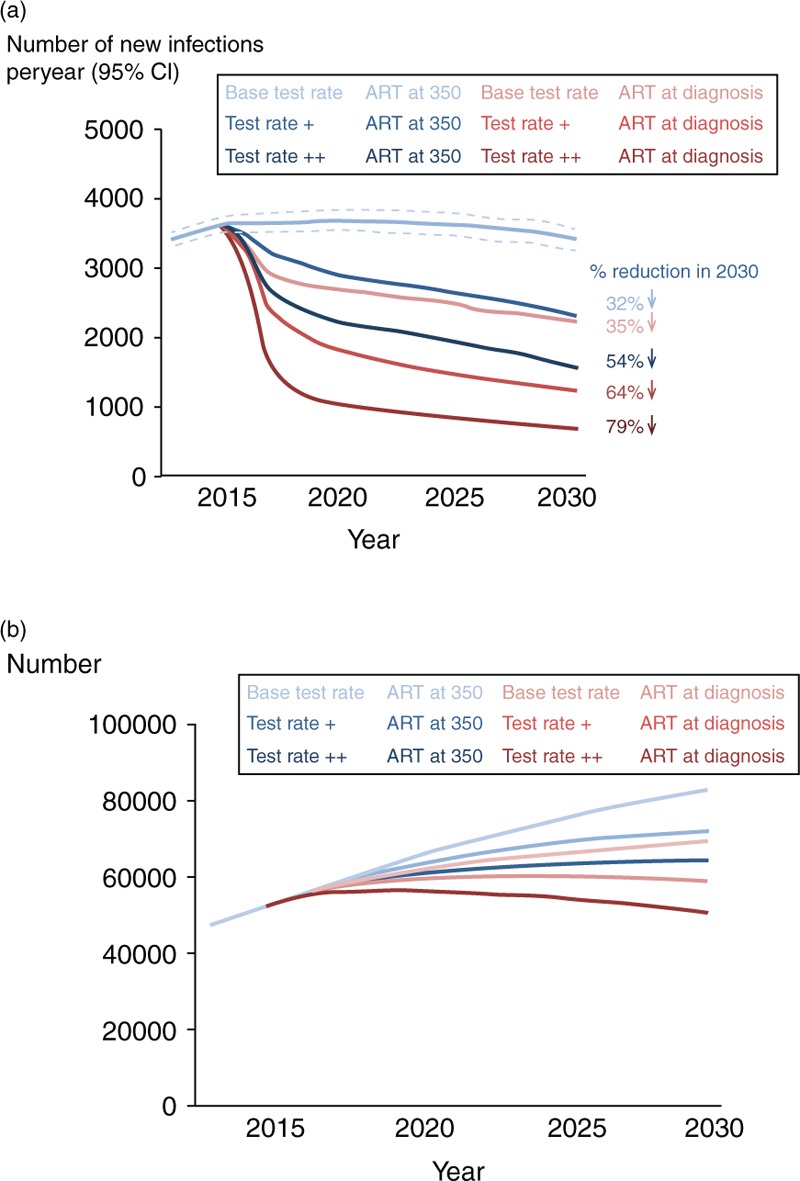
(a) HIV incidence according to test rate and ART initiation strategy between 2015 and 2030. (b) Number of men living with HIV between 2015 and 2030 according to test rate and ART initiation strategy. ART, antiretroviral therapy.
Levels of retention and adherence are currently very high in the United Kingdom, but to understand the importance of this, we consider what would be the effect were these to be reduced. Suppl Fig. 5 shows the reduction in adherence and retention that is considered, which is characterized by an approximately 10% reduction in the proportion of all MSM living with HIV who have suppressed viral load by 2020, from a value of 63%, if there is no change in adherence/retention, to 53%. It is predicted that this would result on average in 1000 extra new infections per year by 2030. This is the case both in the context of the current testing rate and ART initiation strategy (Suppl Fig. 5b) and in the context of the test rate ++/ART initiation at diagnosis scenario (Suppl Fig. 5c).
Over a range of simulations of scenarios involving different testing rates, different ART initiation criteria (also including scenarios with ART initiation at CD4+ cell count <500/μl) and with the current level of adherence/retention or the poorer level just described, it is possible to summarize the mean number of new infections per year we would expect according to the mean proportion of MSM living with HIV (diagnosed and undiagnosed) who have viral suppression (Fig. 3). In this figure, the current UK situation is indicated – about 60% of infected MSM have viral suppression on ART and this is associated with just over 3000 new infections per year. The relationship is approximately linear on a log scale – each 10% higher proportion of MSM with HIV with viral suppression is associated with approximately 37% fewer new infections per year. This graph suggests that we would need an increase in the proportion of all men with viral suppression from below 60 to 90% to see a reduction in incidence to below 1 per 1000 person-years.
Fig. 3.
Relationship between proportion of HIV+ men with viral suppression and number of new infections.
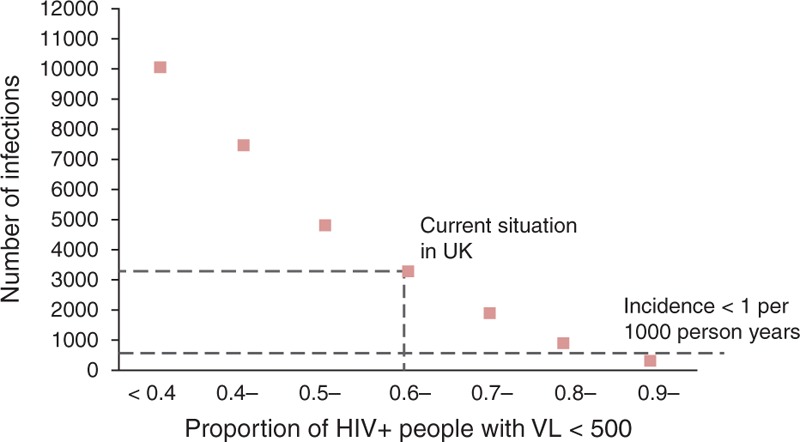
Mean number of new HIV infections per year (over 2015–2030) according to mean proportion of men with HIV who have VL below 500 copies/ml. VL, viral load.
Thus far, we have assumed that future levels of condomless sex will remain as they are currently. In Fig. 4, we illustrate a modest increase in condomless sex, such that the proportion of men with a condomless anal sex partner in the past year is 62% by 2030, instead of the present 52% (Suppl Fig. 2). This is modelled as a general, indiscriminate increase in condomless sex, not one specifically involving those on ART or those with viral suppression, for example, and is apart from any increases in condomless sex due to men with diagnosed HIV, specifically selecting partners who also have diagnosed HIV. The detrimental impact on HIV incidence of the increase in condomless sex is very large in the context of the current testing rate and ART initiation strategy (a doubling of HIV incidence by 2030; Fig. 4b), and much less in the context of the test rate ++, ART initiation at diagnosis scenario Fig. 4c. In Suppl Fig. 5, we consider the association, across multiple scenarios between the number of condomless partnerships per 3 months with viral load above 500 copies/ml and HIV incidence. At present, we estimate there are approximately 10 000 such partnerships per 3 months. This number is strongly associated with HIV incidence, and a reduction to 2000 is required for the HIV incidence to be below 1/1000 per year. Decreases in condomless sex and increases in the proportion of MSM with HIV who are on ART with viral load below 500 copies/ml are two key means of reducing the number of condomless partnerships with viral load above 500 copies/ml.
Fig. 4.
Possible increases in condomless sex and their impact on HIV incidence.
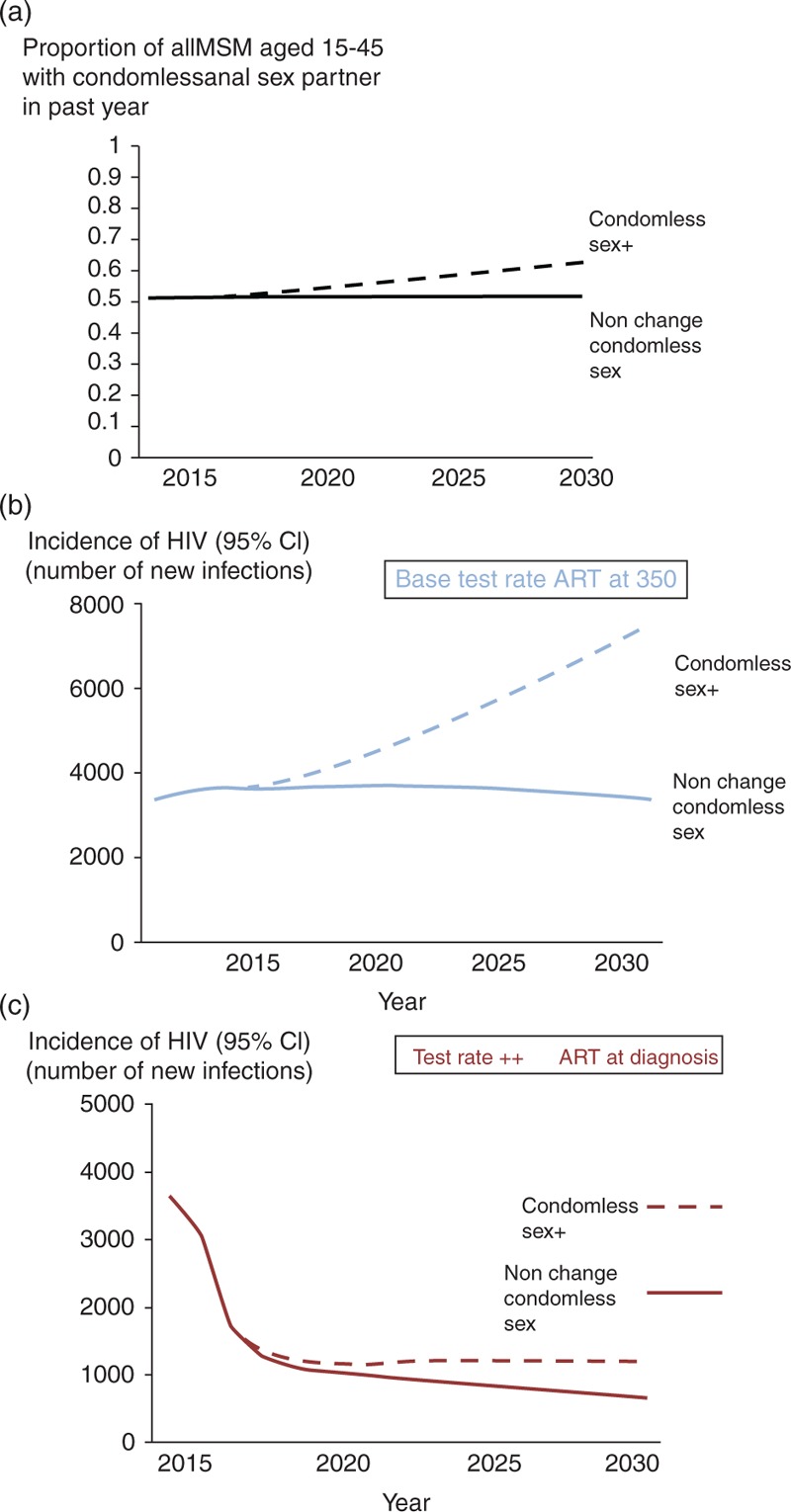
(a) Increase in condomless sex considered (condomless sex +, such that proportion of men with a condomless anal sex partner in the past year is 62% by 2030, instead of the present 52%) and influence on HIV incidence, in the context of (b) base test rate and ART initiation at 350/μl, and (c) test rate ++ and ART at diagnosis. ART, antiretroviral therapy.
Overall, the number of healthy life-years (i.e. QALYs) lived in the MSM population age 15–65 years (including HIV-positive and HIV-negative men) between 2015 and 2030, taking into account discounting, is predicted to be 6 393 500, with continuation of the current testing rate and ART initiation strategy. The total discounted cost of HIV care over this period is £5.5 billion, including around £4.7 billion in ART costs based on current ART list prices (Suppl Fig. 8). Differences in QALYs between the testing and ART initiation scenarios are illustrated in Suppl Fig. 7. Benefits of increased testing become greater with increased time. The scenario which results in most QALYs is the base rate ++/ART at diagnosis scenario. In Fig. 5, the cost-effectiveness plane is shown. Considering current list ART drug prices (Fig. 5a), and if ART at diagnosis can be considered, the most cost-effective policy (lowest ICER) is the test rate ++/ART at diagnosis scenario, with an ICER of £19 077 per QALY gained over our 15-year time horizon. Considering drug price discounts and generic drug prices, the ICER is significantly lower at £12 335. Suppl Fig. 11 shows the cost-effectiveness acceptability curve for the scenario of test rate ++ and ART at diagnosis. This assessment does not include any additional costs for expanding testing, except for that accounted for by the unit test cost of £20 per test done. An extra £8m spent per year on implementation of policies to increase testing would increase the total incremental cost over 15 years to £319m, but will result in an ICER still below £20 000. In a sensitivity analysis in which we assumed that the decline in drug prices from 2015 to 2030 would be to 50% rather than 80% of the current costs, the ICER was £12 428.
Fig. 5.
Cost-effectiveness analysis.
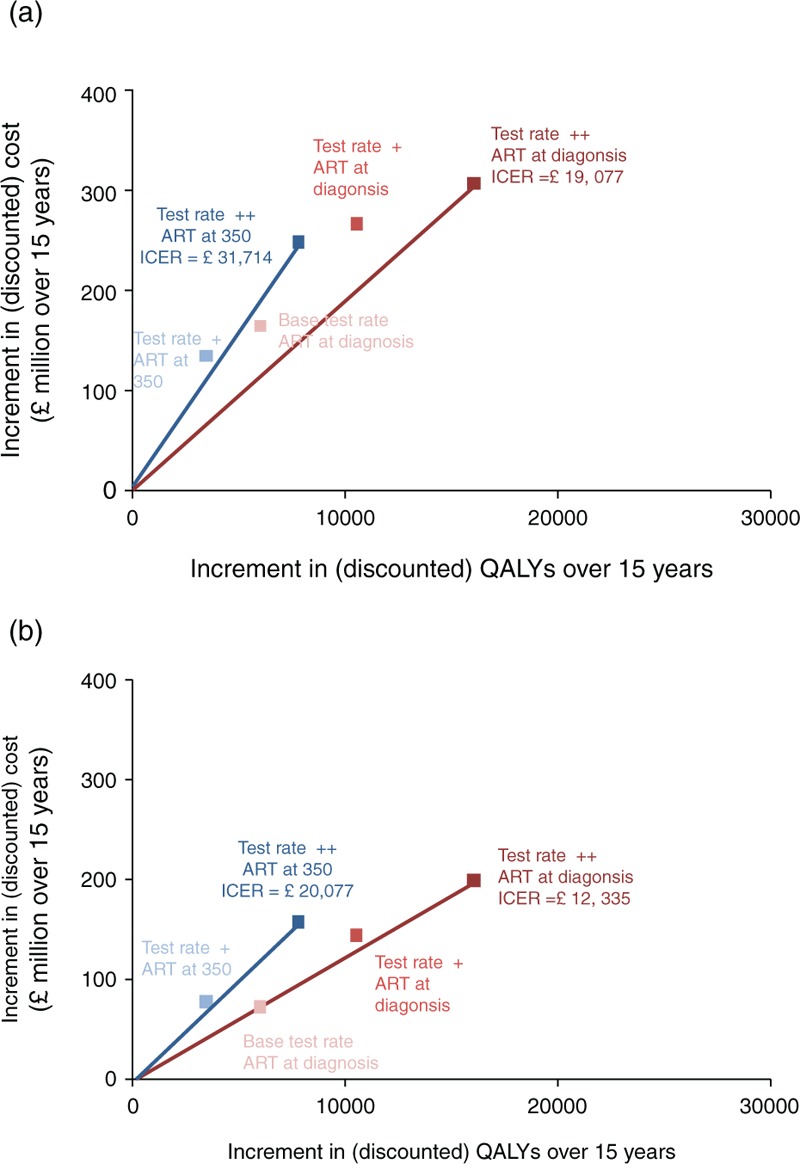
Incremental costs and QALYs over 15 years for MSM in the United Kingdom by potential changes in testing rate and ART initiation criteria (a) with current ART drug prices (b) with a linear decline in drug prices from a current price in 2015 of 30% below the full list price to 80% below the current full list price, reflecting the potential reduction due to price discounts and use of generic drugs. ART, antiretroviral therapy; QALY, quality-adjusted life-years.
Still on Fig. 5, when restricting to scenarios involving changes in HIV testing rates only, then considering current list ART prices, the ICER for increased testing is around £30 000 over the 15-year time horizon, although the ICER is likely to decrease with a longer time horizon due to the increasing QALY benefit (Suppl Fig. 7) and reduced number on ART (Suppl Fig. 4) compared with the base test rate. When considering discounted/generic drug costs, the ICER for the test rate ++ scenario is £20 077, suggesting that testing is cost-effective even over the 15-year time horizon.
Discussion
We find that for increased testing and earlier ART use to lead to an HIV incidence below 1 per 1000 person-years in the United Kingdom, an increase from 60% to 90% is needed in the overall proportion of MSM living with HIV who are virally suppressed on ART. This will involve over 90% of men being diagnosed within 1 year of infection, and assumes no increase in condomless sex. Given the high life expectancy of people with HIV [20], a reduction in incidence to around this level is required for us to see a reduction in the number of MSM living with HIV. We set out the cost conditions under which increased testing is cost-effective. Considering discounts in drug costs and use of generic drugs, policies of increased testing are cost-effective (based on a threshold of £20 000 per QALY) from a UK NHS perspective, if they can be delivered at a cost below £8m per year. Initiation of ART at diagnosis is also cost-effective.
Achieving such high levels of viral suppression is likely to require a cultural change in the approach to HIV testing, such that over 90% of men with HIV are diagnosed within a year of infection, together with initiation of ART at diagnosis. An offer to patients to initiate ART at diagnosis is now appropriate, given the recent results of trials of individual health benefits of earlier ART (http://insight.ccbr.umn.edu/start/) [15], and, further, it is known that some men would wish to start ART to reduce infectiousness even if there were no benefits to health [21]. Increases in testing, even without a change in ART initiation policy, can have a substantial impact on incidence. The increase in testing required would depend on an ambitious campaign, community-rooted and led, designed to reach the highest risk groups within the gay community. Initiatives might include widespread free self-sampling and self-testing, greater testing outreach, simplified fast-track testing for MSM at clinics combined with regular phone texting recall, improved partner notification and testing, and promotion of greater awareness of symptoms of primary HIV infection. It has been reported that online advertising campaigns directly lead to increased uptake of requests for use of free self-sampling kits [22].
Our modelling predicts that HIV incidence would be greatly reduced with a substantial increase in HIV testing, despite the fact that for most men, diagnosis of HIV and treatment initiation cannot be achieved within the period of primary infection, which has been seen as a major obstacle to use of ART to prevent transmission [8]. This is likely because many chains of transmission will contain links which involve transmission from MSM who have been infected for years – these links can be broken with a policy of high levels of testing and immediate ART initiation, thus reducing the frequency of outbreaks of primary infection [7].
There has been extensive previous modelling of effects of increased testing and/or ART HIV epidemics in MSM [23–26], and some linked cost-effectiveness analyses [27–33]. Generally, models show high levels of support for increased HIV testing in MSM. Our analysis adds to that literature by modelling at a greater level of detail and modelling at an individual level variables for which multiple data sources are available for calibration (e.g. viral load, use of specific drugs, acquisition and transmission of resistance specific to drugs, CD4+ cell count), enhancing confidence that the modelled outcomes reflect those that would occur in real life in our specific UK context. Further, we provide specific targets for testing rates and the proportion of men with viral suppression that need to be achieved.
It should be noted that there is appreciable variability between individual model simulation runs in effects on HIV incidence (mean effects were shown), and the variability (shown in Suppl Figs. 9–11) partially reflects the stochastic nature of outbreaks of primary infection and also network-specific effects whereby sometimes the men with very high levels of condomless sex do not get onto ART. A further limitation to consider is that this modelling effectively considers the population as being closed, when this is not the case. MSM from abroad who have sex in the United Kingdom may not have the same high levels of ART coverage as UK residents, so it is important that testing in MSM is increased to high levels and ART is accessible worldwide. We considered future use of generic drugs and assumed that their effectiveness in terms of viral suppression would be equal to originator drug formulations, since we assume patients struggling to adhere due to use of regimens which are not fixed-dose combinations will be switched to single tablet regimens when possible, and that generic single-tablet regimens will be available before too long. Walensky et al.[34] previously modelled some small reduction in viral suppression with generic drugs, but still found them to be highly cost-effective.
In conclusion, we find that substantial investment in increasing HIV testing uptake and frequency in MSM is likely to be a cost-effective means of reducing HIV incidence, as is initiation of ART at diagnosis. However, it is critical that levels of risky condomless sex do not increase, and that ART retention and adherence remain high.
Acknowledgements
We thank all the Public Health England (PHE) HIV surveillance team and Catherine Mercer for providing unpublished data on sexual behaviour from Natsal. We thank colleagues supporting the Legion computing cluster (Legion@UCL) for critical computing support.
Author Contributions: Contributed to the formulation of the research questions, had critical input into interpretation of results and had substantial input into the drafting of the manuscript: A.P., V.C., F.N., Dd.A., O.N.G., A.B., F.L., A.R., A.M., J.E., G.H., S.C., A.J., J.L. and V.D. Led on collection of the HIV surveillance data used in the analysis: V.D., A.B. and O.N.G. Worked on development and programming of the HIV synthesis model: A.P., V.C. and F.N. Performed the modelling analysis: A.P. Conceived and designed the experiments: A.P., V.C., F.N., A.M., A.B., F.L., A.R., J.E., G.H., A.J., O.N.G., Dd.A., S.C., V.D. and J.L. Performed the experiments: A.P., V.C. and F.N. Analyzed the data: A.P. and V.C. Wrote the paper: A.P. ,V.C., F.N., A.M., A.B., F.L., A.R., Dd.A., O.N.G., J.E., G.H., A.J., S.C., V.D. and J.L.
Funding: This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme (RP-PG-0608-10142) (http://www.nihr.ac.uk/research/). The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
Conflicts of interest
Competing interests: No authors have any competing interests.
Ethics statement: An ethics statement was not required for this work.
Supplementary Material
References
- 1.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AAL, Brookmeyer R. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cambiano V, O’Connor J, Phillips AN, Rodger A, Lodwick R, Pharris A, et al. , Antiretroviral therapy for prevention of HIV transmission: implications for Europe. Euro Surveill 2013; 18. [DOI] [PubMed] [Google Scholar]
- 3.Bezemer D, de Wolf F, Boerlijst MC, van Sighem A, Hollingsworth TD, Prins M, et al. A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS 2008; 22:1071–1077. [DOI] [PubMed] [Google Scholar]
- 4.van Sighem A, Jansen I, Bezemer D, De Wolf F, Prins M, Stolte I, Fraser C. Increasing sexual risk behaviour among Dutch men who have sex with men: mathematical models versus prospective cohort data. AIDS 2012; 26:1837–1843. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med 2012; 9:e1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birrell PJ, Gill ON, Delpech VC, Brown A, Desai S, Chadborn T, et al. HIV incidence in men who have sex with men in England and Wales 2001—10: a nationwide population study. Lancet Infect Dis 2013; 13:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AN, Cambiano V, Nakagawa F, Brown AE, Lampe F, Rodger A, et al. , Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelton JD. ARVs as HIV prevention: a tough road to wide impact. Science 2011; 334:1645–1646. [DOI] [PubMed] [Google Scholar]
- 9.Rodger A, Bruun T, Cambiano V, Lundgren J, et al. , HIV transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER study. Conference on Retroviruses and Opportunistic Infections (CROI 2014). Boston, 3–6 March [Abstract 153LB]. [Google Scholar]
- 10.Fisher M, Pao D, Murphy G, Dean G, McElborough D, Homer G, Parry JV. Serological testing algorithm shows rising HIV incidence in a UK cohort of men who have sex with men: 10 years application. AIDS 2007; 21:2309–2314. [DOI] [PubMed] [Google Scholar]
- 11.Brenner B, Wainberg MA, Roger M. Phylogenetic inferences on HIV-1 transmission: implications for the design of prevention and treatment interventions. AIDS 2013; 27:1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/Adultand AdolescentGL.pdf [Accessed 25 May 2014] [Google Scholar]
- 13.Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, et al. BHIVA Guidelines Writing Group. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Update November 2013). HIV Med 2014; 15 Suppl 1:1–85. [DOI] [PubMed] [Google Scholar]
- 14.Collins S, Geffen N. Community views: balancing the public health benefits of earlier antiretroviral treatment with the implications for individual patients - perspectives from the community. Curr Opin HIV AIDS 2014; 9:4–10. [DOI] [PubMed] [Google Scholar]
- 15.Danel C, Gabillard D, Le Carrou J, Anglaret X, Moh R, Eholie S. Early ART and IPT in HV-infected African adults with high CD4 count (Temprano trial). 2015 Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, USA; 2015. ***[abstract 115LB]. [Google Scholar]
- 16.NAM-EATG. Community consensus statement on the use of antiretroviral therapy in preventing HIV transmission. http://www.hivt4p.org/. [DOI] [PubMed] [Google Scholar]
- 17.Brown AE, Gill ON, Delpech VC. HIV treatment as prevention among men who have sex with men in the UK: is transmission controlled by universal access to HIV treatment and care?. HIV Med 2013; 14:563–570. [DOI] [PubMed] [Google Scholar]
- 18.Public Health England. HIV in the United Kingdom: 2013 report. Public Health England 2013 November. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317140300680. [cited 13 April 2014] [Google Scholar]
- 19.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. http://www.nice.org.uk/article/PMG9/chapter/Foreword [Accessed 7 September 2014] [PubMed] [Google Scholar]
- 20.Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012; 26:335–343. [DOI] [PubMed] [Google Scholar]
- 21.Rodger A, Phillips AN, Speakman A, Gilson R, Fisher M, Wilkins E, et al. Attitudes of people in the UK with HIV who are antiretroviral (ART) naïve to starting ART at high CD4 counts for potential health benefit or to prevent HIV transmission. PLoS One 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McOwan A. HIV testing: new technologies and new strategies for those at higher risk. Third Joint Conference of BHIVA with BASHH, Liverpool, April 2014. [Google Scholar]
- 23.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and Antiretroviral Therapy in San Francisco. Science 2000; 287:650–654. [DOI] [PubMed] [Google Scholar]
- 24.Sood N, Wagner Z, Jaycocks A, Drabo E, Vardavas R. Test-and-treat in Los Angeles: a mathematical model of the effects of test-and-treat for the population of men who have sex with men in Los Angeles county. Clin Infect Dis 2013; 56:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlebois ED, Das M, Porco TC, Havlir DV. The effect of expanded antiretroviral treatment strategies on the HIV epidemic among men who have sex with men in San Francisco. Clin Infect Dis 2011; 52:1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punyacharoensin N, Edmunds WJ, DeAngelis D, et al. Modelling the HIV epidemic among MSM in the United Kingdom: quantifying the contributions to HIV transmission to better inform prevention initiatives. AIDS 2015; 29:339–349. [DOI] [PubMed] [Google Scholar]
- 27.Lorenc T, Marrero-Guillamon I, Aggleton P, Cooper C, Llewellyn A, Lehmann A, et al. Promoting the uptake of HIV testing among men who have sex with men: systematic review of effectiveness and cost-effectiveness. Sex Transm Infect 2011; 87:272–278. [DOI] [PubMed] [Google Scholar]
- 28.Wang SH, Moss JR, Hiller JE. Cost-effectiveness of HIV voluntary counseling and testing in China. Asia-Pacific J Public Health 2011; 23:620–633. [DOI] [PubMed] [Google Scholar]
- 29.Juusola JL, Brandeau ML, Long EF, Owens DK, Bendavid E. The cost-effectiveness of symptom-based testing and routine screening for acute HIV infection in men who have sex with men in the USA. AIDS 2011; 25:1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han XX, Xu JJ, Chu ZX, Chu Z, Dai D, Dai D, et al. Screening acute HIV infections among Chinese men who have sex with men from voluntary counseling and testing centers. PLoS One 2011; 6:e28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasry A, Sansom SL, Hicks KA, Uzunangelov V. Allocating HIV prevention funds in the United States: recommendations from an optimization model. PLoS One 2012; 7:e37545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JH, Gilmour S, Zhang HQ, Koyanagi A, Shibuya K. The epidemiological impact and cost-effectiveness of HIV testing, antiretroviral treatment and harm reduction programs. AIDS 2012; 26:2069–2078. [DOI] [PubMed] [Google Scholar]
- 33.Long EF, Mandalia R, Mandalia S, Alistar SS, Beck EJ, Brandeau ML. Expanded HIV testing in low-prevalence, high-income countries: a cost-effectiveness analysis for the United Kingdom. PLoS One 2014; 9:e95735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walensky RP, Sax PE, Nakamura YM, Weinstein M, Pel P, Freedberg K, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


