Supplemental Digital Content is available in the text.
Keywords: aldosterone, angiotensin II, mean arterial pressure, renin-angiotensin system, safety
Abstract
Objective:
Angiotensin II is an endogenous hormone with vasopressor and endocrine activities. This is a systematic review of the safety of IV angiotensin II.
Data Sources:
PubMed, Medline, Scopus, and Cochrane.
Study Selection:
Studies in which human subjects received IV angiotensin II were selected whether or not safety was discussed.
Data Extraction:
In total, 18,468 studies were screened by two reviewers and one arbiter. One thousand one hundred twenty-four studies, in which 31,281 participants received angiotensin II (0.5–3,780 ng/kg/min), were selected. Data recorded included number of subjects, comorbidities, angiotensin II dose and duration, pressor effects, other physiologic and side effects, and adverse events.
Data Synthesis:
The most common nonpressor effects included changes in plasma aldosterone, renal function, cardiac variables, and electrolytes. Adverse events were infrequent and included headache, chest pressure, and orthostatic symptoms. The most serious side effects were exacerbation of left ventricular failure in patients with congestive heart failure and bronchoconstriction. One patient with congestive heart failure died from refractory left ventricular failure. Refractory hypotensive shock was fatal in 55 of 115 patients treated with angiotensin II in case studies, cohort studies, and one placebo-controlled study. One healthy subject died after a pressor dose of angiotensin II was infused continuously for 6 days. No other serious adverse events attributable to angiotensin II were reported. Heterogeneity in study design prevented meta-analysis.
Conclusion:
Adverse events associated with angiotensin II were infrequent; however, exacerbation of asthma and congestive heart failure and one fatal cerebral hemorrhage were reported. This systematic review supports the notion that angiotensin II has an acceptable safety profile for use in humans.
Angiotensin II (ATII) is a naturally occurring octapeptide hormone component of the renin-angiotensin-aldosterone system (RAAS) and is a potent vasoconstrictor (1). ATII has important roles in cardiovascular, neurologic, and renal physiology, including maintenance of blood pressure, thirst sensation, response to the baroreceptor reflex, determination of renal blood flow and glomerular filtration rate, and electrolyte and free water homeostasis (2). ATII has also been implicated as a contributor to pathophysiology in certain conditions. Effects on myocardial structure, particularly adverse remodeling in the setting of congestive heart failure, which are mediated through a variety of receptors and mechanisms, are well described (3).
Since 1941 (4), ATII has been administered to humans in studies of vascular resistance and hypertension and has been given to healthy subjects for up to 11 days (5, 6). In the mid-1960s, IV ATII was widely administered to pregnant women in an attempt to identify those at risk for preeclampsia (7, 8). IV ATII has also been administered to patients with a broad range of medical conditions, including cardiovascular, renal, hepatic, and pulmonary diseases, endocrine and metabolic disorders, and traumatic injuries and shock. ATII has also been administered to children with cancer (9), septic shock (10), and congenital cardiac shunts (11).
ATII has been studied in combination with catecholamines, antihypertensives, anesthetics and analgesics, prostaglandins, indomethacin, and corticosteroids. IV ATII safely restored mean arterial pressure in patients with catecholamine-refractory hypotension following angiotensin converting enzyme (ACE) inhibitor overdose (12–14). A number of case reports and studies reported the successful use of ATII in the treatment of vasopressor-resistant hypotensive septic shock (10, 15–18). A randomized, placebo-controlled pilot study evaluated the use of ATII infusion in high-output shock and showed a decrease in norepinephrine doses in patients receiving ATII (19).
IV ATII has been shown to exacerbate only two underlying diseases, asthma (20) and congestive heart failure (21). In patients with mild asthma, IV ATII has the capacity to worsen bronchospasm induced by methacholine (22) but not by histamine (23); previous studies, however, have documented safe use in patients with chronic obstructive pulmonary disease (24). ATII was noted to exacerbate left ventricular failure when administered to three patients with acute congestive heart failure (21).
Further interest in the use of IV ATII to treat a variety of conditions is likely to grow. To date, there has been no comprehensive documentation of adverse effects associated with administration of exogenous ATII to humans. This systematic review describes the nature, severity, and prevalence of side effects reported in the literature.
METHODS
Search Method
We performed a comprehensive literature search on PubMed, Medline, Scopus, and Cochrane to identify studies that involved IV infusion of ATII in human subjects, initially using the key terms, “IV administration,” “IV injection,” or “IV infusion” combined with “ATII.” Further searches without the “IV” criterion (i.e., “ATII,” filtered to exclude animal and in vitro studies) were conducted to ensure that the search was complete; search results were reviewed and references with non-IV use were discarded. Accepted articles were limited to primary studies of human participants written in the English language. Articles were added to the citation manager, and duplicates and review articles were discarded. Two reviewers (D.M.C., D.Y.) assessed satisfaction of inclusion criteria by abstract review prior to retrieving the full-length article. An additional reviewer (L.W.B.) assessed for appropriateness of including the article in the analysis.
Inclusion and Exclusion Criteria
Studies in which human subjects received IV administered ATII were included whether or not side effects or complications were noted. Side effects were defined as measureable physiologic effects that were unintended or incongruous with the pressor effects of ATII. Adverse events (AEs) were those referred to as such by the authors or described as untoward effects, complications, or symptoms reported by subjects. Exclusion criteria were intentionally minimal and included lack of human subjects, non-IV dosing, or insufficient data on the effect of ATII. Either of two synthetic forms with similar biological activity (6), ile5-ATII identical to the human sequence or val5-ATII amide based on the bovine sequence, was used in most of the studies cited herein; the source or sequence was not identified in others. Studies of other analogs were excluded.
A number of subjects identified by comparisons of authors, chronology, baseline characteristics, and treatments were apparently reported in more than one article but have been counted only once in summaries of number of subjects exposed.
Analysis
After abstract review, full-text articles were retrieved for all relevant studies and analyzed for the physiologic effects, AEs and serious AEs of ATII. Studies were grouped according to the target organ system. Two separate reviewers (D.M.C., D.Y.) extracted the number of subjects, dosages of ATII, any concurrent drug administration, the type of study, and patient comorbidities. A third reviewer (L.W.B.) verified the information.
RESULTS
Study Characteristics
The initial search yielded 18,468 studies. After application of exclusion criteria, 1,124 studies were suitable for analysis (complete reference list in Supplemental Content 1, http://links.lww.com/CCM/C617). These studies, published from 1941 to 2016, encompassed a total of 31,281 subjects who were administered ATII IV at infusion rates ranging from 0.05 (25) to 3,780 ng/kg/min (26). The majority of subjects received less than or equal to 30 ng/kg/min or a bolus injection of a few micrograms. The highest reported doses were administered in the following settings: one patient with pancreatic cancer (3,780 ng/kg/min) (26); 32 adults with sepsis, systemic inflammatory response syndrome, or liver failure (maximum, 1,600 ng/kg/min; mean, 550 ng/kg/min) (17); two children with septic shock (doses of 160 and 800 ng/kg/min, respectively) (10); seven normal volunteers (100 ng/kg/min) (27); six children with cancer (up to 80 ng/kg/min) (9); and 43 pregnant women (up to 64 ng/kg/min) (7). The duration of infusion was generally minutes to hours although one study applied continuous infusion of ATII at pressor doses for up to 11 days (5). Approximately 10% of studies entailed infusion of ATII into a brachial, coronary, or hepatic artery; these studies are not included in this report.
Studies were categorized according to the primary organ system of interest, including cardiovascular (471 studies, 13,993 subjects), endocrine (226 studies, 3,605 subjects), renal (211 studies, 5,924 subjects), reproductive systems/pregnancy (71 studies, 3,278 subjects), gastrointestinal (80 studies, 3,509 subjects), pulmonary (15 studies, 167 subjects), neurologic (26 studies, 460 subjects), hematologic and immune (10 studies, 117 subjects), and other organ systems (three studies, 43 subjects). Eleven studies reported use of ATII with chemotherapy in 185 patients with solid tumors.
Frequency of Physiologic and Side Effects of ATII
Following the availability of synthetic ATII, studies in the early 1960s documented the following rapid physiologic changes with IV administration: increased systemic blood pressure, increased pulmonary arterial and wedge pressures (without change in ventilatory function), reduced heart rate and cardiac output, decreased peripheral blood flow, decreased renal blood flow and glomerular filtration rate, reduction in sodium and water excretion, and increased plasma aldosterone (5, 28–34). These studies also established that patients with cirrhosis and ascites were less sensitive to the pressor effects of ATII and had a paradoxical renal response of natriuresis and diuresis compared with healthy subjects (5, 33).
Among all studies, the most commonly cited physiologic effect of ATII was elevation of blood pressure, which was reported in the majority of studies. The acute pressor effect of ATII is shown for 34 representative studies that illustrate relationships between ATII dose and the magnitude of the pressor response (Supplemental Content 2, http://links.lww.com/CCM/C618). Doses eliciting a blood pressure increase of at least 5% ranged from 0.5 to 75 ng/kg/min. The most commonly cited nonpressor effects of ATII included altered renal function, increased plasma aldosterone and other changes in RAAS, other endocrine perturbations, and alterations of electrolyte balance. Increased plasma aldosterone was reported in 182 studies using ATII doses from 1 to 20 ng/kg/min; comprehensive references and details for representative studies that illustrate dose-response relationships are in Supplemental Content 3 (http://links.lww.com/CCM/C619).
Effects of ATII by Organ System
Discussions of ATII activities specifically related to the cardiovascular, endocrine pulmonary, renal, hematologic, immunological, and neurologic systems, as well as discussions relating to oncology and pregnancy are provided in Supplemental Content 4 (http://links.lww.com/CCM/C620).
Summary of AE Data
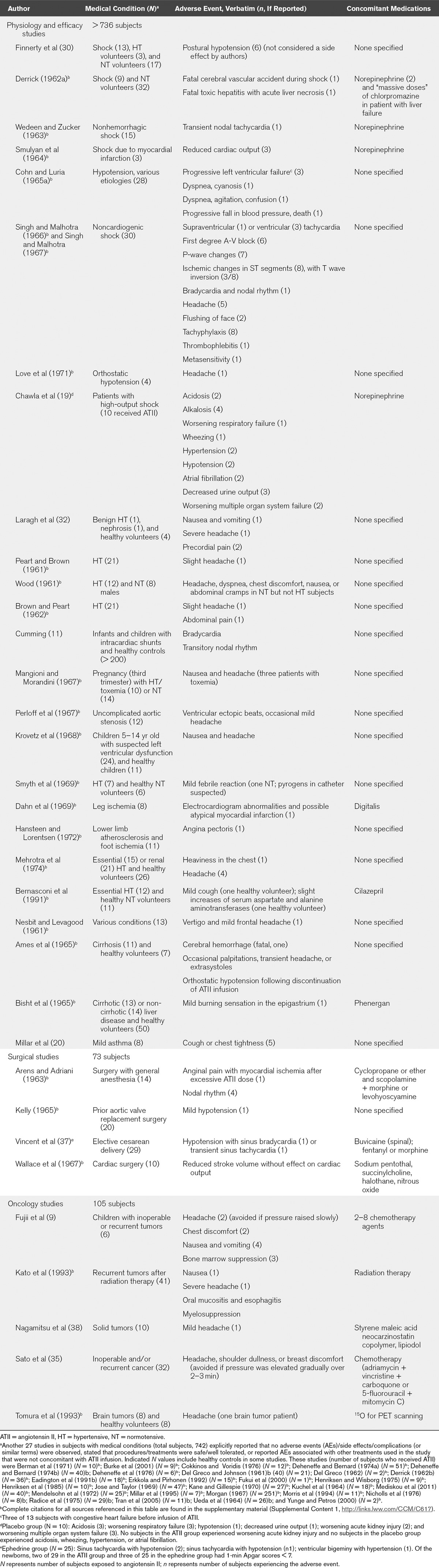
The majority of studies analyzed herein were not prospectively designed to collect safety data. Accordingly, of the 1,124 studies included in this analysis, 982 studies did not mention safety and 75 studies variably reported that there were no complications, AEs, side effects, or patient-reported subjective feelings or symptoms. Eight studies reported ATII dose reductions or discontinuations due to excessive pressor responses. The remaining 59 studies reported the AEs listed in Tables 1 and 2. The most common symptoms (reported in ≥ 2 studies) were headache, sensation of chest pressure/tightness, dyspepsia/nausea, bradycardia, and orthostatic hypotension/dizziness. One occurrence of headache was characterized as severe (32). Patients with mild asthma reported cough or chest tightness (20). ATII was noted to exacerbate the left ventricular failure when administered to three patients with acute congestive heart failure (21). Frequent or severe gastrointestinal AEs occurred only when ATII was coadministered with chemotherapy (9, 35).
TABLE 1.
Reported Adverse Events by Study—Healthy Volunteers
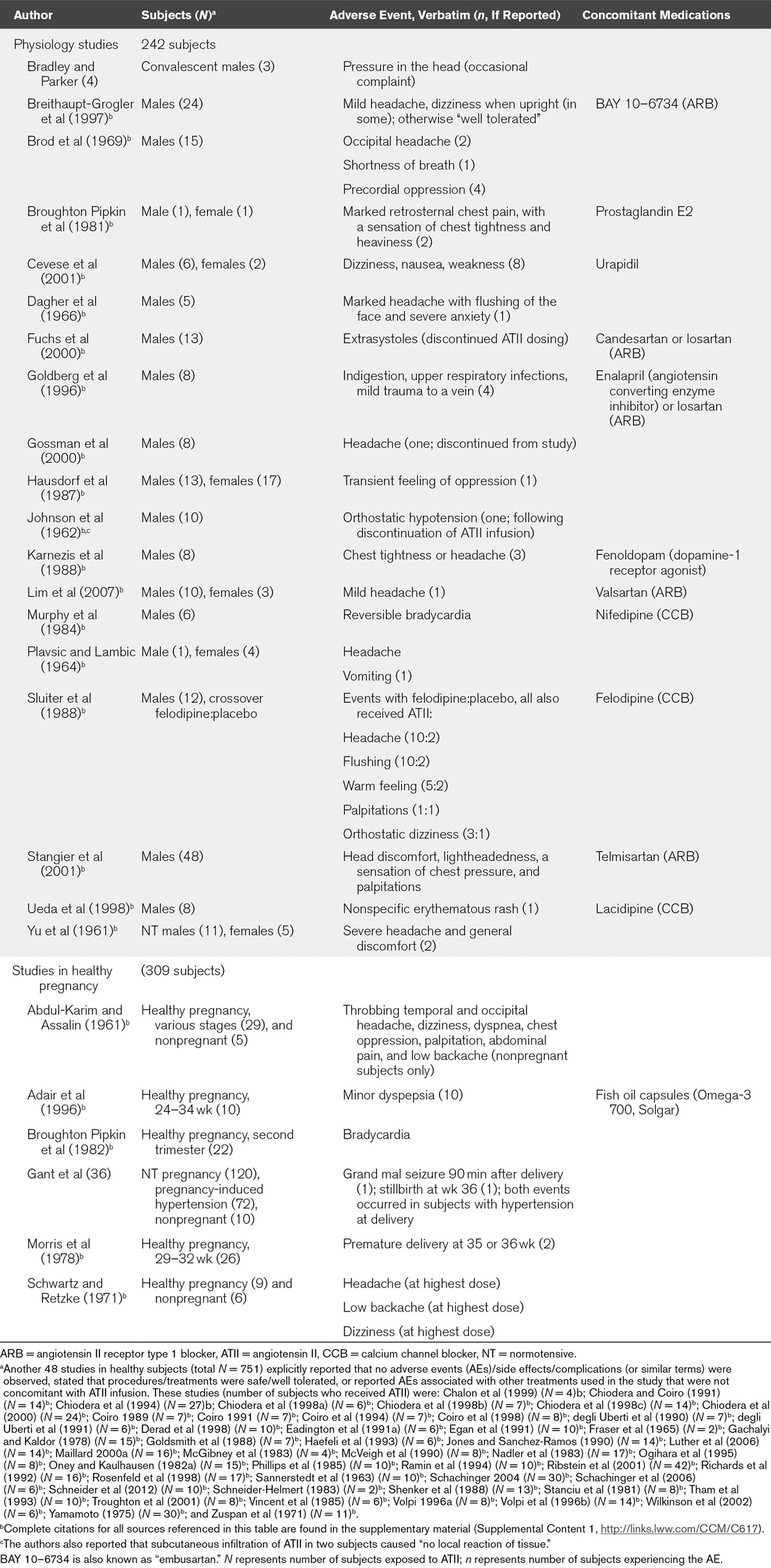
Among 192 pregnant teenaged girls monitored for ATII sensitivity during pregnancy, 72 developed preeclampsia including two who had complications at delivery (one stillbirth with placental abruption and one seizure following delivery of a healthy infant) (36). These events occurred 6 and 12 days after the last ATII infusion, respectively. No AEs or pregnancy complications were reported for the 120 girls who remained normotensive during pregnancy.
A placebo-controlled study of ATII in the treatment of severely ill patients with catecholamine-resistant shock reported the following AEs in the ATII group (n = 10) but not in the placebo group (n = 10): alkalosis (four patients); hypertension (two patients); atrial fibrillation (two patients); and wheezing (one patient) (19). The 30-day mortality rate was similar for the ATII and placebo groups (50% vs 60%; p = 1.00).
In an early study of the effects of prolonged ATII infusion, a death in a 36-year-old healthy subject after 6 days continuous infusion of a pressor dose of ATII (1.5 µg/min to maintain blood pressure at approximately 150/100 mm Hg) occurred following a cerebral hemorrhage during straining at stool (5). The subject experienced sodium retention over the first 5 days of ATII infusion (net +265 mEq) followed by “escape” on day 6 with sodium excretion of 127 mEq/d compared with 2 mEq/d on day 2. A few minutes before the event, blood pressure was 180/100 mm Hg. One patient with preinfusion symptoms of acute heart failure expired after he experienced a decline in cardiac output, which was not restored despite the initiation of IV norepinephrine (21). Upon initiation of ATII infusion, this patient experienced no pressor effect, a decline in cardiac output of 12%, and a decrease in systemic vascular resistance of 9.6%.
The only other deaths reported after ATII administration were associated with preexisting septicemia or shock, or occurred months after ATII was infused alongside chemotherapy in patients with inoperable cancer (35, 38). Among patients with one or more categories of distributive shock diagnosed (or inferred based on reported clinical information), 55 deaths occurred (16, 17, 19, 39–44). However, the reported deaths were not attributed to use of ATII.
DISCUSSION
Overall, 31,281 participants have been exposed to IV ATII in the studies reviewed including healthy normotensive subjects, normotensive and preeclamptic pregnant women, subjects with hypertension, congestive heart failure, diabetes, solid tumors, and other comorbidities, critically ill patients, and children. Excluding subjects with refractory shock and oncology patients receiving ATII with chemotherapy, there have been two deaths and fewer than 300 subjects with AEs reported.
There were two deaths not linked to sepsis, shock, or cancer. One case involved a cerebral hemorrhage in a 36-year-old healthy volunteer receiving a continuous infusion of ATII for 6 days with associated sodium retention and sustained hypertension. The other case involved fulminant left ventricular failure in a patient with preexisting acute decompensated heart failure. Since these studies, both published in 1965, studies in healthy subjects have generally used infusions of much shorter duration. In one study of five healthy volunteers, a continuous infusion of ATII at 2 ng/kg/min was maintained for 66 hours with additional incremental dose challenges on days 1 and 3; with that, sodium and potassium balances were stable and no AEs were reported (6). Beyond the cerebrovascular event and the left ventricular failure, there is no documentation of exogenous ATII association with serious AEs or morbidities, although exacerbation of existing asthma or congestive heart failure remains a potential risk. Two adverse outcomes were reported during pregnancy, placental abruption and eclampsia, but both occurred well after both patients received ATII infusions (6 and 12 d, respectively, postinfusion) and were thus unlikely to be related to ATII. Reports of other AEs were limited. Among the relatively few studies that reported AEs, transient headache (26 studies) and abnormal chest sensations (10 studies) were reported most frequently. A period of orthostatic hypotension following discontinuation of ATII infusion was reported in several studies, suggesting that dose tapering might minimize symptoms.
Our review is limited by the heterogeneity of study design, which precluded formal meta-analysis or calculation of integrated AE rates. Studies differed in objectives; inclusion/exclusion criteria, comorbidities, and baseline characteristics; ATII amino acid sequence, dose, and duration; endpoints; and the collection of AE data. Most studies were designed to understand the physiologic changes induced by exogenous ATII and did not prospectively collect safety data. Hence, the data reported herein are obtained from a limited subset of included studies, which could represent a reporting bias. Additionally, there was no standard terminology for patient-reported symptoms.
Most of the studies used one of two synthetic forms of ATII, an amide derivative of the bovine amino acid sequence or an acetate salt of the human sequence. The human form showed greater pressor activity than the bovine form in one comparative study (45), but pressor and aldosterone-stimulating activities were similar in other limited comparisons (46, 47). No studies have directly compared the safety of the two forms in humans. Finally, although multiple broad search terms were used, there is a possibility that we may have missed relevant articles, including those in languages other than English.
ATII administered IV equilibrates rapidly in the circulation and has a half-life in plasma of approximately 1 minute or less (48, 49). Pharmacodynamics is such that blood pressure increases within minutes, is sustained at a fixed infusion rate, and returns to preinfusion values within minutes after cessation of the infusion (50). As such, direct links between dosages and changes in organ system responses could not be obtained across all studies.
In general, dosages of 1–10 ng/kg/min in normotensive or hypertensive subjects were required to elicit systemic pressor responses of 10–30 mm Hg. The magnitude of the pressor response was variable between subjects for a given dose but was dose dependent when individuals were administered successive incremental doses. In the physiologic studies, ATII was typically infused for a few minutes to a few hours in both normotensive and hypertensive subjects. In studies evaluating hypotensive subjects, much larger therapeutic dosages (50–1,600 ng/kg/min) were administered for 1–7 days to achieve hemodynamic stabilization and included administration of other vasoconstrictors (norepinephrine, vasopressin). Safety risks under these two radically different scenarios are likely to be very different. The only placebo-controlled study designed to assess safety as well as efficacy in severely ill patients found a reduced norepinephrine requirement, low frequency of AEs, and similar mortality rates (19).
These findings have important implications for clinical practice. ATII has demonstrated the ability to restore arterial pressure and reduce the need for potentially harmful doses of catecholamines in patients with refractory septic shock, improve natriuresis and diuresis in patients with liver failure, rescue subjects following ACE inhibitor overdose, and enhance chemotherapy delivery to solid tumors. Although a discussion of the efficacy of ATII is beyond the scope of this analysis, it should be noted that among patients included in this report with septic shock, catecholamine-resistant shock, ACE inhibitor overdose, and anesthesia-induced hypotension, outcomes seem to have been changed in a number of cases (10, 12–19, 21, 37, 51).
To our knowledge, ATII formulated for clinical use is currently available for research purposes only and is not an approved therapy for any indication. (Angiotensin amide was previously marketed by Ciba-Geigy under the trade name Hypertensin for “states of shock and circulatory collapse.”) A phase 3 study assessing the safety and efficacy of ATII in catecholamine-resistant hypotension is ongoing (NCT02338843, www.clinicaltrials.gov). Where current literature falls short is in establishing a safe therapeutic range, with minimal AEs, for the potential clinical applications of ATII.
In summary, ATII has multiple potential clinical applications, with the recent focus being its use as a rescue vasopressor in catecholamine-resistant high-output shock. Establishing the dose range and tolerability of ATII is of fundamental importance, and the data included herein indicate that IV ATII may be safe in a variety of clinical circumstances. Further prospective trials are needed to more completely establish the appropriate dosing and duration of therapy.
TABLE 2.
Reported Adverse Events by Study—Patients With Medical Conditions
ACKNOWLEDGMENT
Assistance with database searching and manuscript revision was provided by Richard Boehme, PhD, and Christopher Barnes, PhD, of Articulate Science LLC and funded by La Jolla Pharmaceutical Company.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by La Jolla Pharmaceutical Co.
Drs. Busse and Wang served as the principal investigators (PIs) for their institutions in a global multi-institution phase 3 trial evaluating angiotensin II as a pressor drug in catecholamine resistant shock. This phase 3 trial was sponsored by La Jolla Pharmaceuticals, which manufactures the drug and supported this manuscript. However, neither physician nor their institutions received any compensation for time and contributions to the manuscript. Dr. Busse reports having received consulting fees from La Jolla. Dr. Khanna was the site PI for the Angiotensin II in High Output Shock trial (ATHOS3). Drs. Dana and Chawla are employed by La Jolla Pharmaceuticals. Dr. Dana was a former employee of Merck & Co and Cubist Pharmaceuticals, as well as a current shareholder in Ligand Pharmaceuticals and La Jolla Pharmaceutical Company. Dr. Chawla is a current shareholder of La Jolla Pharmaceutical Company. The authors discuss angiotensin II in this article, an investigational drug. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Basso N, Terragno NA. History about the discovery of the renin-angiotensin system. Hypertension 2001; 38:1246–1249.. [DOI] [PubMed] [Google Scholar]
- 2.Struthers AD, MacDonald TM. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res 2004; 61:663–670.. [DOI] [PubMed] [Google Scholar]
- 3.Weber KT. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation 1997; 96:4065–4082.. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SE, Parker B. The hemodynamic effects of angiotonin in normal man. J Clin Invest 1941; 20:715–719.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames RP, Borkowski AJ, Sicinski AM, et al. Prolonged infusions of angiotensin II and norepinephrine and blood pressure, electrolyte balance, and aldosterone and cortisol secretion in normal man and in cirrhosis with ascites. J Clin Invest 1965; 44:1171–1186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oelkers W, Schöneshöfer M, Schultze G, et al. Effect of prolonged low-dose angiotensin II infusion on the sensitivity of adrenal cortex in man. Circ Res 1975; 36(6 Suppl 1):49–56.. [DOI] [PubMed] [Google Scholar]
- 7.Conti C, Tranquilli AL, Garzetti GG, et al. Modulation of vascular reactivity after acute calcium antagonist administration in pregnant women moderately sensitive to angiotensin infusion. Boll Soc Ital Biol Sper 1994; 70:243–248.. [PubMed] [Google Scholar]
- 8.Oney T, Kaulhausen H. The value of the angiotensin sensitivity test in the early diagnosis of hypertensive disorders in pregnancy. Am J Obstet Gynecol 1982; 142:17–20.. [DOI] [PubMed] [Google Scholar]
- 9.Fujii Y, Hongo T, Masui H, et al. Angiotensin-induced hypertension chemotherapy in children with advanced solid tumors. Acta Paediatr Jpn 1991; 33:381–383.. [DOI] [PubMed] [Google Scholar]
- 10.Yunge M, Petros A. Angiotensin for septic shock unresponsive to noradrenaline. Arch Dis Child 2000; 82:388–389.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumming GR. Acute hemodynamic effects of angiotensin II. Preliminary report. Can Med Assoc J 1963; 88:827–832.. [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson T, Corke C, Agar J. Enalapril overdose treated with angiotensin infusion. Lancet 1993; 341:703. [DOI] [PubMed] [Google Scholar]
- 13.Newby DE, Lee MR, Gray AJ, et al. Enalapril overdose and the corrective effect of intravenous angiotensin II. Br J Clin Pharmacol 1995; 40:103–104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trilli LE, Johnson KA. Lisinopril overdose and management with intravenous angiotensin II. Ann Pharmacother 1994; 28:1165–1168.. [DOI] [PubMed] [Google Scholar]
- 15.Ryding J, Heslet L, Hartvig T, et al. Reversal of ‘refractory septic shock’ by infusion of amrinone and angiotensin II in an anthracycline-treated patient. Chest 1995; 107:201–203.. [DOI] [PubMed] [Google Scholar]
- 16.Thomas VL, Nielsen MS. Administration of angiotensin II in refractory septic shock. Crit Care Med 1991; 19:1084–1086.. [DOI] [PubMed] [Google Scholar]
- 17.Whiteley SM, Dade JP. Treatment of hypotension in septic shock. Lancet 1996; 347:622. [PubMed] [Google Scholar]
- 18.Wray GM, Coakley JH. Severe septic shock unresponsive to noradrenaline. Lancet 1995; 346:1604. [DOI] [PubMed] [Google Scholar]
- 19.Chawla LS, Busse L, Brasha-Mitchell E, et al. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): A pilot study. Crit Care 2014; 18:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar EA, Angus RM, Hulks G, et al. Activity of the renin-angiotensin system in acute severe asthma and the effect of angiotensin II on lung function. Thorax 1994; 49:492–495.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn JN, Luria MH. Studies in clinical shock and hypotension. II. Hemodynamic effects of norepinephrine and angiotensin. J Clin Invest 1965; 44:1494–1504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar EA, Nally JE, Thomson NC. Angiotensin II potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J 1995; 8:1838–1841.. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay SG, Clayton RA, Dagg KD, et al. Effect of angiotensin II on histamine-induced bronchoconstriction in the human airway both in vitro and in vivo. Respir Med 1997; 91:609–615.. [DOI] [PubMed] [Google Scholar]
- 24.Jezek V, Schrijen F. Haemodynamic reaction to exercise and increased afterload in the detection of right heart failure in pulmonary diseases. Cor Vasa 1980; 22:272–280.. [PubMed] [Google Scholar]
- 25.Radice M, Folli G, Giani P, et al. Latent myocardial contractile impairment in patients with angina pectoris. Acta Cardiol 1975; 30:333–341.. [PubMed] [Google Scholar]
- 26.Louis WJ, Doyle AE. The effects of varying doses of angiotensin on renal function and blood pressure in man and dogs. Clin Sci 1965; 29:489–504.. [PubMed] [Google Scholar]
- 27.Goldsmith SR, Dodge-Brown D, Pentel P. Effects of infused norepinephrine and angiotensin-II on vasopressin levels in humans. Am J Med Sci 1988; 295:513–516.. [DOI] [PubMed] [Google Scholar]
- 28.Depasquale NP, Burch GE. Angiotensin II, digital blood flow, and the precapillary and postcapillary blood vessels of man. Ann Intern Med 1963; 58:278–292.. [DOI] [PubMed] [Google Scholar]
- 29.Depasquale NP, Burch GE. Effect of angiotensin II on the intact forearm veins of man. Circ Res 1963; 13:239–245.. [DOI] [PubMed] [Google Scholar]
- 30.Finnerty FA, Jr, Massaro GD, Chupkovich V, et al. Evaluation of the pressor, cardiac, and renal hemodynamic properties of angiotensin II in man. Circ Res 1961; 9:256–263.. [DOI] [PubMed] [Google Scholar]
- 31.Gill JR, Jr, Barbour BH, Slater JD, et al. Effect of angiotensin II on urinary dilution in normal man. Am J Physiol 1964; 206:750–754.. [DOI] [PubMed] [Google Scholar]
- 32.Laragh JH, Angers M, Kelly WG, et al. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA 1960; 174:234–240.. [DOI] [PubMed] [Google Scholar]
- 33.Laragh JH, Cannon PJ, Bentzel CJ, et al. Angiotensin II, norepinephrine, AND renal transport of electrolytes and water in normal man and in cirrhosis with ascites. J Clin Invest 1963; 42:1179–1192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segel N, Harris P, Bishop JM. The effects of synthetic hypertensin of the systemic and pulmonary circulations in man. Clin Sci 1961; 20:49–61.. [PubMed] [Google Scholar]
- 35.Sato H, Sato K, Sato Y, et al. Induced hypertension chemotherapy of cancer patients by selective enhancement of drug delivery to tumor tissue with angiotensin II. Sci Rep Res Inst Tohoku Univ Med 1981; 28:32–44.. [PubMed] [Google Scholar]
- 36.Gant NF, Daley GL, Chand S, et al. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973; 52:2682–2689.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent RD, Jr, Werhan CF, Norman PF, et al. Prophylactic angiotensin II infusion during spinal anesthesia for elective cesarean delivery. Anesthesiology 1998; 88:1475–1479.. [DOI] [PubMed] [Google Scholar]
- 38.Nagamitsu A, Greish K, Maeda H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: Cases of advanced solid tumors. Jpn J Clin Oncol 2009; 39:756–766.. [DOI] [PubMed] [Google Scholar]
- 39.Cohn JN, Luria MH. Studies in clinical shock and hypotension. 3. Comparative effects of vasopressor drugs and dextran. Arch Intern Med 1965; 116:562–566.. [PubMed] [Google Scholar]
- 40.Del Greco F, Johnson DC. Clinical experience with angiotensin II in the treatment of shock. JAMA 1961; 178:994–999.. [DOI] [PubMed] [Google Scholar]
- 41.Nassif AC, Nolan TR, Corcoran AC. Angiotensin II in treatment of hypotensive states. JAMA 1963; 183:751–754.. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Malhotra RP. Comparative study of angiotensin and nor-adrenaline in hypotensive states (shock). J Assoc Physicians India 1966; 14:639–645.. [PubMed] [Google Scholar]
- 43.Tristani FE, Cohn JN. Studies in clinical shock and hypotension. VII. Renal hemodynamics before and during treatment. Circulation 1970; 42:839–851.. [DOI] [PubMed] [Google Scholar]
- 44.Udhoji VN, Weil MH. Circulatory effects of angiotensin, levarterenol and metaraminol in the treatment of shock. N Engl J Med 1964; 270:501–505.. [DOI] [PubMed] [Google Scholar]
- 45.Kono T, Taniguchi A, Imura H, et al. Relative biological activities of Asn1-,Val5-angiotensin II, Ile5-angiotensin II and Sar1-angiotensin II in man. Life Sci 1985; 37:365–369.. [DOI] [PubMed] [Google Scholar]
- 46.Boyd GW, Adamson AR, Arnold M, et al. The role of angiotensin II in the control of aldosterone in man. Clin Sci 1972; 42:91–104.. [DOI] [PubMed] [Google Scholar]
- 47.Oelkers W, Brown JJ, Fraser R, et al. Sensitization of the adrenal cortex to angiotensin II in sodium-deplete man. Circ Res 1974; 34:69–77.. [DOI] [PubMed] [Google Scholar]
- 48.Donato L, Coli A, Pasqualini R, et al. Metabolic clearance rate of radioiodinated angiotensin II in normal men. Am J Physiol 1972; 223:1250–1256.. [DOI] [PubMed] [Google Scholar]
- 49.Magness RR, Cox K, Rosenfeld CR, et al. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am J Obstet Gynecol 1994; 171:668–679.. [DOI] [PubMed] [Google Scholar]
- 50.Vittorio TJ, Lang CC, Katz SD, et al. Vasopressor response to angiotensin II infusion in patients with chronic heart failure receiving beta-blockers. Circulation 2003; 107:290–293.. [DOI] [PubMed] [Google Scholar]
- 51.Ramin SM, Ramin KD, Cox K, et al. Comparison of prophylactic angiotensin II versus ephedrine infusion for prevention of maternal hypotension during spinal anesthesia. Am J Obstet Gynecol 1994; 171:734–739.. [DOI] [PubMed] [Google Scholar]


