Supplemental Digital Content is available in the text.
Keywords: endotoxin, hemoperfusion, mortality, polymyxin B, sepsis
Abstract
Objective:
Several studies have reported a survival benefit for polymyxin B hemoperfusion treatment in patients with severe sepsis and septic shock. However, recently, a propensity-matched analysis and a randomized controlled trial reported no survival benefit for polymyxin B hemoperfusion treatment. We performed an up-to-date meta-analysis to determine the effect of polymyxin B hemoperfusion treatment on mortality in patients with severe sepsis and septic shock.
Data Sources:
PubMed, Embase, and Cochrane Library were searched from inception to May 2016.
Study Selection:
Studies investigating the effect of polymyxin B hemoperfusion on mortality were considered eligible. We searched for terms related to severe sepsis and septic shock and terms related to polymyxin B hemoperfusion.
Data Extraction:
The following data were extracted from the original articles: the name of the first author and publication year, subjects and setting, inclusion and exclusion criteria, mean age and size of the study population, male percentage, mortality, blood pressure, Sequential Organ Failure Assessment score, pulmonary oxygenation, and levels of endotoxin and humoral cytokines.
Data Synthesis:
A total of 17 trials were included. The pooled risk ratio for overall mortality was 0.81 (95% CI, 0.70–0.95), favoring polymyxin B hemoperfusion (p = 0.007). Disease severity subgroup meta-analysis revealed a significant reduction of mortality in the intermediate- and high-risk groups (risk ratio, 0.84; 95% CI, 0.77–0.92 and risk ratio, 0.64; 95% CI, 0.52–0.78, respectively), but not in the low-risk group (risk ratio, 1.278; 95% CI, 0.888–1.839). The nonlinear meta-regression with restricted cubic spline showed an almost linear inverse association between the baseline mortality rate and reduction in the risk of mortality.
Conclusion:
The present study demonstrated that polymyxin B hemoperfusion treatment may reduce mortality in patients with severe sepsis and septic shock in specific disease severity subgroups.
Since the first definition of sepsis and septic shock was established (1), mortality and morbidity have remained high in patients with severe sepsis and septic shock despite decades of medical advances. Lipopolysaccharide, a bacterial endotoxin, is thought to play a key role in the pathogenesis of sepsis (2). Danner et al (3) found that multiple organ failure and left ventricular depression occurred more frequently in endotoxemic septic shock patients than in endotoxin-undetectable septic shock patients, and that endotoxemia was associated with high mortality. Polymyxin B is a cyclic cationic polypeptide antibiotic derived from Bacillus polymyxa; it exhibits antimicrobial activity against Gram-negative bacteria and can bind and neutralize endotoxin (4). An endotoxin removal cartridge has been developed using polymyxin B as an immobilized adsorbent for polymyxin B hemoperfusion (PMX-HP) (5).
After the introduction of PMX-HP, several studies have determined its clinical efficacy for patients with severe sepsis and septic shock (6–8). Nemoto et al (6) reported that PMX-HP treatment significantly improved the overall survival compared with that of the control group (41% vs 11%; p = 0.002). The Early use of polymyxin B hemoperfusion in abdominal septic shock (EUPHAS) trial also showed reduced mortality and improved hemodynamics and pulmonary oxygenation (7). A meta-analysis published in 2011 confirmed these beneficial effects (8). However, two recent studies have shown controversial results. The first study by Iwagami et al (9) did not show any survival benefit for PMX-HP treatment in patients with abdominal septic shock. The second study, a randomized controlled trial (RCT), showed a nonsignificant increase in mortality after PMX-HP treatment in patients with peritonitis-induced septic shock (10).
The discrepancy in the results of studies may be attributed to the severity of severe sepsis and septic shock. In the present study, we hypothesized that PMX-HP treatment improves clinical outcomes only in the target population of patients with high disease severity. We performed a systematic review and meta-analysis and a disease severity subgroup meta-analysis and a meta-regression analysis of the effects of PMX-HP treatment on mortality in patients with severe sepsis and septic shock.
METHODS
Search Strategy and Study Selection
We searched for the following terms in the databases of PubMed, Embase, and Cochrane Library from inception to May 2016: “PMX” or “polymyxin B hemoperfusion” and “septic shock.” We did not apply any language restriction when searching for these terms. For study selection, we initially screened the titles and abstracts. We included studies that met the following criteria: 1) adult patients with septic shock; 2) RCT, propensity-matched cohort study (prospective or retrospective), or historically controlled study; 3) patients received at least one course of PMX-HP treatment; and 4) studies reporting the outcomes of the investigation of the prognostic and hemodynamic variables of PMX-HP treatment in patients with sepsis. The exclusion criteria were as follows: 1) inadequate study type or modality; 2) animal studies; 3) trials involving neonates or pediatric patients; and 4) data on mortality could not be obtained. Two reviewers (T.C., C-T.L.) independently performed screening, and disagreements were resolved by consensus. If an agreement could not be reached, the opinion of a third reviewer (Y-C.Y.) was considered at the conclusion of the screening process.
Data Extraction and Quality Assessment
We extracted data into one file modified from the data extraction template of the Cochrane Consumers and Communication Review Group. The following data were extracted from original articles: name of the first author and publication year, subjects and setting, grouping strategy, enrollment period, mean age of the study population, sample size, male percentage, clinical results before and after intervention (including mortality and blood pressure, either as systolic blood pressure or mean arterial pressure [MAP]), Sequential Organ Failure Assessment (SOFA) score, pulmonary oxygenation (Pao2/Fio2 ratio), and blood levels of endotoxin and cytokines. Any concomitant intervention was also extracted and recorded.
For RCTs, the quality of eligible trials was assessed using the tool of risk of bias summary according to Review Manager software, version 5.3 (Review Manager; The Nordic Cochrane Centre, Copenhagen, Denmark). For non-RCTs, the risk of bias was assessed using the assessment tool of Risk Of Bias in Non-randomized Studies of Interventions (11) and was further graphically summarized using Review Manager software, version 5.3 (The Nordic Cochrane Centre). Publication bias was assessed by visual inspection of a funnel plot and by using Egger test. Two investigators independently performed extraction and risk of bias assessment.
Data Synthesis and Analysis
Study characteristics, the timing of mortality assessment of each study, and the reported mortality of the PMX-HP and conventional treatment groups were summarized. In addition, pre/posttreatment change in MAP, Pao2/Fio2 ratio, SOFA score, and levels of endotoxin and humoral cytokines of PMX-HP and conventional treatment groups were summarized. The outcomes were analyzed using the DerSimonian-Laird random effect models, concerning potential high heterogeneity among studies. Risk ratios (RRs) for overall mortality, with a 95% CI, for the PMX-HP and conventional treatment groups were calculated and presented as summary statistics.
Disease severity subgroup random effect meta-analysis was performed to assess the effects of PMX-HP treatment on mortality in patients with different disease severities. The included studies were stratified into three groups based on the mortality rates of the conventional treatment group: low-risk group (mortality rate < 0.3), intermediate-risk group (0.3–0.6), and high-risk group (> 0.6). RRs with a 95% CI for the mortality-stratified analysis between the PMX-HP and conventional treatment groups were calculated and presented as summary statistics. Furthermore, studies reporting 28- or 30-day mortality rates were selected specifically for disease severity subgroup meta-analysis. RCTs and non-RCTs were separated and compared by subgroup meta-analysis using random effects analysis. In addition, inverse variance method was also used to determine if there is heterogeneity between the two subgroups. The RCTs and non-RCTs were specifically divided into two individual disease severity subgroup meta-analyses.
Both linear and restricted cubic splines nonlinear meta-regression analyses were performed to test the relationship between the baseline mortality rate in the conventional treatment group on the mortality ratio between PMX-HP group and conventional treatment group. Statistical heterogeneity was assessed using Cochran’s Q through the chi-square test and was quantified using the I2 test. Publication bias was assessed by examining asymmetry of the funnel plot. Statistical analysis was performed using STATA/SE 13 (Stata Corp LP, College Station, TX). The results were considered statistically significant when the two-sided p value was less than 0.05.
RESULTS
Search Results and Trial Characteristics
A total of 554 publications were identified using our search strategy, and only 17 papers were included for systematic review and meta-analysis. Figure 1 shows the study selection flowchart. Table 1 summarizes the characteristics of the included individual studies (6, 7, 9, 10, 12–24). Figure 2 shows the quality assessment of eligible RCTs and non-RCTs, respectively. Supplementary Figure 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/C506; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513) presents the funnel plot. According to Egger test, publication bias was considered absent (p = 0.453). The effects of PMX-HP treatment on plasma endotoxin, cytokine levels, hemodynamics, pulmonary oxygenation, and SOFA score are detailed in Supplementary Table 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/C507).
Figure 1.
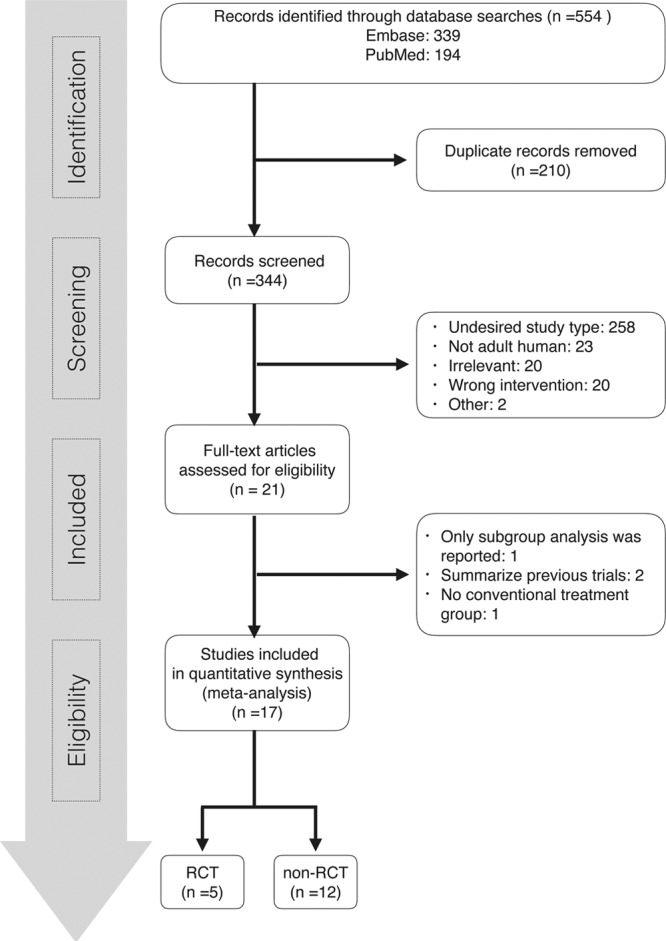
Flowchart of study selection. RCT = randomized controlled trial.
TABLE 1.
Characteristics of Study Design, Patient Population, Timing of Intervention and Outcome Assessment, Baseline Mortality, and Risk Ratio of Included Studies
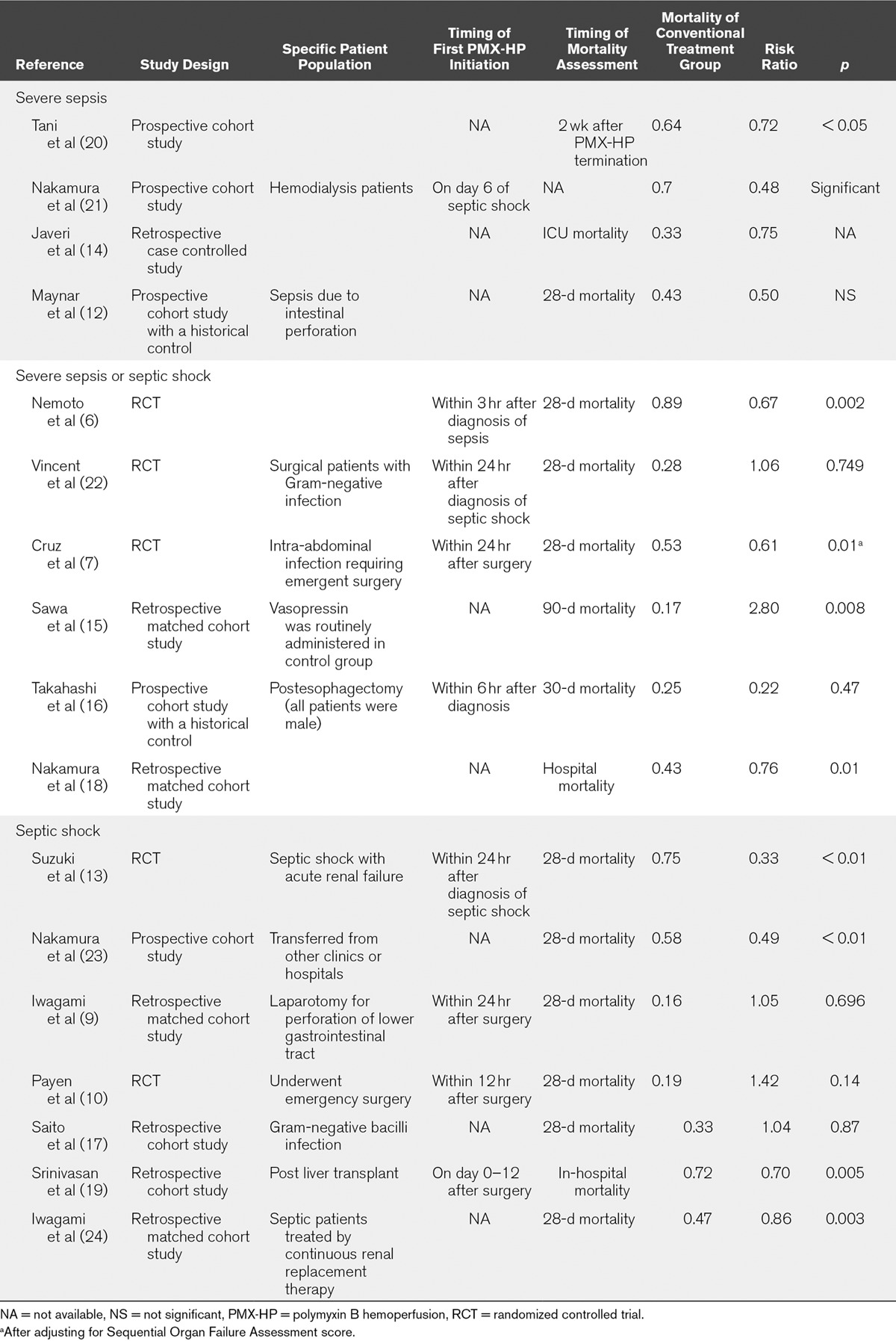
Figure 2.
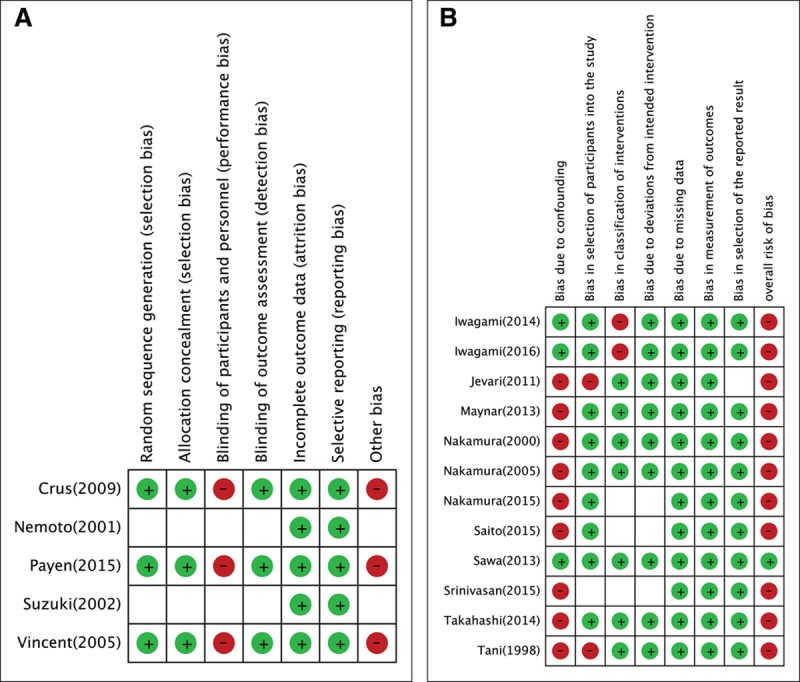
Quality assessment of eligible trials. A, Randomized controlled trials. B, Nonrandomized controlled trials.
Effect on Mortality
Supplementary Figure 2 (Supplemental Digital Content 3, http://links.lww.com/CCM/C508; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513) illustrates the meta-analysis of the 17 included studies. The pooled RR of overall mortality was 0.81 (95% CI, 0.70–0.95; p = 0.007) for PMX-HP treatment. Figure 3 demonstrates the disease severity subgroup meta-analysis of overall mortality. Significant risk reductions were observed in both intermediate- and high-risk groups (RR, 0.84; 95% CI, 0.77–0.92 and RR, 0.64; 95% CI, 0.52–0.78, respectively), but not in the low-risk group (RR, 1.28; 95% CI, 0.89–1.84). Tests of heterogeneity were insignificant in all groups. Supplementary Figure 3 (Supplemental Digital Content 4, http://links.lww.com/CCM/C509; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513) presents the disease severity subgroup meta-analysis of 28- or 30-day mortality. Supplementary Figure 4 (Supplemental Digital Content 5, http://links.lww.com/CCM/C510; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513) presents the separate subgroup meta-analysis of RCTs and non-RCTs, and the RR was 0.73 (95% CI, 0.47–1.15; p = 0.172) in the RCTs and 0.85 (95% CI, 0.73–0.98; p = 0.03) in the non-RCTs. The separate disease severity subgroup meta-analysis of overall mortality for the RCTs and non-RCTs are depicted in Supplementary Figure 5 (Supplemental Digital Content 6, http://links.lww.com/CCM/C511; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513) and Supplementary Figure 6 (Supplemental Digital Content 7, http://links.lww.com/CCM/C512; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/C513), respectively. In disease severity subgroup meta-analysis for RCTs, there is a trend of greater risk reduction in the higher risk group (RR, 0.509; 95% CI, 0.25–1.05; p = 0.067). Linear meta-regression analysis revealed an inverse relationship between the baseline mortality rate in the conventional treatment group and reduction in the risk of mortality (RR, 0.67; 95% CI, 0.55–0.82; p = 0.001). The results of separate linear meta-regression analysis of RCTs and non-RCTs were as follows: RCTs (RR, 0.55; 95% CI, 0.23–1.29; p = 0.11) and non-RCTs (RR, 0.71; 95% CI, 0.55–0.93; p = 0.018). The nonlinear meta-regression with restricted cubic spline showed an almost linear inverse association between the baseline mortality rate and reduction in the risk of mortality (Fig. 4).
Figure 3.
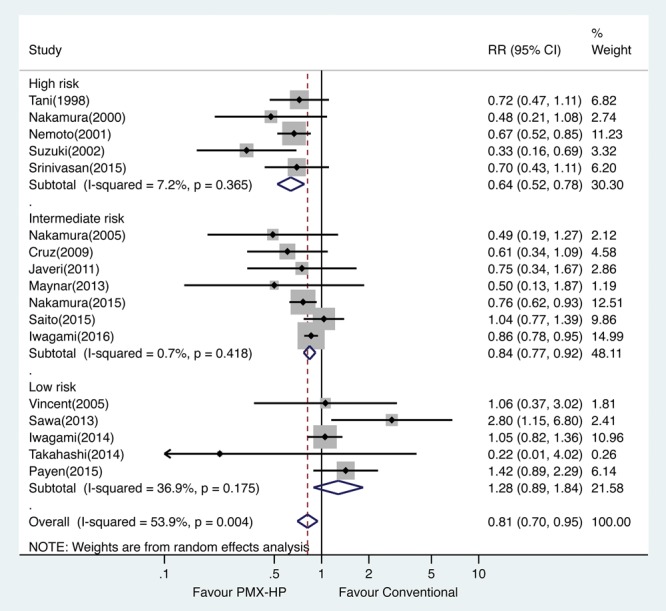
Risk ratios (RRs) of mortality by disease severity subgroup meta-analysis. PMX-HP = polymyxin B hemoperfusion.
Figure 4.
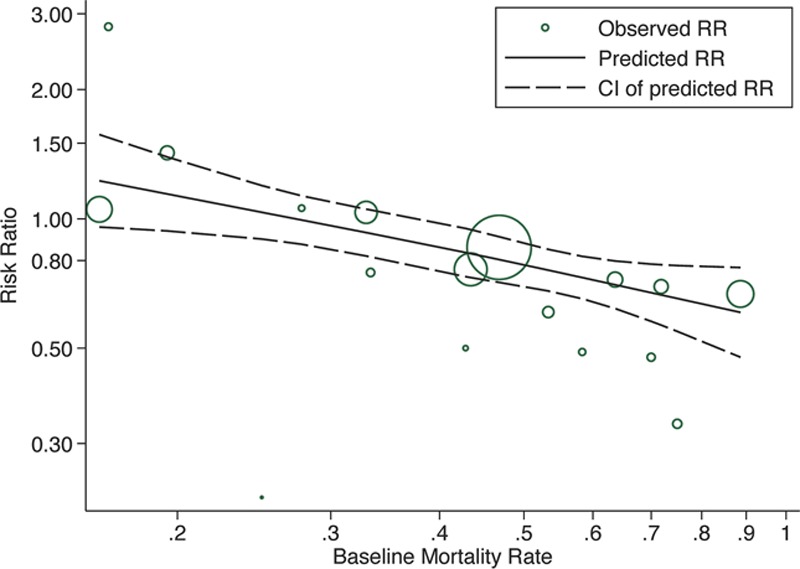
Meta-regression of the effect of the baseline mortality rate of the control group on risk ratio (RR) of mortality after polymyxin B hemoperfusion (PMX-HP) treatment. Cl = confidence limits.
DISCUSSION
This systematic review demonstrates that PMX-HP treatment may reduce mortality in patients with severe sepsis and septic shock. In disease severity subgroup meta-analysis, a significant risk reduction of overall mortality was observed in the intermediate- and high-risk groups, but not in the low-risk group. Meta-regression analysis revealed an almost linear inverse association between the baseline mortality rate in the conventional treatment group and reduction in the risk of mortality.
There are three major differences between this study and the study by Mitaka and Tomita (8). First, we used disease severity subgroup meta-analysis to demonstrate that PMX-HP treatment reduces morality in the intermediate- and high-risk groups, but not in the low-risk group. Second, we referred to more recent studies with less favorable outcomes. Third, we used meta-regression analysis to reveal the inverse association between the baseline mortality rate and reduction in the risk of mortality. One notably concern is that the older studies demonstrated more favorable outcomes after PMX-HP treatment compared with recent studies. Because of early diagnosis and resuscitation of severe sepsis and septic shock in current critical care, the mortality rate has markedly decreased overtime in recent studies. However, it may indicate that the mean mortality is lower, and there still remains a population of septic patients with higher disease severity currently. We must ensure that these patients benefit from the PMX-HP treatment.
In addition to the severity of severe sepsis and septic shock, several other factors may contribute to the discrepancy in the effects of PMX-HP treatment on mortality among studies. First, time to initiation of PMX-HP treatment is crucial. Takeyama et al (25) reported that patients who received PMX-HP treatment within 6 hours after being diagnosed with septic shock had a significantly shorter duration of ventilatory support and a lower catecholamine requirement. Second, acute kidney injury has been reported to amplify the sepsis cascade induced by endotoxin (26–28). Iwagami et al (9) failed to demonstrate any survival benefit of PMX-HP treatment in their retrospective propensity-matched analysis. More recently, however, they reported a significant survival benefit of PMX-HP treatment for septic shock patients complicated with acute kidney injury using the same database (24). This finding is consistent with our meta-analysis, which indicates that selecting the appropriate target population is crucial for PMX-HP treatment. Furthermore, we identified one study in our systematic review that reported a higher mortality rate in their PMX-HP–treated group (15). Thus, we suggest that the complications of PMX-HP treatment, including hemodynamic instability, coagulation, and technical problems, should be carefully monitored (10).
A major limitation of our systematic reviews is that we included RCTs and non-RCTs for meta-analysis. The included non-RCTs in the present review may be at risk of bias because of confounding factors. However, the number of double-blinded, large-scale RCTs of PMX-HP treatment for severe sepsis and septic shock is limited.
The present review has several other limitations. First, the timing of the initiation of PMX-HP treatment differed among the included studies. Second, our study focused on short-term outcomes. Third, although our study suggests that patients with high disease severity may benefit more from PMX-HP treatment, most studies might exclude patients with extremely high disease severity with a mortality rate of greater than 90%.
CONCLUSION
This study revealed that PMX-HP treatment may reduce mortality in patients with severe sepsis and septic shock. Furthermore, the disease severity subgroup meta-analysis indicated a survival benefit related to PMX-HP treatment in the intermediate- and high-risk groups, but not in the low-risk group. We believe that selecting appropriate patients for PMX-HP treatment is crucial to improve patient survival. Additional RCTs targeting selected patients with high disease severity may be warranted to define the clinical role of PMX-HP in current critical care.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.. [PubMed] [Google Scholar]
- 2.Heumann D, Glauser MP, Calandra T. Molecular basis of host-pathogen interaction in septic shock. Curr Opin Microbiol 1998; 1:49–55.. [DOI] [PubMed] [Google Scholar]
- 3.Danner RL, Elin RJ, Hosseini JM, et al. Endotoxemia in human septic shock. Chest 1991; 99:169–175.. [DOI] [PubMed] [Google Scholar]
- 4.Davies B, Cohen J. Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis 2011; 11:65–71.. [DOI] [PubMed] [Google Scholar]
- 5.Hanasawa K, Tani T, Oka T, et al. Selective removal of endotoxin from the blood by extracorporeal hemoperfusion with polymyxin B immobilized fiber. Prog Clin Biol Res 1988; 264:337–341.. [PubMed] [Google Scholar]
- 6.Nemoto H, Nakamoto H, Okada H, et al. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif 2001; 19:361–368.. [DOI] [PubMed] [Google Scholar]
- 7.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009; 301:2445–2452.. [DOI] [PubMed] [Google Scholar]
- 8.Mitaka C, Tomita M. Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock. Shock 2011; 36:332–338.. [DOI] [PubMed] [Google Scholar]
- 9.Iwagami M, Yasunaga H, Doi K, et al. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: A propensity-matched analysis. Crit Care Med 2014; 42:1187–1193.. [DOI] [PubMed] [Google Scholar]
- 10.Payen DM, Guilhot J, Launey Y, et al. ; ABDOMIX Group: Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med 2015; 41:975–984.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Higgins JPT, Reeves BC, et al. A Tool for Assessing Risk Of Bias in Non-Randomized Studies of Interventions, Version 7 March 2016. Available at http://www.riskofbias.info. Accessed August 28, 2016. [Google Scholar]
- 12.Maynar J, Martínez-Sagasti F, Herrera-Gutiérrez M, et al. Direct hemoperfusion with polymyxin B-immobilized cartridge in severe sepsis due to intestinal perforation: Hemodynamic findings and clinical considerations in anticoagulation therapy. Rev Esp Quimioter 2013; 26:151–158.. [PubMed] [Google Scholar]
- 13.Suzuki H, Nemoto H, Nakamoto H, et al. Continuous hemodiafiltration with polymyxin-B immobilized fiber is effective in patients with sepsis syndrome and acute renal failure. Ther Apher 2002; 6:234–240.. [DOI] [PubMed] [Google Scholar]
- 14.Javeri Y, Madaan A, Juneja D, et al. Study of the role of polymyxin B direct hemoperfusion as an adjuvant therapy in severe sepsis of varied etiology. Intensive Care Med 2011; 37:S41. [Google Scholar]
- 15.Sawa N, Ubara Y, Sumida K, et al. Direct hemoperfusion with a polymyxin B column versus vasopressin for gram negative septic shock: A matched cohort study of the effect on survival. Clin Nephrol 2013; 79:463–470.. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Takeuchi H, Kawakubo H, et al. Effectiveness of polymyxin B-direct hemoperfusion (PMX-DHP) therapy using a polymyxin B-immobilized fiber column in patients with post-esophagectomy sepsis. Esophagus 2014; 11:189–196.. [Google Scholar]
- 17.Saito N, Sugiyama K, Ohnuma T, et al. Effectiveness of polymyxin B immobilized fiber hemoperfusion in patients with septic shock due to Gram-negative bacillus infection: The PMXHP study. Critical Care 2015; 19:S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura Y, Kiyomi F, Hoshino K, et al. The efficacy of the polimyxin B immobilized fiber column direct hemoperfusion for septic shock. Crit Care Med 2015; 43:266. [Google Scholar]
- 19.Srinivasan T, Singh MK, Goja S, et al. Toraymyxin filtration in septic shock in living donor liver transplantation-significant improvement in outcome when used at the right time and the right patient. Transplantation 2015; 99:282.25594557 [Google Scholar]
- 20.Tani T, Hanasawa K, Endo Y, et al. Therapeutic apheresis for septic patients with organ dysfunction: Hemoperfusion using a polymyxin B immobilized column. Artif Organs 1998; 22:1038–1044.. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Ushiyama C, Suzuki S, et al. Polymyxin b-immobilized fiber reduces increased plasma endothelin-1 concentrations in hemodialysis patients with sepsis. Ren Fail 2000; 22:225–234.. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005; 23:400–405.. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Kawagoe Y, Suzuki T, et al. Changes in plasma interleukin-18 by direct hemoperfusion with polymyxin B-immobilized fiber in patients with septic shock. Blood Purif 2005; 23:417–420.. [DOI] [PubMed] [Google Scholar]
- 24.Iwagami M, Yasunaga H, Noiri E, et al. Potential survival benefit of polymyxin B hemoperfusion in septic shock patients on continuous renal replacement therapy: A propensity-matched analysis. Blood Purif 2016; 42:9–17.. [DOI] [PubMed] [Google Scholar]
- 25.Takeyama N, Noguchi H, Hirakawa A, et al. ; Japan Sepsis Study Group: Time to initiation of treatment with polymyxin B cartridge hemoperfusion in septic shock patients. Blood Purif 2012; 33:252–256.. [DOI] [PubMed] [Google Scholar]
- 26.Klein CL, Hoke TS, Fang WF, et al. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 2008; 74:901–909.. [DOI] [PubMed] [Google Scholar]
- 27.Doi K, Leelahavanichkul A, Hu X, et al. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int 2008; 74:1017–1025.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 2007; 18:155–164.. [DOI] [PubMed] [Google Scholar]


