Abstract
Background Intracranial hemangiopericytomas (HPCs) are characterized by high recurrence rates and extracranial metastases. Radiotherapy provides an adjunct to surgery, but the timing of therapy and the patients most likely to benefit remain unclear.
Methods A retrospective review of 20 patients with HPC treated at the University of Texas Southwestern Medical Center between 1985 and 2014 was conducted. Recurrence and metastasis rates along with overall survival (OS) were characterized based on therapeutic approach and tumor pathology using Kaplan-Meier and Cox regression analyses.
Results The mean age was 45.6 years (range: 19–77). Gross total resection (GTR) was achieved in 13 patients, whereas 5 patients underwent subtotal resection. Median follow-up was 91.5 months (range: 8–357). The 5-, 10-, and 15-year recurrence-free survival (RFS) rates were 61, 41, and 20%, respectively. Six patients developed metastases at an average of 113 months (range: 42–231). OS at last follow-up was 80%. Importantly, immediate postoperative adjuvant radiotherapy (IRT) did not influence RFS compared with surgery alone or OS compared with delayed radiotherapy at the time of recurrence.
Conclusion HPCs have high recurrence rates necessitating close follow-up. Surgery remains an important first step, but the timing of radiotherapy for optimal control and OS remains uncertain.
Keywords: hemangiopericytoma, intracranial, radiotherapy, recurrence, survival
Introduction
The term “hemangiopericytoma” (HPC) was first coined in 1942 by Stout and Murray when they identified tumors arising from pericytes that had just been described by Zimmermann. 1 The first report of an intracranial HPC was published 5 years later in 1953. 2 Since then, several revisions have been made to the histopathologic classification of HPCs. In 1993, the World Health Organization (WHO) recognized HPC as a separate pathologic entity for the first time. 3 In 2007, the WHO designated HPCs as meningeal tumors of mesenchymal origin most frequently classified as grade II with more aggressive variants receiving a grade III classification (anaplastic HPC). Pathologically, they are essentially high-grade fibrosarcomas arising from the pericytes in the walls of dural capillaries. More recently, HPCs, along with solitary fibrous tumors, have been found to harbor a genomic inversion at the 12q13 locus fusing the NAB2 and STAT6 genes and leading to STAT6 nuclear expression that can be detected through immunohistochemistry. Identification of STAT6 nuclear expression or the NAB2-STAT6 fusion gene is now recommended to confirm the diagnosis. Differentiating between the solitary fibrous and HPC phenotypes can be more subjective and is currently based on histopathologic patterns. The solitary fibrous tumor phenotype is characterized by a patternless architecture of alternating hypo- and hypercellular areas with thick bands of collagen. In contrast, the HPC phenotype has high cellularity and a delicate, rich network of reticulin fibers and individual cells. 4 Immunohistochemistry staining for CD34 may also provide distinction between the two phenotypes as CD34 expression is strong in solitary fibrous tumors but patchy and weak in HPCs. 5 Despite receiving their own classification, HPCs still only account for less than 1% of all intracranial tumors. 4 6
While HPCs present with similar imaging characteristics to meningiomas, they have a significantly different clinical course with 5-year recurrence rates between 20 and 70%. 3 7 8 9 10 11 12 13 Therefore, these tumors require a unique therapeutic strategy. Most of the recent literature has focused on addressing the role of adjuvant radiotherapy either in the immediate postoperative period after resection or at the time of recurrence. 7 9 10 14 15 Several studies have shown improved RFS with adjuvant radiotherapy, but few have demonstrated improved OS. 7 11 12 15
A better understanding of which HPCs are likely to recur and metastasize may aid in future treatment decisions regarding timing of radiotherapy. In an attempt to address these questions and add patients to the cohort in the literature for this rare tumor, we retrospectively reviewed patients with HPCs treated surgically at our institution.
Methods
Patient Population
A retrospective review was conducted of patients with a tissue diagnosis of HPC treated at the University of Texas Southwestern Medical Center between 1985 and 2014 with at least 6 months of clinical and radiographic follow-up. All tumor specimens were reviewed by faculty neuropathologists. Histopathologic diagnosis was made based on morphologic appearance on hematoxylin and eosin staining as well as immunohistochemistry results based on the most recent World Health Organization (WHO) diagnostic criteria at the time of surgery. The study was approved by the institutional review board at the University of Texas Southwestern Medical Center.
Variables Assessed
Patient records were reviewed, and data on demographics, tumor characteristics, tumor location, treatment modalities, recurrence, metastases, survival, and length of follow-up were collected. Local recurrence was assessed through the use magnetic resonance imaging (MRI). New or increased nodular enhancement within the prior surgical cavity was defined as local recurrence. All imaging was reviewed by faculty neuroradiologists. Metastasis was defined as extracranial disease. Tumor location was subdivided into three groups: convexity, skull base, and parasagittal. Dural venous sinus involvement was also assessed. Operative reports and initial postoperative MRI were reviewed to determine extent of resection that was subdivided into gross total resection (GTR) and subtotal resection (STR). If either the operative report indicated subtotal resection or the initial postoperative MRI revealed nodular enhancement, the resection was deemed to be subtotal. Methylation-inhibited binding protein 1 labeling index (MIB-1) was also recorded from pathology reports (15/20 patients). MIB-1 was not available in five of the reports. For determining which variables were associated with tumor recurrence or metastasis, age, sex, tumor location, extent of resection, and MIB-1 were analyzed.
Statistical Analysis
Quantitative variables were described using mean, standard deviation, and range. Categorical variables were assessed using frequency and percentage. Overall patient survival was determined from the date of surgery to death or last follow-up. Patients alive at last follow-up who missed their next follow-up were censored from the survival group.
The Kaplan-Meier method was used to evaluate the association of the various patient and tumor characteristics with recurrence as well as the efficacy of various treatment approaches. Univariate analysis was performed using the Cox regression analysis with statistical significance set at a p valve of ≤ 0.05. All statistical analyses were completed using SPSS Statistics version 22 (IBM Corporation, New York, United States).
Results
Patient Characteristics
Using the aforementioned criteria, 20 patients with pathology-confirmed HPC were identified ( Table 1 ). Eighty percent (16/20) of the patients were female. The mean patient age was 45.6 ± 15.4 years (range: 19–77 years). The most common tumor location was skull base (40%) followed by convexity (30%) and parasagittal (30%). Thirty-five percent (7/20) had dural venous sinus involvement. Information regarding presenting symptoms was available for 16 patients. Headache was the most common presentation ( n = 6, 38%) followed by vision disturbance ( n = 5, 31%) and seizure ( n = 3, 19%). GTR was documented in 13 (65%) patients whereas subtotal resection was achieved in 5 (25%) patients. Records were incomplete on the remaining two patients. At the time of initial diagnosis, eight tumors were grade 2 and three were grade 3. Pathologic grade was not available for nine of the tumors. Four (20%) patients underwent adjuvant radiotherapy in the immediate postoperative period (within 3 months of surgery). Four patients underwent adjuvant chemotherapy at some point during their treatment course with varying regimens, all of whom had extracranial metastases. One patient underwent treatment with sunitinib and zoledronic acid and was switched to bevacizumab, temozolomide, and zoledronic acid after developing cardiotoxicity with sunitinib. This patient was then transitioned to dacarbazine and doxorubicin after progression was noted. A second patient was treated with a combination of bevacizumab and irinotecan. The third patient was treated with bevacizumab and temozolomide, followed by pazopanib and then gemcitabine. The fourth patient was started on pazopanib followed by sunitinib and then bevacizumab and temozolomide. The median follow-up was 91.5 months (range: 8–357 months).
Table 1. Demographic data and tumor characteristics of 20 patients with intracranial hemangiopericytomas evaluated at a single institution from 1985 to 2014.
| Number (%) | |
|---|---|
| Total | 20 |
| Mean age (y) (range) | 45.6 (19–77) |
| Median follow-up (mo) (range) | 91.5 (8–357) |
| Female | 16 (80) |
| Tumor location | |
| Convexity Parasagittal Skull base |
6 (30) 6 (30) 8 (40) |
| Tumor grade | |
| II III Not delineated |
8 (40) 3 (15) 9 (45) |
| Extent of resection | |
| GTR STR Unknown |
13 (65) 5 (25) 2 (10) |
| MIB-1 | |
| < 5% ≥ 5% Unknown |
8 7 5 |
| Patients with recurrence | 11 (55) |
| Mean time to recurrence (mo) | 62.2 (4–154) |
| Patients with metastases | 6 (30) |
| Mean time to metastasis (mo) | 113 (42–231) |
| Mortality | 4 (20) |
Abbreviations: GTR, gross total resection; MIB-1, methylation-inhibited binding protein 1 labeling index; STR, subtotal resection.
Outcome Data
During the follow-up period, 11 (55%) patients had tumor recurrence at a mean of 62.2 ± 56.2 months (range: 4–154 months) after initial surgery. Three of these patients developed disseminated intracranial disease remote from the primary site. Two patients underwent repeat surgery alone, four patients underwent radiotherapy alone (Gamma Knife radiosurgery, CyberKnife radiosurgery, or intensity-modulated radiotherapy), and five patients underwent a combination of surgery and radiotherapy. Six (30%) patients had tumor metastasis at a mean of 113 ± 74.5 months (range: 42–231 months) from initial surgery. Metastatic disease was found in the liver in four patients, axial spine in three, long bones in two, and soft tissue in one patient. Three patients had multiple metastatic sites.
The median recurrence-free survival (RFS) for the overall patient cohort was 107 months with 5-, 10-, and 15-year actuarial rates of 61, 41, and 20%, respectively ( Fig. 1 ). The median metastasis-free survival (MFS) was 231 months with 5-, 10-, and 15-year actuarial rates of 94, 73, and 59%, respectively. OS at last follow-up was 80% (16/20). The 5-, 10-, and 15-year actuarial rates for OS were 94, 73, and 73%, respectively.
Fig. 1.

Kaplan-Meier curves for recurrence-free, metastasis-free, and overall survivals for the patient cohort. Ten-year actuarial rates for recurrence-free, metastasis-free, and overall survivals were 41, 73, and 73%, respectively.
Prognostic Factors and Treatment Efficacy
Univariate analysis revealed a significant increase in RFS for males relative to females (hazard ratio [HR] 2.41 with 95% confidence interval [CI] 1.06–5.49, p = 0.04). There was a trend toward an increase in RFS for patients with parasagittal tumor locations compared with convexity lesions (HR 8.03 with 95% CI 0.91–70.46, p = 0.06; Fig. 2 ). On the other hand, dural venous sinus involvement, age ≥ 45, and MIB-1 did not affect RFS, MFS, or OS ( Table 2 ). A cutoff of 5% was used for MIB-1 as this was near the median value for the cohort and allowed for similar patient numbers for comparison.
Fig. 2.
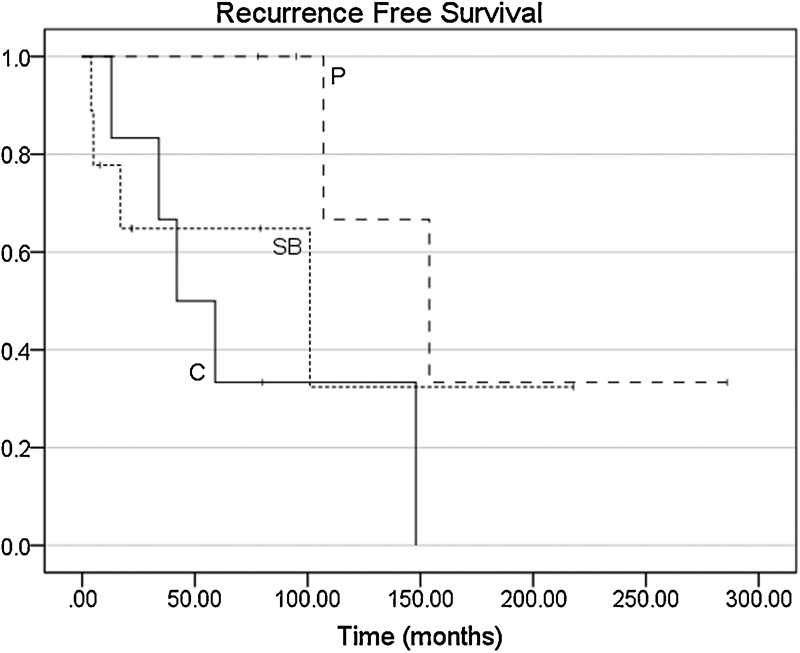
Kaplan-Meier curves of recurrence-free survival by tumor location. C, convexity; P, parasagittal; SB, skull base. Recurrence-free survival trended higher in parasagittal tumors compared with convexity lesions (hazard ratio [HR] 8.03 with 95% confidence interval [CI] 0.91–70.46, p = 0.06).
Table 2. Hazard ratios for prognostic factors regarding RFS, MFS, and OS.
| Variable | Recurrence-free survival ( p Value) | Metastasis-free survival ( p Value) | Overall survival ( p Value) |
|---|---|---|---|
| Male sex | 2.41 (0.04) | 0.86 (0.79) | 0.19 (0.56) |
| Age ≥ 45 | 1.40 (0.27) | 1.41 (0.45) | 0.67 (0.49) |
| Dural venous sinus involvement | 0.66 (0.24) | 0.88 (0.77) | 0.73 (0.58) |
| MIB-1 (continuous variable) | 1.05 (0.69) | 1.21 (0.20) | 1.27 (0.16) |
| MIB-1 ≥ 5% | 0.73 (0.47) | 1.66 (0.41) | 1.42 (0.57) |
| Parasagittal vs. convexity location | 8.03 (0.60) | 1.77 (0.54) | 0.42 (0.48) |
| Tumor grade 2 vs. 3 | 1.10 (0.94) | 2.739 (0.48) | N/A |
Abbreviations: MFS, metastasis-free survival; MIB-1, methylation-inhibited binding protein 1 labeling index; OS, overall survival; RFS, recurrence-free survival.
No patients with documented tumor grade died in the follow-up period.
Analysis of various treatment modalities revealed that extent of resection did not affect RFS, MFS, or OS. A total of 11 patients underwent radiotherapy at some point during the course of their treatment ( Table 3 ). Four of the patients who underwent intensity-modulated radiotherapy received doses > 50 Gy. Five patients underwent stereotactic radiosurgery (Gamma Knife or CyberKnife) with an average dose of 22.2 Gy (range: 16–25 Gy). Treatment information was not available for the remaining two patients. Immediate postoperative adjuvant radiotherapy (IRT) was not associated with improved RFS over surgery alone (HR 1.05, p = 0.90). More importantly, compared with delayed radiotherapy at the time of recurrence (DRT), IRT was not associated with increased MFS (HR 6.94, p = 0.31) or OS (HR 1.11, p = 0.87) ( Fig. 3 ). Furthermore, in those patients with recurrence, repeat surgical resection in addition to radiotherapy did not improve progression-free survival (PFS) over radiotherapy alone (HR 2.05, p = 0.26; Fig. 4 ).
Table 3. Radiotherapy treatment characteristics of patients who underwent adjuvant therapy.
| Patient | Age (y)/sex | Type of radiotherapy | Dose (Gy) | Fractions | Timing |
|---|---|---|---|---|---|
| 1 | 56 F | Unknown | Unknown | Unknown | IRT |
| 2 | 47 F | SRS (CK) | 25 | 5 | DRT |
| 3 | 41 F | Unknown | Unknown | Unknown | DRT |
| 4 | 58 F | SRS (GK) | 20 | 1 | IRT |
| 5 | 45 F | IMRT | 50.6 | 23 | DRT |
| 6 | 40 F | IMRT | 54 | 30 | DRT |
| 7 | 36 F | SRS (GK) | 16 | 1 | DRT |
| 8 | 69 M | SRS (CK) | 25 | 5 | DRT |
| 9 | 42 F | IMRT/SRS (CK) | 50.4 (IMRT), 8 (CK) | 28 | IRT |
| 10 | 61 M | SRS (CK) | 25 | 5 | DRT |
| 11 | 70 F | IMRT | 59.4 | 33 | IRT |
Abbreviations: CK, CyberKnife; DRT, delayed radiotherapy at the time of recurrence; GK, Gamma Knife; IMRT, intensity-modulated radiation therapy; IRT, immediate postoperative radiotherapy; SRS, stereotactic radiosurgery.
Fig. 3.
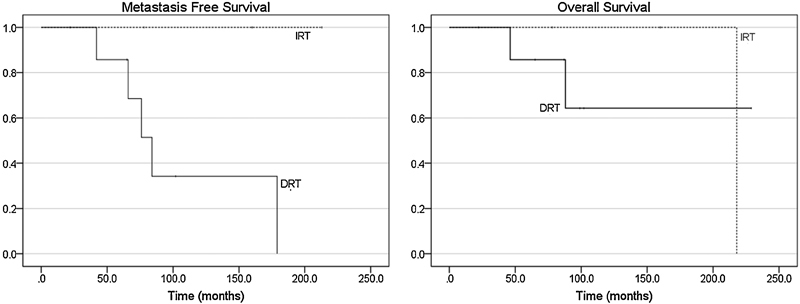
Kaplan-Meier curves of metastasis-free (hazard ration [HR] 6.94 with 95% confidence interval [CI] 0.17–287.13, p = 0.31) and overall survivals (HR 1.11 with 95% CI 0.33–3.74, p = 0.87) with respect to timing of radiotherapy. DRT, delayed radiotherapy at the time of recurrence; IRT, immediate postoperative radiotherapy.
Fig. 4.

Kaplan-Meier curves of progression-free survival after retreatment of recurrent hemangiopericytomas. There was no difference between surgery plus radiotherapy (S + RT) and radiotherapy alone (RT; hazard ratio [HR] 2.05 with 95% confidence interval [CI] 0.59–7.09, p = 0.26).
Discussion
Similar to higher-grade meningiomas, HPCs present a significant therapeutic challenge due to the high rates of local recurrence (55% in the current study). Despite having similar imaging characteristics and local behavior to higher grade meningiomas, HPCs present a unique challenge due to their ability to metastasize to extracranial locations (30% in this study). Moreover, the significantly lower incidence of HPCs compared with meningiomas makes assessing prognostic factors and efficacy of various treatment modalities more difficult.
Factors Associated with Recurrence and Metastasis
The ability to determine which HPCs have a predisposition for recurrence or metastasis is critical to guiding further therapy such as early radiotherapy or reoperation. In this series, only a trend toward increased recurrence was seen with a parasagittal tumor location compared with a tumor located at the convexity. In contrast to other case series, skull base tumor location or dural venous sinus involvement did not have an impact on RFS or MFS. 11 Furthermore, a lower MIB-1 did not correlate with increased RFS, which is in contrast with case series of atypical meningiomas. 16 Other studies have also suggested that GTR may improve RFS and OS compared with subtotal resection. 8 11 The lack of significant difference in local recurrence based on extent of resection in the current study is likely due to insufficient cohort size. Furthermore, the decreased RFS seen in females may be explained by the fact that no male patients had a histopathologic grade 3 tumor whereas three of the female patients did.
Treatment Paradigm
Treatment of HPCs has focused on surgical resection and radiotherapy along with the occasional use of chemotherapy in tumors with multiple recurrences. Surgical resection, either gross total or subtotal, is most frequently the first line of therapy as it allows for immediate reduction in tumor volume. Furthermore, surgery provides important diagnostic information given that HPCs and meningiomas are essentially indistinguishable on imaging. 17 18 19 20 Most authors have approached surgery with the goal of achieving as complete a resection as possible. Nonetheless, patient outcomes based on extent of resection have been variable. Some series have seen longer RFS but no improvement in OS. 7 8 12 13 Other series have indicated improved OS. 11 The most robust analysis of extent of resection was performed in a meta-analysis by Rutkowski et al of 277 patients. 15 This study concluded that patients who had GTR of their tumors lived significantly longer compared with those who had subtotal resections (median survival of 13 years compared with 9.75 years, p < 0.01). Additionally, adjuvant radiotherapy after subtotal resection was still inferior to GTR. Independent of those mixed results, it is clear that surgery has an irreplaceable role in the diagnosis of these tumors. Whenever safe, GTR should be achieved, but the current evidence does not support aggressive surgical resection in high risk or eloquent areas such as dural venous sinuses, the brainstem, or in proximity to the cranial nerves.
Radiotherapy has become an important adjunct to surgery, particularly when GTR is not achievable. However, the efficacy of early versus late utilization of this treatment modality remains unclear. Several studies have shown increased RFS in patients who undergo IRT, although this was not observed in this cohort of patients. 3 7 8 11 12 14 None of these series, however, were able to show a significant increase in OS with early radiotherapy although trends toward improved outcome were reported. 12 14 Fig. 5 demonstrates the general treatment algorithm used at our institution for a patient who presents with a homogenously enhancing dural based mass on magnetic resonance imaging whose histopathologic diagnosis after resection is HPC. Grades 2 and 3 lesions will go through the same algorithm with the caveat that it is often difficult to obtain a GTR of grade 3 lesions, and therefore these lesions more frequently require IRT.
Fig. 5.

Treatment algorithm for a patient who presents with a homogenously enhancing dural-based mass on magnetic resonance imaging (MRI). FLAIR, fluid-attenuated inversion recovery.
Though the responsiveness of HPCs to radiotherapy is clear, its role in improving overall outcome could benefit from further elucidation to prevent unnecessary radiation side effects. 9 10 For example, a certain subset of HPCs may be more radiosensitive and benefit from early radiotherapy. Some of this categorization may be possible at the histologic level, in similar fashion to what has been done with atypical meningiomas and necrosis. 21 The ultimate characterization, however, will require determination of the mutated genes that drive replication through genome sequencing. In 2013, a gene fusion of the NAB2 and STAT6 proteins was identified as one of these driver mutations. 22 Additionally, identification of this and other pathways should lead to more effective chemotherapeutic agents that may improve outcome more definitively than surgery and radiotherapy alone.
Limitations
The retrospective nature and single institution experience of this study are both limitations. The rarity of HPCs makes it difficult to accumulate large numbers at a single institution, and the small cohort in this study limits the statistical conclusions. At our institution, all these cases are reviewed by a multidisciplinary committee, but a selection bias about the treatment paradigm for each tumor recurrence based on physician preference cannot be excluded. Given the long-time interval of the study and the numerous histologic classification changes involving HPCs, variations in the histologic diagnosis are possible. Patient follow-up varied widely as well. Some of these issues could be addressed in the future through the creation of a multi-institutional patient registry for HPC patients. Determining the true efficacy of IRT in HPC patients will almost certainly require a multi-institutional randomized controlled trial.
Conclusion
Despite advancements in surgery and radiotherapy, HPCs continue to have high rates of recurrence and thus require close clinical follow-up. In our cohort, IRT did not improve RFS, MFS, or OS. In patients with recurrence, surgery with radiotherapy did not significantly improve PFS compared with radiotherapy alone. The establishment of a multicenter, prospectively collected registry of patients with HPCs would increase our understanding of the role of different treatment modalities on the long-term RFS.
References
- 1.Stout A P, Murray M R. Hemangiopericytoma: a vascular tumor featuring Zimmermann's pericytes. Ann Surg. 1942;116(01):26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg C F, Garret R. Hemangiopericytoma occurring in the meninges: case report. Cancer. 1954;7(03):602–606. doi: 10.1002/1097-0142(195405)7:3<602::aid-cncr2820070319>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G. Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. 2014;21(08):1310–1314. doi: 10.1016/j.jocn.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Louis D N, Ohgaki H, Wiestler O D, Cavenee W K, Ellison D W. Lyon, France: World Health Organization; 2016. WHO Classification of Tumours of the Central Nervous System. Revised Fo; p. 408. [Google Scholar]
- 5.Perry A, Scheithauer B W, Nascimento A G. The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol. 1997;21(11):1354–1360. doi: 10.1097/00000478-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Louis D N, Ohgaki H, Wiestler O D et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(02):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghia A J, Chang E L, Allen P Ket al. Intracranial hemangiopericytoma: patterns of failure and the role of radiation therapy Neurosurgery 20137304624–630., discussion 630–631 [DOI] [PubMed] [Google Scholar]
- 8.Kim J H, Jung H-W, Kim Y-Set al. Meningeal hemangiopericytomas: long-term outcome and biological behavior Surg Neurol 2003590147–53., discussion 53–54 [DOI] [PubMed] [Google Scholar]
- 9.Kim J W, Kim D G, Chung H-T et al. Gamma Knife stereotactic radiosurgery for intracranial hemangiopericytomas. J Neurooncol. 2010;99(01):115–122. doi: 10.1007/s11060-010-0114-z. [DOI] [PubMed] [Google Scholar]
- 10.Olson C, Yen C-P, Schlesinger D, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat Gamma Knife surgery. J Neurosurg. 2010;112(01):133–139. doi: 10.3171/2009.3.JNS0923. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski M J, Jian B J, Bloch O et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118(06):1628–1636. doi: 10.1002/cncr.26411. [DOI] [PubMed] [Google Scholar]
- 12.Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg. 2011;114(03):747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]
- 13.Chen L F, Yang Y, Yu X G, Gui Q P, Xu B N, Zhou D B. Multimodal treatment and management strategies for intracranial hemangiopericytoma. J Clin Neurosci. 2015;22(04):718–725. doi: 10.1016/j.jocn.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Dufour H, Métellus P, Fuentes Set al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy Neurosurgery 20014804756–762., discussion 762–763 [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski M J, Sughrue M E, Kane A J et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010;113(02):333–339. doi: 10.3171/2010.3.JNS091882. [DOI] [PubMed] [Google Scholar]
- 16.Klinger D R, Flores B C, Lewis J J et al. Atypical Meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84(03):839–845. doi: 10.1016/j.wneu.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama M, Sakai H, Onoue H, Miyazaki Y, Abe T. Imaging intracranial haemangiopericytomas: study of seven cases. Neuroradiology. 2004;46(03):194–197. doi: 10.1007/s00234-003-1157-z. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Chen Z Y, Ma L, Lou X, Li S J, Wang Y L. Intracranial hemangiopericytoma: MR imaging findings and diagnostic usefulness of minimum ADC values. J Magn Reson Imaging. 2013;38(05):1146–1151. doi: 10.1002/jmri.24075. [DOI] [PubMed] [Google Scholar]
- 19.Righi V, Tugnoli V, Mucci A, Bacci A, Bonora S, Schenetti L. MRS study of meningeal hemangiopericytoma and edema: a comparison with meningothelial meningioma. Oncol Rep. 2012;28(04):1461–1467. doi: 10.3892/or.2012.1919. [DOI] [PubMed] [Google Scholar]
- 20.Towner J E, Johnson M D, Li Y M. Intraventricular hemangiopericytoma: a case report and literature review. World Neurosurg. 2016;89:7.28E7–7.28E12. doi: 10.1016/j.wneu.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Sun S Q, Cai C, Murphy R KJet al. Management of atypical cranial meningiomas, part 2: predictors of progression and the role of adjuvant radiation after subtotal resection Neurosurgery 20147504356–363., discussion 363 [DOI] [PubMed] [Google Scholar]
- 22.Robinson D R, Wu Y M, Kalyana-Sundaram S et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(02):180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]


