Abstract
Purpose This study aims to report long-term clinical outcomes after Gamma Knife radiosurgery (GKRS) for intracranial grade 2 meningiomas.
Methods In this Institutional Review Board approved study, we reviewed records of all patients with grade 2 meningiomas treated with GKRS between 1998 and 2014.
Results A total of 97 postoperative histopathologically confirmed grade 2 meningiomas in 75 patients were treated and are included in this study. After a mean follow-up of 41 months, 28 meningiomas had local recurrence (29.79%). Median time to local recurrence was 89 months (mean: 69, range: 47–168). The 3- and 5-year actuarial local control (LC) rates were 68.9 and 55.7%, respectively. The 3- and 5-year overall survival rates were 88.6 and 81.1%, respectively. There was a trend toward worse LC with tumors treated with radiation doses ≤ 13 versus > 13 Gy. There was no radiation necrosis or second malignant tumors noted in our series.
Conclusion This report, one of the largest GKRS series for grade 2 meningiomas, demonstrates that GKRS is a safe and effective treatment modality for patients with grade 2 meningiomas with durable tumor control and minimal toxicity. Adjuvant GKRS could be considered as a reasonable treatment approach for patients with grade 2 meningiomas.
Keywords: Gamma Knife, radiosurgery, meningioma, grade 2, stereotactic
Introduction
Meningiomas are the most common brain tumors reported to the Central Brain Tumor Registry in the United States before gliomas. 1 There has been an increase in the incidence of meningiomas over the past few years. 2
World Health Organization (WHO) grade 2 meningiomas include atypical, clear cell, and chordoid meningiomas. Atypical meningiomas have increased mitotic activity (> 4 mitoses but < 20 per 10 high powered fields) or three or more of the following features: increased cellularity, small cells with a high nuclear to cytoplasmic ratio, prominent nucleoli, uninterrupted patternless or sheet-like growth, or foci of spontaneous or geographic necrosis. 3 About 4 to 7% of meningiomas are of atypical histologic subtype. 4
WHO grade 2 meningioma results in significantly worse local control (LC) and survival compared with WHO grade 1 meningioma. 5 6 Although treatment for meningioma has evolved over the years, gross total surgical resection has been the mainstay for meningiomas treatments. 7 8 According to the NCCN guidelines, external beam radiation therapy may be considered for resected or incompletely resected grade 2 meningiomas. ( https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdf ). 5 6 9 10 11 12 13 14
Stereotactic radiosurgery (SRS) refers to the delivery of large doses of radiation targeting a precisely defined target, utilizing multiple, nonparallel radiation beams that converge on the target lesion. The Gamma Knife (GK) system consists of an array of more than 192 cobalt-60 sources that has a treatment delivery accuracy of between 0.1 and 1 mm. 15
There have been a considerable number of studies reporting the role of SRS as an effective and safe treatment modality for patients with meningiomas 15 16 17 18 19 ; however, very few have reported the treatment outcomes of postoperative GK SRS for grade 2 meningiomas. 5 6 9 10 11 12 13 14 Furthermore, most of the studies reporting SRS treatment outcomes for grade 2 meningiomas included small sample sizes ranging from 25 to 35 patients. 9 10 11 12 13 14 In this study, we report the long-term clinical outcomes and treatment-induced adverse events among patients with histopathologically confirmed grade 2 meningiomas treated consecutively with GK SRS from 1998 to 2014.
Patients and Methods
Patient Selection and Staging
Our Institutional Review Board approved this study, which includes patients with meningiomas treated postoperatively with GK. These patients presented to our department from January 1998 to August 2014. Patient charts were reviewed and patients' and tumor characteristics, treatment outcomes, and treatment-induced adverse events are all reported in this study.
Treatment Planning
All patients were immobilized with a GK head frame that was placed by an expert neurosurgeon on the morning of the treatment day. All patients underwent a planning magnetic resonance imaging scan with intravenous contrast with a slice thickness of 1 mm. The treatment target volume included the surgical bed of the operated area as well as any residual or recurrent disease. Radiation doses were prescribed to the 50% isodose curve encompassing the target volume that was approved by both the neurosurgeon and radiation oncologist ( Fig. 1 ).
Fig. 1.
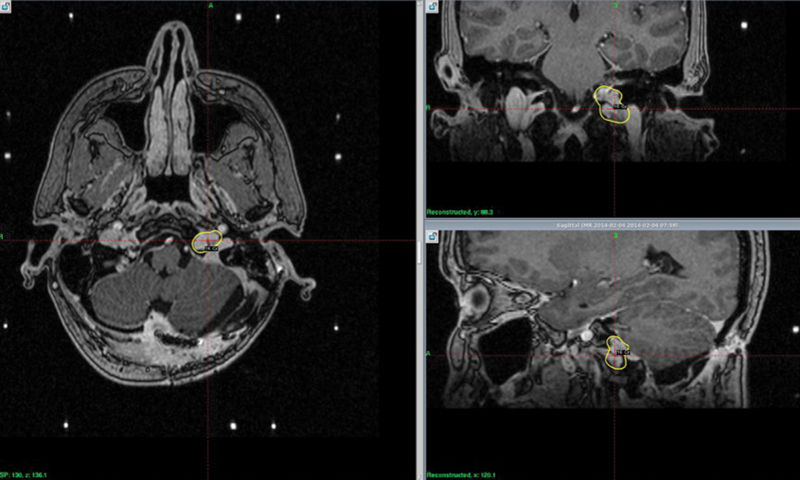
The 50% isodose line encompassing the target volume.
Statistical Analyses
Continuous variables were summarized by means, standard deviations (SDs), medians, and ranges. Categorical variables were summarized as frequencies and percentages. LC and overall survival (OS) rates were estimated via the Kaplan–Meier method and differences between groups of interest in these rates were assessed via the log-rank test.
Results
Patient and Tumor Characteristics
From January 1998 to August 2014, a total of 97 postoperative histopathologically confirmed grade 2 meningiomas in 75 patients were treated and are included and analyzed for this study. The mean follow-up for all patients was 40.7 months (range: 3.0–173.6 months, median: 30.8 months). Table 1 lists patient demographics, including sex, age, race, smoking history, performance status, tumor characteristics (including site and size), and treatment description (including dose, number of isocenters, and prescription isodose). Approximately 34% of the patients had residual disease treated with SRS within 6 months of the surgery date, while 66% of patients were treated after tumor recurrence based on radiological progression. Thirty-five patients received external beam radiation for childhood tumors or other meningiomas—not including the reported meningiomas—and none of the patients in this report received fractionated external beam radiation therapy for the meningioma reported in this study.
Table 1. Patient demographics, tumor characteristics, and treatment modalities.
| Age | ||
|---|---|---|
| Median (y) | 62 | |
| Range | 20–87 | |
| N | % | |
| Gender | ||
| Male | 54 | 55.7 |
| Female | 43 | 44.3 |
| Smoking | ||
| Smoker | 21 | 21.7 |
| < 20 p/y | 13 | 13.4 |
| 20–40 p/y | 6 | 6.2 |
| > 40 p/y | 2 | 2.1 |
| Nonsmoker | 54 | 55.6 |
| Unknown | 22 | 22.6 |
| Race | ||
| White | 71 | 73.2 |
| Black | 13 | 13.4 |
| Other | 3 | 3.1 |
| N/A | 10 | 10.3 |
| Tumor status | ||
| Residual (< or = 6 mo) | 33 | 34 |
| Recurrent (> 6 mo) | 64 | 66 |
| Tumor site | ||
| Anterior cranial fossa | 11 | 11.3 |
| Middle cranial fossa | 12 | 12.3 |
| Posterior cranial fossa | 16 | 16.5 |
| Convexity | 32 | 33 |
| Parasagittal | 13 | 13.4 |
| Temporal | 10 | 10.3 |
| Others | 3 | 3.1 |
| Initial presentation | ||
| Asymptomatic | 43 | 44.3 |
| Ataxia | 1 | 1 |
| Nausea | 2 | 2 |
| Headache | 21 | 21.7 |
| Hearing loss | 5 | 5.2 |
| Visual impairment | 13 | 13.4 |
| Sensory deficit | 10 | 10.3 |
| Facial palsy | 2 | 2.1 |
| Number of isocenters per treatment (number of shots) | ||
| 1–10 | 29 | 30 |
| 10–20 | 32 | 33 |
| 20–30 | 17 | 17.5 |
| 30–40 | 10 | 10.3 |
| > 40 | 9 | 9.3 |
| Dose (Gy) | ||
| < 12 | 1 | 1 |
| 12 | 5 | 5.2 |
| 13 | 8 | 8.2 |
| 14 | 35 | 36.1 |
| 15 | 4 | 4.1 |
| 16 | 35 | 36.1 |
| > 16 | 9 | 9.3 |
| Tumor size (cc) | ||
| < 2 | 23 | 23.7 |
| 2–4 | 17 | 17.5 |
| 4–6 | 12 | 12.3 |
| > 6 | 46 | 47.4 |
Of 57 (58.76%) symptomatic patients, common symptoms included headache (21.65%), visual impairment (14.43%), hearing deficit (5.15%), motor deficit (9.28%), and sensory deficit (10.31%). The median GKRS dose was 14.5 Gy (mean: 14.9, SD ± 1.7) prescribed to the 50% isodose line, utilizing a median number of 18 isocenters.
Treatment Outcomes
After a mean follow-up of 41 months (range: 3–174 months), 28 meningiomas had local recurrence (29.79%). Median time to local recurrence was 89 months (mean: 69, range: 47–168). The 3- and 5-year actuarial LC rates were 68.9 and 55.7%, respectively. The 3- and 5-year OS rates were 88.6 and 81.1%, respectively. For patients with residual meningiomas treated within 6 months of their surgery, 8 out of 36 (22.2%) had local recurrence. Table 2 shows the LC and OS rates stratified by various patients and tumors characteristics. There were significant associations between older age and previous radiation during childhood or elsewhere with worse OS. There was a trend toward worse LC with tumors treated with radiation doses ≤ 13 versus > 13 Gy. In a univariate analysis, there was a significant statistical association between larger tumor sizes and worse LC. In addition, there was a trend toward worse outcomes with radiation doses ≤ 13 Gy and anterior cranial fossa tumors. There were also significant statistical associations between worse OS and age at presentation > 60 years, history of previous radiation during childhood or elsewhere, and anterior cranial fossa tumors.
Table 2. Treatment outcomes.
| Treatment outcomes | Median (mo) | 2 y (%) | 3 y (%) | 5 y (%) | Log-rank p -value | |
|---|---|---|---|---|---|---|
| LC | ||||||
| LC according to tumor site | Anterior cranial fossa | NA | 50% (13.7%, 78.5%) | 50% (13.7%, 78.5%) | NA | 0.42 |
| Middle cranial fossa | 53.7 (32.8, 60.1) | 90.9% (50.8%, 98.7%) | 68.2% (16.3%, 92.2%) | 45.5% (6.1%, 80.1%) | ||
| Posterior cranial fossa | NA | 63.3% (21.5%, 87.3%) | 63.3% (21.5%, 87.3%) | 63.3% (21.5%, 87.3%) | ||
| Convexity | 109.6 (51.8, 167.8) | 90.6% (67.3%, 97.6%) | 84.1% (57.7%, 94.7%) | 74.8% (43.5%, 90.4%) | ||
| Parasagittal | 46.7 (22.2, 114.3) | 88.9% (43.3%, 98.4%) | 66.7% (28.17%, 87.8%) | 41.7% (10.9%, 70.8%) | ||
| Temporal | NA | 64.3% (15.1%, 90.2%) | 64.3% (15.1%, 90.2%) | 64.3% (15.1%, 90.2%) | ||
| Others | NA | 50.0% (0.6%, 91.0%) | 50.0% (0.6%, 91.0%) | NA | ||
| LC according to initial symptoms | No | 53.7 (32.8, 93.1) | 78.7% (57.5%, 90.1%) | 58.4% (32.5%, 77.3%) | 34.1% (7.8%, 63.6%) | 0.39 |
| Yes | 88.7 (46.7, 167.8) | 77.5% (59.9%, 88.1%) | 73.6% (55.1%, 85.5%) | 64.1% (43.3%, 78.9%) | ||
| LC according to age (y) | ≤ 60 | 88.7 (46.7, 167.8) | 75.7% (56.8%, 87.2%) | 71.3% (51.2%, 84.2%) | 65.8% (44.3%, 80.6%) | 0.73 |
| > 60 | 51.8 (32.8, 109.6) | 80.4% (60.8%, 90.9%) | 64.8% (41.3%, 80.8%) | 39.3% (14.7%, 63.4%) | ||
| LC according to gender | Female | 60.1 (37.4, 114.3) | 85.9% (68.6%, 94.1%) | 75.2% (52.7%, 88.1%) | 56.4% (31.4%, 75.4%) | 0.59 |
| Male | 88.7 (35.0, 167.8) | 72.9% (54.1%, 85%) | 64.5% (44.2%, 79.1%) | 58.1% (36.1%, 74.8%) | ||
| LC according to smoking history | Never smoker | 60.1 (37.4, 167.8) | 72.9% (56.6%, 83.9%) | 68.9% (51.3%, 81.2%) | 54.7% (34.5%, 71%) | 0.32 |
| Ever smoker | NA | 100% (100%, 100%) | 75.0% (12.8%, 96.1%) | 75.0% (12.8%, 96.1%) | ||
| LC according to race | White | 99.0 (51.8, 167.8) | 79.2% (64.5%, 88.4%) | 76.6% (61.3%, 86.5%) | 60.0% (40.3%, 75%) | 0.56 |
| Nonwhite | 60.1 (3.1, 60.1) | 85.2% (51.9.4%, 96.2%) | 63.9% (17.5%, 89.2%) | 63.9% (17.5%, 89.2%) | ||
| LC according to previous radiation | No | 60.1 (37.4, 114.3) | 79.3% (61.3%, 89.6%) | 71.3% (51.3%, 84.2%) | 59.1% (35.7%, 76.5%) | 0.42 |
| Yes | 88.7 (33.3, 167.8) | 73.7% (52%, 86.7%) | 67.0% (43.0%, 82.7%) | 52.1% (26.7%, 72.4%) | ||
| LC according to tumor size (cc) | < 2 | NA | 79.1% (51.8%, 92%) | 79.1% (51.8%, 92%) | 79.1% (51.8%, 92%) | 0.59 |
| 2–4 | 60.1 (13.5, 109.6) | 80.2% (40.3%, 94.8%) | 80.2% (40.3%, 94.8%) | 64.2% (22.5%, 87.7%) | ||
| 4–6 | 99.0 (4.4, 99.0) | 83.3% (27.3%, 97.5%) | 62.5% (14.2%, 89.3%) | 62.5% (14.2%, 89.3%) | ||
| > 6 | 53.7 (33.3, 167.8) | 76.0% (55.7%, 87.9%) | 62.6% (40.9%, 78.2%) | 46.5% (24.9%, 65.6%) | ||
| LC according to radiation dose (Gy) | ≤ 13 | 32.8 (15.0, 114.3) | 59.7% (23.5%, 83.2%) | 47.8% (15.3%, 74.8%) | 17.9% (1.1%, 51.7%) | 0.08 |
| > 13 | 99.0 (51.8, 167.8) | 81.9% (68.6%, 90.0%) | 73.0% (57.0%, 83.6%) | 64.5% (46.0%, 78.1%) | ||
| LC according to radiation Rx (mo) | ≤ 6 | 88.7 (51.8, 109.6) | 79.4% (50.8%, 92.5%) | 79.4% (50.8%, 92.5%) | 69.5% (37.5%, 87.4%) | 0.98 |
| > 6 | 99.0 (35.0, 167.8) | 75.0% (58.2%, 85.9%) | 65.0% (44.7%, 79.4%) | 54.2% (28%, 74.5%) | ||
| OS | ||||||
| OS according to tumor site | Anterior cranial fossa | 49.9 (33.5, 64.9) | 90.9% (50.8%, 98.7%) | 77.9% (35.4%, 94.2%) | 46.8% (11.4%, 76.7%) | 0.06 |
| Middle cranial fossa | 88.1 (34.4, 122.6) | 100% (100%, 100%) | 80% (20.4%, 96.9%) | 80% (20.4%, 96.9%) | ||
| Posterior cranial fossa | NA | 100% (100%, 100%) | 100% (100%, 100%) | 100% (100%, 100%) | ||
| Convexity | NA | 100% (100%, 100%) | 92.3% (56.6%, 98.9%) | 92.3% (56.6%, 98.9%) | ||
| Parasagittal | NA | 92.3% (56.6%, 98.9%) | 92.3% (56.6%, 98.9%) | 92.3% (56.6%, 98.9%) | ||
| Temporal | NA | 75.0% (29.8%, 93.4%) | 75.0% (29.8%, 93.4%) | 75.0% (29.8%, 93.4%) | ||
| Others | NA | 100% (100%, 100%) | 100% (100%, 100%) | 66.7% (5.4%, 94.5%) | ||
| OS according to initial symptoms | No | 88.1 (48.3, 122.6) | 100% (100%, 100%) | 93.3% (61.3%, 99.0%) | 84.0% (48.7%, 95.9%) | 0.81 |
| Yes | NA | 90.4% (76.5%, 96.3%) | 84.2% (67.9%, 92.7%) | 77.6% (59.6%, 88.3%) | ||
| OS according to age (y) | ≤ 60 | NA | 97.6% (84.3%, 99.7%) | 97.6% (84.3%, 99.7%) | 92.2% (69.6%, 98.2%) | 0.004 |
| > 60 | 88.1 (49.9, 122.6) | 92.8% (78.4%, 97.7%) | 78.9% (57.1%, 90.4%) | 68.9% (45.7%, 83.8%) | ||
| OS according to gender | Female | 122.6 (72.8, 122.6) | 96.1% (85.2%, 99.0%) | 92.1% (75.8%, 97.6%) | 82.1% (59.9%, 92.7%) | 0.55 |
| Male | NA | 94.3% (78.6%, 98.6%) | 84.9% (63.3%, 94.3%) | 80.2% (57.6%, 91.5%) | ||
| OS according to smoking history | Nonsmokers | NA | 92.7% (81.3%, 97.3%) | 83.1% (66.7%, 91.9%) | 71.8% (52.5%, 84.4%) | 0.14 |
| Ever smokers | NA | 100% (100%, 100%) | 100% (100%, 100%) | 100% (100%, 100%) | ||
| OS according to race | White | NA | 93.6% (83.5%, 97.6%) | 90.8% (78.5%, 96.2%) | 84.7% (69.4%, 92.7%) | 0.38 |
| Nonwhite | NA | 100% (100%, 100%) | 71.4% (25.8%, 92.0%) | 53.6% (13.2%, 82.5%) | ||
| OS according to previous radiation | No | NA | 100% (100%, 100%) | 91.7% (70.6%, 97.9%) | 91.7% (70.6%, 97.9%) | 0.02 |
| Yes | NA | 87.0% (68.3%, 95.0%) | 81.9% (60.6%, 92.3%) | 65.0% (40.3%, 81.5%) | ||
| OS according to tumor size (cc) | < 2 | NA | 100% (100%, 100%) | 88.9% (43.3%, 98.4%) | 88.9% (43.3%, 98.4%) | 0.64 |
| 2–4 | NA | 100% (100%, 100%) | 87.5% (38.7%, 98.1%) | 75.0% (31.5%, 93.1%) | ||
| 4–6 | NA | 87.5% (38.7%, 98.1%) | 87.5% (38.7%, 98.1%) | 65.6% (15.7%, 90.9%) | ||
| > 6 | 122.6 (72.8, 173.6) | 92.2% (76.9%, 97.5%) | 88.2% (70.4%, 95.6%) | 83.6% (63.4%, 93.2%) | ||
| OS according to radiation dose (Gy) | ≤ 13 | 88.1 (33.5, 122.6) | 100% (100%, 100%) | 90.0% (47.3%, 98.5%) | 80.0% (40.9%, 94.6%) | 0.20 |
| > 13 | NA | 94.1% (84.5%, 97.8%) | 88.5% (75.0%, 94.9%) | 82.1% (65.8%, 91.1%) | ||
| OS according to radiation Rx (mo) | ≤ 6 | NA | 93.9% (77.9%, 98.4%) | 93.9% (77.9%, 98.5%) | 73.1% (37.2%, 90.5%) | 0.78 |
| > 6 | NA | 95.6% (83.4%, 98.9%) | 85.0% (66.9%, 93.7%) | 81.3% (62.3%, 91.4%) | ||
Abbreviations: LC, local control; NA, not available; OS, overall survival.
Statistically significant values have been boldfaced.
Treatment-Induced Adverse Events
GK SRS was very well tolerated. Acute adverse events (within 3 months of treatment) included headache (1%) and visual impairment (1%). Chronic adverse events included transient seizures (3%), headache (2%), sensory deficit (3%, two patients, one experienced bilateral lower extremities mild numbness and the other reported occasional facial numbness), visual impairment (2%, one patient reported occasional right eye blurry vision), and motor deficit (3%, three patients experienced tremors or muscle weakness).
Discussion
WHO grade 2 meningiomas are relatively aggressive tumors with 3-year LC rates ranging from 35 to 70%. 9 10 11 13 14 As salvage surgical interventions after tumor recurrence are accompanied by high morbidity and possible neurological dysfunction, many neuro-oncologists advocate for adjuvant early radiation therapy in patients with grade 2 meningiomas. 9 There have not been many studies analyzing histopathologically confirmed WHO grade 2 meningiomas with a large sample size of patients. To our knowledge, this is the biggest cohort of homogenous histopathologically confirmed grade 2 meningiomas treated with GK RS. Most of the studies utilized local-regional control as an end point, while a few others used OS. 9 10 11 13 14 Our study analyzed both the LC and OS.
This study included 97 meningiomas in 75 patients who have been treated postoperatively either adjuvantly or after tumor progression. Median time to local recurrence was 89 months (mean: 69, range: 47–168). The 3- and 5-year actuarial LC rates were 68.9 and 55.7%, respectively. The 3- and 5-year OS rates were 88.6 and 81.1%, respectively. Our LC rates come on the higher side of the published studies with a LC rate ranges from 40 to 70%. 9 10 11 12 13 14
Our results compare favorably to the study by Ferraro et al, 12 which reported the treatment outcomes of 31 patients with WHO grade 2 meningiomas, all of whom had surgery and were treated with GK. The 3-year OS and progression-free survival (PFS) were 83.4 and 70.1%, compared with 88.6 and 68.9% in our series. Their median OS was 36 months and PFS was 25.8 months.
Our outcomes are superior to the results reported by Kim et al, 13 who reported the outcomes of 35 Korean patients with 49 atypical or anaplastic meningiomas treated with RS. The mean tumor volume and marginal irradiation dose were 3.5 cm 3 (range: 0.3–25.3) and 16 Gy (range: 12–21), respectively. The 3-year actuarial local tumor control rate for patients with atypical meningiomas after RS was 36%. 13 In Aboukais et al 9 series, with a mean follow-up of 56.4 months (range: 12–108 months), the 1-, 2-, and 3-year actuarial LC rates for all patients were 75, 52, and 40%, respectively, and the regional control rates were 75, 48, and 33%. The mean PFS after RS was 32.4 months among those with progression in a target irradiated volume in Aboukais et al series. 9 Attia et al 10 reported the outcomes and pattern of failure of 24 patients after treatment for atypical meningioma with GK RS. The overall LC rates at 2 and 5 years were 51% and 44%, respectively. With a median follow-up time of 42.5 months, 14 of 24 patients experienced a treatment failure at the time of last follow-up. 10 In our series, at a mean follow-up of 41 months, only 28 of the 97 meningiomas (29.79%) had local recurrence. Median time to local recurrence in our series was 89 months compared with 24.8 months in the series reported by Attia et al. 10
Ferraro et al 12 published data of 31 patients with atypical and 4 patients with malignant meningiomas treated with GK RS. In their report, for WHO grade 2 tumors, the 3-year OS and PFS were 83.4 and 70.1% compared with 88.6 and 68.9% in our series. In a univariate analysis, LC was adversely related to prior history of benign meningioma, nuclear atypia, high mitotic rate, spontaneous necrosis, and WHO grade 3 diagnosis. The same analysis demonstrated that OS was adversely affected in patients with WHO grade 3 diagnosis, prior history of benign meningioma, prior fractionated radiotherapy, larger tumor volume, and higher isocenter number. The univariate analysis also demonstrated that larger tumor sizes resulted in worse LC. Also, older patients, patients with tumors in the anterior cranial fossa, and patients who had received prior cranial radiation had worse survival.
The treatment-induced adverse events in our cohort are comparable to other published reports. Only two patients experienced transient acute headaches, and one patient had transient acute visual impairment. Chronic adverse events were also minimal and included headache (1%) and visual impairment (1%). Chronic adverse events included transient seizures (3%), headache (2%), sensory deficit (3%, two patients, one was diagnosed with parasagittal meningioma, received 16 Gy ad experienced bilateral lower extremities mild numbness and the other was diagnosed with anterior cranial fossa—sino-orbital—meningioma, received 16 Gy and reported occasional facial numbness), visual impairment (2%, one patient reported occasional right eye blurry vision; this patient was diagnosed with cavernous sinus meningioma and received 14 Gy), and motor deficit (3%, three patients experienced tremors or muscle weakness; those patients were diagnosed with parasagittal meningioma, intraventricular meningioma, and sphenoid wing meningiomas, and received 14, 18, and 13 Gy, respectively).
In our subgroup analysis, early SRS compared with later SRS intervention upon radiological evidence of recurrence did not impact the LC or the OS ( Tables 2 and 3 ). There has been a trend toward better LC with doses > 14 Gy compared with lower doses.
Table 3. Results of a univariate model aimed to analyze various factors that might impact local control and overall survival.
| Univariate models | Local control | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | LCL | UCL | p -Value | HR | LCL | UCL | p -Value |
| Presenting symptoms | 0.7 | 0.3 | 1.6 | 0.39 | 0.9 | 0.3 | 2.7 | 0.81 |
| Age at presentation > 60 y | 1.2 | 0.5 | 2.5 | 0.73 | 6.7 | 1.5 | 30.5 | 0.01 |
| Smoking | 0.4 | 0.1 | 2.8 | 0.34 | NA | |||
| Female vs. male | 0.8 | 0.4 | 1.8 | 0.59 | 1.4 | 0.5 | 4.3 | 0.55 |
| Previous RT | 1.4 | 0.6 | 3.1 | 0.42 | 4.2 | 1.1 | 15.5 | 0.03 |
| Race: nonwhite vs. white | 1.4 | 0.5 | 4.2 | 0.56 | 1.8 | 0.5 | 6.7 | 0.38 |
| Tumor size | 1.04 | 1.01 | 1.1 | 0.003 | 1.02 | 0.98 | 1.1 | 0.28 |
| Tumor size > 4 mL | 1.4 | 0.6 | 3.1 | 0.44 | 1.2 | 0.4 | 4.1 | 0.75 |
| Radiation dose > 13 (Gy) | 0.5 | 0.2 | 1.1 | 0.09 | 0.5 | 0.2 | 1.5 | 0.21 |
| Radiation dose > 6 mo | 1.0 | 0.4 | 2.4 | 0.98 | 0.8 | 0.2 | 2.9 | 0.78 |
| Anterior cranial fossa | 3.0 | 1.0 | 9.4 | 0.06 | 4.4 | 1.2 | 16.8 | 0.03 |
| Middle cranial fossa | 1.6 | 0.5 | 4.9 | 0.42 | 2.2 | 0.6 | 9.1 | |
| Posterior cranial fossa | 1.9 | 0.5 | 6.8 | 0.34 | NA | |||
Abbreviation: NA, not available.
This study, however, does come with the limitations of being a retrospective single institution study, with patients treated in both the adjuvant and salvage settings. Nevertheless, it is the largest published report of homogenous histopathologically confirmed grade 2 meningiomas. The study highlights the value of treating with higher doses (> 14 Gy) whenever feasible as this might increase tumor control. This study did not show an advantage for adjuvant versus salvage SRS for patients with grade 2 meningiomas.
Conclusion
This report, one of the largest GKRS series for grade 2 meningiomas, demonstrates that GKRS is a safe and effective treatment modality for patients with grade 2 meningiomas with durable tumor control and minimal toxicity. Adjuvant GKRS could be considered as a reasonable treatment approach for patients with grade 2 meningiomas.
Footnotes
Conflict of Interest This work was not supported, funded, or sponsored by any extrainstitutional source, nor are there any actual or potential conflicts of interest with the production and publication of this work. No author has a direct or indirect commercial financial incentive or any conflict of interest associated with publishing this article.
References
- 1.Baldi I, Engelhardt J, Bonnet C et al. Epidemiology of meningiomas. Neurochirurgie. 2014 doi: 10.1016/j.neuchi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom Q T, Gittleman H, Farah P et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-oncol. 2013;15 02:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry A, Louis D N, Scheithauer B W . Lyon: IARC Press; 2007. Meningiomas; p. 164. [Google Scholar]
- 4.Whittle I R, Smith C, Navoo P, Collie D.Meningiomas Lancet 2004363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 5.Milker-Zabel S, Zabel-du Bois A, Huber P, Schlegel W, Debus J. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas : long-term experience and review of the literature. Strahlenther Onkol. 2006;182(11):635–640. doi: 10.1007/s00066-006-1548-2. [DOI] [PubMed] [Google Scholar]
- 6.Pasquier D, Bijmolt S, Veninga T et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71(05):1388–1393. doi: 10.1016/j.ijrobp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Newman S A. Meningiomas: a quest for the optimum therapy. J Neurosurg. 1994;80(02):191–194. doi: 10.3171/jns.1994.80.2.0191. [DOI] [PubMed] [Google Scholar]
- 8.Goyal L K, Suh J H, Mohan D S, Prayson R A, Lee J, Barnett G H. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(01):57–61. doi: 10.1016/s0360-3016(99)00349-1. [DOI] [PubMed] [Google Scholar]
- 9.Aboukais R, Zairi F, Lejeune J P et al. Grade 2 meningioma and radiosurgery. J Neurosurg. 2015;122(05):1157–1162. doi: 10.3171/2014.9.JNS14233. [DOI] [PubMed] [Google Scholar]
- 10.Attia A, Chan M D, Mott R T et al. Patterns of failure after treatment of atypical meningioma with Gamma Knife radiosurgery. J Neurooncol. 2012;108(01):179–185. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi C Y, Soltys S G, Gibbs I C et al. Cyberknife stereotactic radiosurgery for treatment of atypical (WHO grade II) cranial meningiomas. Neurosurgery. 2010;67(05):1180–1188. doi: 10.1227/NEU.0b013e3181f2f427. [DOI] [PubMed] [Google Scholar]
- 12.Ferraro D J, Funk R K, Blackett J Wet al. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas Radiat Oncol 2014938. Doi: 10.1186/1748-717X-9-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J W, Kim D G, Paek S H et al. Radiosurgery for atypical and anaplastic meningiomas: histopathological predictors of local tumor control. Stereotact Funct Neurosurg. 2012;90(05):316–324. doi: 10.1159/000338253. [DOI] [PubMed] [Google Scholar]
- 14.Mori Y, Tsugawa T, Hashizume C, Kobayashi T, Shibamoto Y. Gamma Knife stereotactic radiosurgery for atypical and malignant meningiomas. Acta Neurochir Suppl (Wien) 2013;116:85–89. doi: 10.1007/978-3-7091-1376-9_13. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz M. Stereotactic radiosurgery: comparing different technologies. CMAJ. 1998;158(05):625–628. [PMC free article] [PubMed] [Google Scholar]
- 16.Kondziolka D, Mathieu D, Lunsford L Det al. Radiosurgery as definitive management of intracranial meningiomas Neurosurgery 2008620153–58., discussion 58–60 [DOI] [PubMed] [Google Scholar]
- 17.Pollock B E, Stafford S L, Utter A, Giannini C, Schreiner S A. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55(04):1000–1005. doi: 10.1016/s0360-3016(02)04356-0. [DOI] [PubMed] [Google Scholar]
- 18.Starke R M, Przybylowski C J, Sugoto M et al. Gamma Knife radiosurgery of large skull base meningiomas. J Neurosurg. 2015;122(02):363–372. doi: 10.3171/2014.10.JNS14198. [DOI] [PubMed] [Google Scholar]
- 19.Przybylowski C J, Raper D M, Starke R M, Xu Z, Liu K C, Sheehan J P. Stereotactic radiosurgery of meningiomas following resection: predictors of progression. J Clin Neurosci. 2015;22(01):161–165. doi: 10.1016/j.jocn.2014.07.028. [DOI] [PubMed] [Google Scholar]


