Abstract
Purpose:
Head and neck cancer (HNC) patients suffer from significant morbidity, which may introduce challenging care demands and subsequent stress-induced mind–body interactions for informal caregivers. This prospective study evaluated patient and caregiver predictors of diurnal cortisol rhythm among HNC caregivers during radiation treatment.
Method:
Patient–caregiver dyads completed measures at radiation treatment start (T1; n = 32) and 5 weeks into treatment (T2; n = 29). Measures included the Functional Assessment of Cancer Therapy–Head and Neck, the Caregiver Quality of Life (QOL) Index–Cancer, the Caregiver Reaction Assessment, the Medical Outcomes Social Support Survey, and the Eating Assessment tool. Patients also received a clinical swallowing evaluation using the Functional Assessment of Oral Intake Scale. Caregiver cortisol concentrations were assessed from salivary samples at T1 and T2.
Results:
Caregiver cortisol slope became significantly flatter during radiation treatment. Greater caregiver schedule burden was associated with a flatter cortisol slope (β = .35, p = .05) in caregivers at T1. Lower patient functional QOL (β = −.41, p = .05) and lower overall caregiver QOL at T1 (β = −.39, p = .04) were each separately associated with a flatter cortisol slope in caregivers over treatment.
Conclusions:
Results suggest the presence of a mind–body interaction in HNC caregivers. Dysregulation in diurnal cortisol rhythm in caregivers was significantly associated with increased caregiver schedule burden and lower patient and caregiver QOL. Targeted interventions developed for HNC caregivers may help to prevent negative health outcomes associated with dysregulated cortisol.
Keywords: cortisol, dyads, head and neck cancer, caregiving, quality of life, burden
It is estimated that more than 62,000 Americans will be diagnosed with head and neck cancer (HNC) in 2016 (American Cancer Society, 2016). Although the disease only accounts for approximately 3% of all cancers in the United States, the morbidity associated with it is significant (Eades, Chasen, & Bhargava, 2009). Up to 75% of HNC patients suffer from dysphagia (difficulty swallowing; Carnaby-Mann, Crary, & Amdur, 2012; Murphy & Gilbert, 2009). Likewise, approximately 95% of HNC patients treated with radiation therapy report xerostomia (dry mouth; Dirix, Nuyts, Vander Poorten, Delaere, & Van den Bogaert, 2008). Additional associated morbidities may include neuropathic and nociceptive pain (Epstein, Wilkie, Fischer, Kim, & Villines, 2009), fibrosis (Moloney, Brunner, Alexander, & Clark, 2015), and osteoradionecrosis (Silvestre-Rangil & Silvestre, 2011).
Providing informal care for patients with HNC can be a challenging experience. Patients with HNC report higher care demands compared to many other cancer patients (Chen et al., 2009), and caregivers assist with unique care tasks, which may include special food preparation (Penner, 2009), gastronomy feeding (Penner, McClement, Lobchuk, & Daeninck, 2012), and tracheostomy management (Mayre-Chilton, Talwar, & Goff, 2011). Not surprisingly, HNC caregivers experience negative psychosocial effects. Drabe and colleagues (2008) reported that HNC caregivers experience a high prevalence of psychiatric disorders, particularly agoraphobia (22.6%), and other studies have reported greater psychological distress and poorer psychological well-being in HNC caregivers in comparison to the general population (Ross, Mosher, Ronis-Tobin, Hermele, & Ostroff, 2010). Further, studies have also demonstrated that psychological distress and caregiver burden increase during the care recipient’s oncologic treatment for HNC (Badr, Gupta, Sikora, & Posner, 2014; Nightingale, Lagorio, & Carnaby, 2014). Collectively, these findings suggest an increased risk for a negative psychosocial sequela associated with the role of informal caregiving for HNC patients.
Informal caregiving has negative health consequences as well. Lee, Colditz, Berkman, and Kawachi (2003) found that spouse caregiving was an independent risk factor for cardiovascular disease. Similarly, Capistrant, Moon, and Glymour (2012) reported that spouse caregiving predicted the incidence of hypertension. Most concerning, research has also shown that caregiving increases the risk of mortality (Schulz & Beach, 1999). Although the exact cause of these outcomes is unknown, contributory factors may in part include stress-induced mind–body interactions (Sherwood et al., 2008). Sherwood et al. (2008) present a biobehavioral model adapted from the Pittsburgh Mind–Body Center model that proposes mind–body interactions affecting caregivers’ health. This model depicts the impact of care recipient disease characteristics and caregiver personal characteristics on caregivers’ psychological (e.g., depression) and behavioral responses (e.g., sleep patterns). In turn, caregivers’ psychobehavioral responses may trigger a biological response, ultimately affecting the caregiver’s overall physical health.
Abnormal secretion of cortisol, a steroid hormone or glucocorticoid, is an example of a biological response to stressful stimuli (Aldwin, 2007). Under normal circumstances, cortisol secretion is highest in the morning at approximately 30–45 min postawakening (i.e., the cortisol awakening response or CAR) and lowest in the evening, demonstrating a negative diurnal slope (Nicholson, 2008). Thus, lower slope values reflect a more rapid decline and indicate a normal response. Steeper declines are typically associated with more optimal psychosocial and physical health (Adam & Kumari, 2009). In contrast, flattened slopes with values approaching 0 reflect either consistently high or low cortisol levels and indicate dysregulation of the hypothalamic pituitary adrenal axis (HPA) (Nicholson, 2008).
Dysregulated cortisol production is a potential mediator of the relationship between chronic stress and compromised physical health among informal caregivers (McEwen, 2015). However, few studies have evaluated stress–cortisol associations among caregivers, especially cancer caregivers. Further, among the few studies that have been conducted, findings have been unclear. For example, Miller, Cohen, and Ritchey (2002) found that parents of children with cancer (n = 25) demonstrated significantly flatter (more abnormal) diurnal cortisol slopes in comparison to parents of healthy children (n = 25). Correspondingly, the authors also reported that parents of children with cancer had more psychological distress than parents of healthy children. In another study, Thomas et al. (2012) reported that partners of men with prostate cancer (n = 19) demonstrated lower daily cortisol output than controls (n = 26). The authors also found that partners who reported at least subthreshold post-traumatic stress disorder (PTSD) symptoms had significantly lower cortisol production than those without PTSD symptoms. However, in contrast to the authors’ hypothesis, there were no differences in diurnal cortisol slope between caregivers and controls. Likewise, Rohleder, Marin, Ma, and Miller (2009) found no difference in diurnal cortisol slopes between 18 family caregivers (83% spouses) of brain cancer patients undergoing treatment and 19 matched controls across four time points. Finally, in a study comparing glioblastoma cancer caregivers and controls, Miller et al. (2014) reported similar cortisol responses (CAR, total cortisol secretion, and diurnal cortisol slopes) across the two groups. These mixed findings and the lack of investigation into HNC caregiving underscore the need for further investigation into the psychoneuroendocrine responses of HNC caregivers.
With the present study, we address this gap in the literature. The purpose of this study was to prospectively evaluate diurnal cortisol rhythm in caregivers of patients with HNC undergoing radiation treatment. The study aims were to (1) describe and compare diurnal cortisol rhythm in HNC caregivers from the initiation of radiation treatment toward the conclusion of the care recipients’ treatment and (2) identify care recipient and caregiver predictors of diurnal cortisol rhythm in HNC caregivers. We hypothesized that diurnal cortisol rhythm would become significantly flatter toward the conclusion of treatment, when caregiver psychological distress and burden would likely be highest (Badr et al., 2014; Nightingale et al., 2014). Additionally, we hypothesized that poorer psychosocial functioning (demonstrated by lower quality of life [QOL], greater burden, and lower perceived social support) among care recipients and caregivers would predict a flatter cortisol slope among HNC caregivers. Finally, we hypothesized that care recipient factors potentially associated with a more stressful caregiving experience (more aggressive treatment [combined radiation and chemotherapy], presence of dysphagia, and a more advanced disease stage) would be associated with a flatter cortisol slope.
Method
Participants
We recruited patient–caregiver combinations (dyads) sequentially from an academic outpatient radiation clinic over a 6-month period. The treating radiation oncologist identified care recipients who were appropriate for inclusion in the study, and the care recipients subsequently identified their informal caregivers. We approached each dyad at the clinic to review study procedures and obtain written informed consent prior to the beginning of the care recipient’s radiation therapy. Inclusion criteria for care recipients were as follows: (1) HNC, confirmed by clinical history and exam, with positive cross-sectional study and histopathological biopsy excluding other pathology, (2) planned treatment with external beam radiotherapy (with or without chemotherapy), and (3) age of at least 21 years. We excluded care recipients from the study if they were (1) receiving palliative treatment with a noncurative intent, (2) older than 90 years of age, and (3) unable to read and communicate in English. Caregivers who were providing care for a patient with HNC meeting the inclusion criteria above and were at least 21 years of age were eligible to participate. Conversely, we excluded caregivers if they (1) had a current cancer diagnosis, (2) were older than 90 years of age, and (3) were unable to read and communicate in English.
Procedure
For this study, we used a prospective parallel arm design. Caregivers and care recipients participated in a standard intake interview at baseline for the collection of sociodemographic characteristics, caregiver comorbidities and prescription medications, and information regarding the relationship between care recipient and caregiver. We obtained care recipient disease and treatment-related data, including treatment type (radiation or combined chemoradiation), tumor stage, and tumor location via medical chart review. Assessments occurred at the following time points: (a) the beginning of the care recipient’s radiation treatment (T1) and (b) 5 weeks following the onset of the care recipient’s radiation treatment (T2). We selected 5 weeks as our second time point to provide an approximate end-of-treatment estimate of caregiver outcomes, as our lab has experienced difficulty obtaining complete data from participants during the final week of treatment. Participants completed all instruments at the outpatient radiation clinic or in a patient-preferred location and returned them to the study researcher in person at the clinic or by mail. The local institutional review board approved all procedures before implementation.
Assessments
Caregiver salivary cortisol
Caregivers completed salivary cortisol collections 2 times a day (on waking and at 21:00 hr) for 3 consecutive days following the T1 and T2 assessments. Previous research has determined that this frequency of salivary cortisol collection (i.e., 2 times per day for 3 days) provides an accurate measure of diurnal cortisol slope (Kraemer et al., 2006) while minimizing participant burden. Moreover, research has further determined that salivary cortisol is a reliable measure of free circulating cortisol in the body (Kirschbaum & Hellhammer, 1994). The cortisol collection procedure entailed the participant placing a cotton swab in her or his mouth for approximately 2 min and then storing the sample in a salivette (plastic tube) using supplies from Salimetrics (State College, PA). We instructed caregivers to refrain from eating, drinking, smoking, brushing their teeth, or using mouthwash for 30 min prior to saliva collection. We provided caregivers with a journal to record compliance with the abovementioned procedures as well as the dates and times of saliva collection and asked them to return these study behavior journals with the saliva samples. Caregivers were to store their samples in a freezer until they returned them to the study researcher in an insulated cooler that we provided to them. We also gave them the opportunity to return saliva samples via mail (1- to 3-day delivery) with prepaid postage provided. Saliva is considered bioexempt and can safely be mailed without special precautions. Moreover, research indicates that saliva samples can be kept at room temperature for extended periods without freezing and remain stable (Clements & Parker, 1998).
We stored the returned saliva samples at −80°C until we shipped them on dry ice via overnight shipping to Salimetrics, Inc. for assaying in accordance with Salimetrics procedures. Saliva samples were assayed singularly to determine cortisol levels using a highly sensitive enzyme immunoassay (Salimetrics). The test uses 25 μl of saliva per determination, has a lower limit of sensitivity of 0.003 μg/dl, uses a standard curve range from 0.012 μg/dl to 3.0 μg/dl, and has an average intraassay coefficient of variation of 3.5% and an average interassay coefficient of variation of 5.1%. Method accuracy determined by spike and recovery averaged 100.8% and linearity determined by serial dilution averaged 91.7%.
Caregiver burden
We assessed caregiver burden at both time points (T1 and T2) using the Caregiver Reaction Assessment (CRA; Given et al., 1992). The CRA assesses the positive and negative aspects of caregiving with five subscales including Esteem, Lack of Family Support, Impact on Finances, Impact on Daily Schedule, and Impact on Health. The instrument provides a score for each subscale only. The CRA has 24 items, each of which uses a 5-point Likert-type scale ranging from strongly disagree to strongly agree. Initially tested in a range of caregivers, including cancer caregivers, the instrument has demonstrated adequate construct validity and internal reliability and has been recommended as an instrument to measure caregiver burden (Deeken, Taylor, Mangan, Yabroff, & Ingham, 2003; Given et al., 1992). In the present study, internal consistency at T1 was adequate for the majority of the subscales (α = .72 [Lack of Family Support] to α = .92 [Finances]) and approached acceptability for the Esteem subscale (α = .63). At T2, all subscales demonstrated adequate internal consistency (α = .72 [Esteem] to α = .88 [Lack of Family Support]).
Caregiver and care recipient’s QOL
We measured QOL in caregivers at both time points using the Caregiver Quality of Life Index–Cancer Scale (CQOLC; Weitzner, Jacobsen, Wagner, Friedland, & Cox, 1999). The CQOLC was originally developed for and tested in caregivers of patients with lung, breast, and prostate cancers. The instrument assesses burden, disruptiveness, positive adaptation, and financial concerns and provides a score for each subscale as well as a total-scale score. The CQOLC has 35 items, the responses for which use a 5-point Likert-type scale (i.e., not at all to very much). It has demonstrated adequate construct validity, internal consistency, and test–retest reliability, and previous authors have strongly recommended it as a QOL instrument for cancer caregivers (Deeken et al., 2003). Internal consistency for the total scale in the present study was strong (α = .85 at T1 and α = .91 at T2). Internal consistency for the subscales at T1 was adequate and ranged from α = .69 (positive adaptation) to .87 (burden). Likewise, internal consistency for the subscales at T2 ranged from α = .76 (disruptiveness) to α = .89 (financial concerns).
We also measured QOL in care recipients at both time points using the Functional Assessment of Cancer Therapy–Head and Neck (FACT-HN) instrument (Cella et al., 1993; D’Antonio, Zimmerman, Cella, & Long, 1996). The FACT-HN is a 27-item instrument and measures intensity of response using a 5-point Likert-type scale (i.e., not at all to very much). This instrument has five subscales including Physical, Social/Family, Emotional, Functional, and Additional Concerns related to HNC. Scores are calculated for each subscale as well as summed for total performance on the instrument. The FACT-HN has demonstrated adequate internal consistency and construct validity (D’Antonio et al., 1996; List et al., 1999). Internal consistency in the present study for the overall instrument was strong (α = .94 at T1 and α = .91 at T2). The majority of the subscale scores were adequate at T1 and ranged from α = .77 (HNC concerns) to α = .89 (emotional well-being). The Social Well-Being subscale approached acceptability at T1 (α = .69). Similarly, the majority of the subscales demonstrated adequate internal consistency at T2, with scores ranging from α = .79 (social well-being) to α = .91 (physical well-being). Internal consistency for the Head and Neck subscale at T2 approached acceptability (α = .66).
Caregiver and care recipient’s social support
We assessed social support in caregivers and care recipients at a single time point (T2) using the Medical Outcomes Study Social Support Survey (Sherbourne & Stewart, 1991). This instrument assesses the perceived availability of several types of social support including emotional, informational, tangible, positive social interaction, and affection. The instrument has 19 items and uses a 5-point Likert-type scale for responses (none of the time to all of the time). Higher rankings indicate greater social support. The instrument can be scored using a total summed score as an overall index, although authors recommend evaluating each dimension separately due to demonstrated independence among subscales. This tool has been used in caregiver populations (Cumming, Cadilhac, Rubin, Crafti, & Pearce, 2008; Sherbourne & Stewart, 1991). It has demonstrated adequate internal reliability, test–retest reliability, and convergent and discriminant validity (Sherbourne & Stewart, 1991). Internal consistency in the present study was high for both care recipients and caregivers (α ≥ .87).
Care recipient swallowing function
We assessed swallowing ability in care recipients at both time points using the Eating Assessment Tool (EAT-10; Belafsky et al., 2008) and the Functional Oral Intake Scale (FOIS; Crary, Mann, & Groher, 2005). The Eat-10 is a validated self-report measure of a patient’s perception of his or her swallowing ability. The EAT-10 has 10 items using a 5-point Likert-type scale ranging from no problem to severe problem. A score of 3 or greater is indicative of dysphagia (Belafsky et al., 2008). The FOIS is a 7-point ordinal scale that is completed by the study researcher. It describes the functional oral intake of food and liquid with consideration for alternate food sources, modifications in food/liquid, and required maneuvers or compensations used by the patients to sustain a particular level of intake. A score of 5 or below is indicative of dysphagia. This scale has demonstrated strong concurrent and content validity compared to disability and clinical swallowing measures and interrater reliability across time points (Crary et al., 2005).
Data Analysis
Initially, we used descriptive statistics and graphic analyses to understand the distribution of the data, assess assumptions, and search for outliers. We checked outliers for all data, excluding cortisol, for errors, and if their values were correct we used them. We log-transformed cortisol data to control for skew and subsequently reviewed them for time and concentration value outliers. We considered values to be outliers if they were four standard deviations above the mean. However, we retained outliers that were within normal concentration values for the participant’s age and gender, and there were no additional behavioral compliance issues (e.g., smoked a cigarette) or medications or comorbidity known to interfere with cortisol concentration values that were reported in the participant’s journal. We performed all hypothesis testing at the two-sided .05 level.
For both aims, we conducted analysis to review the relationship between cortisol slopes and collection times. We calculated unstandardized β coefficients for cortisol slope at T1 and T2 by regressing raw cortisol concentration values on collection times, consistent with prior research (Sephton, Sapolsky, Kraemer, & Spiegel, 2000). To determine whether caregiver demographic variables, care recipient treatment and disease-related variables, or caregiver and care recipient psychosocial variables were associated with caregiver cortisol slope, we used correlation analyses and descriptive statistics. Caregiver demographic variables included age, gender, and socioeconomic status (identified via education and income levels). Care recipient treatment and disease-related variables included stage of disease, type of treatment (radiation or chemoradiation), and swallowing ability (dysphagia or no dysphagia). Lastly, psychosocial variables included caregiver burden, caregiver and care recipient QOL, and caregiver and care recipient social support.
For Aim 1, we used a paired samples t-test to evaluate mean difference in cortisol slope as a continuous variable from T1 to T2. For Aim 2, we entered variables that were significantly associated with the dependent variable (diurnal cortisol rhythm) into separate linear regression analyses as predictor variables, while controlling for age and gender. Age and gender are associated with diurnal cortisol and are therefore typically used as covariates in cortisol analyses (Adam & Kumari, 2009). We used histograms, scatterplots, and residual plots to evaluate the regression models for linearity, outliers, normality of residuals, and homoscedasticity. We performed analyses using version 21.0 of SPSS (IBM Analytics).
Results
Participants
A total of 33 caregivers consented to the study, one of which we excluded from analyses due to poor compliance. The remaining participants included 32 caregivers who completed T1 assessments and 29 who completed T2 assessments. Participant loss (9%) was attributed to caregivers feeling overwhelmed (n = 1), the care recipient being medically unable to finish radiation treatment (n = 1), and care recipient death (n = 1). All participating caregivers at T1 (n = 32) and at T2 (n = 29) returned surveys. All caregivers returned complete saliva samples (6 each) at T1. At T2, 26 caregivers returned all samples and 3 caregivers returned five samples. Caregiver characteristics are displayed in Table 1.
Table 1.
Participant Characteristics at Study Entry.
| Characteristic | Caregiver | Care Recipient |
|---|---|---|
| Age (years), M (SD); Range | 57.41 (14.60); 24–81 | 60.59 (14.99); 21–83 |
| Gender, female, n (%) | 27 (84.4) | 8 (25.0) |
| Race, n (%) | ||
| White | 29 (90.6) | 28 (87.5) |
| Other | 3 (9.4) | 4 (12.5) |
| Ethnicity, not Hispanic/Latino, n (%) | 30 (93.8) | 31 (96.9) |
| Education, n (%) | ||
| <High school | 1 (3.1.) | 4 (12.5) |
| High school/GED | 10 (31.3) | 12 (37.5) |
| College/tech. degree | 16 (50.0) | 10 (31.3) |
| Graduate school | 5 (15.6) | 6 (18.8) |
| Income, annual, n (%) | ||
| <US$29,999 | 9 (28.1) | |
| US$30,000–59,999 | 9 (28.1) | |
| US≥60,000 | 13 (43.8) | |
| Employment, not employed, n (%) | 17 (53.1) | |
| Relationship to care recipient, n (%) | ||
| Spouse/partner | 23 (72.0) | |
| Parent | 4 (12.5) | |
| Child | 2 (6.2) | |
| Other family | 2 (6.2) | |
| Friend | 1 (3.1) | |
| Treatment, n (%) | ||
| Radiation only | 11 (34.40) | |
| Combined chemoradiation | 21 (65.60) | |
| Prior surgery, n (%) | 12 (37.50) | |
| AJCC stage, n (%) | ||
| 0 | 2 (6.2) | |
| I | 3 (9.4) | |
| II | 2 (6.2) | |
| III | 6 (18.8) | |
| IV | 16 (50.0) | |
| Not staged | 3 (9.4) | |
| Recurrence, yes, n (%) | 3 (9.4) |
Note. n = 32 dyads. AJCC = American Joint Committee on Cancer; GED = general equivalency diploma.
We enrolled each caregiver-matched care recipient in the study, although four declined survey participation at both T1 and T2 and an additional care recipient declined survey participation at T1 only. Of these five care recipients, two indicated that they were not feeling well enough to complete their surveys and the remaining three indicated that they did not feel comfortable sharing their feelings or reflecting on their cancer experience. Therefore, we obtained surveys from 27 care recipients at T1 and 28 care recipients at T2. The study researcher obtained FOIS scores for 28 care recipients at T1 and 27 care recipients at T2. Care recipient characteristics are displayed in Table 1.
Change in Diurnal Cortisol Rhythm of Caregivers
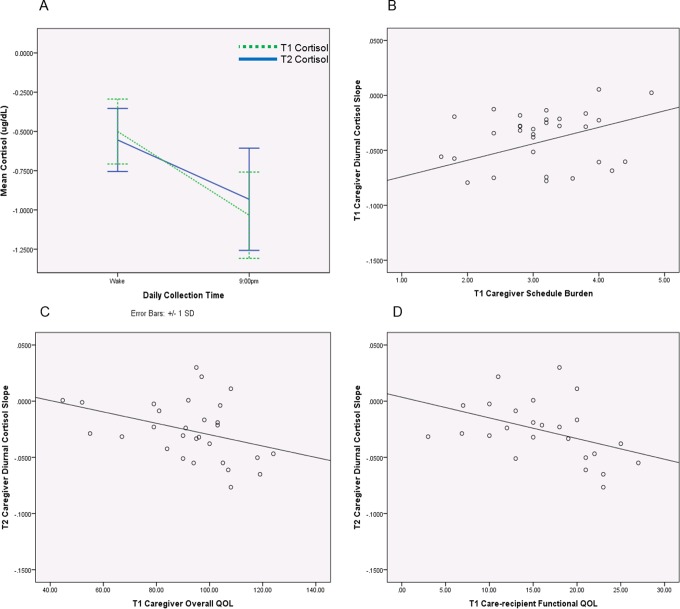
A paired samples t-test revealed a significantly flatter caregiver diurnal cortisol slope at T2 (M = −.03, SD = .03) in comparison to T1 (M = −.04, SD = .04), t(28) = −2.56, p = .02, d = −.60 (see Figure 1A).
Figure 1.
(A) Caregivers’ diurnal cortisol rhythm at T1 and T2. Caregivers’ diurnal cortisol rhythm became significantly flatter at T2 in comparison to T1. (B) Caregivers’ schedule burden and diurnal cortisol rhythm at T1. Controlling for age and gender, caregiver schedule burden at T1 was significantly associated with cortisol slope at T1. (C) Caregivers’ overall quality of life (QOL) at T1 and diurnal cortisol rhythm at T2. Controlling for age and gender, caregiver’s overall QOL at T1 was significantly associated with diurnal cortisol rhythm at T2. (D) Care recipients’ functional QOL at T1 and caregivers’ diurnal cortisol slope at T2. Controlling for age and gender, care recipient’s functional QOL at T1 was significantly associated with diurnal cortisol slope at T2. Cortisol data are log transformed.
Predictors of Cortisol Slope in Caregivers
Caregiver diurnal cortisol slope at T1 was positively correlated with caregiver schedule burden at T1, r(30) = .34, p = .05. A linear regression analysis indicated that, when controlling for age and gender, caregiver schedule burden at T1 was significantly associated with cortisol slope at T1 (β = .35, p = .05), accounting for 18.0% of the variance (see Table 2 and Figure 1B). These results indicate that for each standard deviation increase in caregiver schedule burden at T1, there was a .35 increase in diurnal cortisol slope.
Table 2.
Significant Associations Between T1 Predictors and T1 and T2 Caregiver Diurnal Cortisol Rhythm.
| Variable | r | β | p | R 2 |
|---|---|---|---|---|
| Associations with T1 diurnal cortisol rhythm | ||||
| Caregiver schedule burden | .34 | .35 | .05 | .18 |
| Associations with T2 diurnal cortisol rhythm | ||||
| Caregiver overall QOL | −.37 | −.39 | .04 | .17 |
| Care-recipient functional QOL | −.42 | −.41 | .05 | .19 |
Note. Separate linear regression analyses controlled for age and gender. Caregiver demographic variables, care recipient treatment and disease-related variables, and caregiver and care recipient social support were not significantly associated with caregiver cortisol response. QOL = quality of life.
In contrast, T2 diurnal cortisol slope was negatively correlated with the caregiver’s overall QOL at T1, r(27) = −.37, p = .05, care recipient’s overall QOL at T1, r(27) = −.40, p = .05, care recipient’s emotional QOL at T1, r(27) = −.39, p = .05, and care recipient’s functional QOL at T1, r(27) = −.42, p = .04. We used separate linear regression analyses to predict cortisol slope at T2 from each of the four correlated predictor variables individually. When controlling for age and gender, caregiver’s overall QOL at T1 was significantly associated with diurnal cortisol rhythm at T2 (β = −.39, p = .04), accounting for 17.0% of the variance (see Table 2 and Figure 1C). For each standard deviation increase in caregiver’s overall QOL at T1, there was a .39 decrease in cortisol slope at T2. In addition, when controlling for age and gender, care recipient’s functional QOL at T1 was significantly associated with diurnal cortisol slope at T2 (β = −.41, p = .05), accounting for 19.0% of the variance (see Table 2 and Figure 1D). For each standard deviation increase in care recipient’s functional QOL at T1, there was a .41 decrease in caregiver’s diurnal cortisol slope at T2. Caregiver demographic variables (age, gender, and socioeconomic status), care recipient treatment and disease-related variables (American Joint Committee on Cancer [AJCC] stage, treatment type, and swallowing ability), and caregiver and care recipient social support were not significantly associated with caregiver cortisol response at T1 or T2.
Discussion
The first aim of this pilot investigation was to evaluate change in diurnal cortisol rhythm over the radiation treatment period in caregivers of patients with HNC. Consistent with the hypothesis, our results demonstrated that, on average, caregivers’ diurnal cortisol slope became flatter (more abnormal) from the initiation of treatment (T1) toward the conclusion of treatment (5 weeks posttreatment initiation, T2), indicating overstimulation of the HPA axis. Moreover, the change in diurnal cortisol slope represented a moderate-to-large effect size.
Also noteworthy is the average diurnal cortisol slope value for caregivers at the initiation of the study. In comparison to a study that evaluated both caregivers of children with cancer in active treatment and parents of healthy children (Miller, Cohen, & Ritchey, 2002), the caregivers in the present study demonstrated a flatter diurnal cortisol slope at T1 when their care recipients’ radiation treatment was just beginning. In other words, caregivers in the present study experienced more dysregulation in their diurnal cortisol rhythm at the start of their care recipients’ treatment in comparison to caregivers of pediatric cancer patients who were in active treatment. It is unclear what factors may be associated with this discrepancy in cortisol slope values; however, it is possible that the difference is attributable to inconsistent procedures for collecting saliva across studies. Likewise, another possible explanation is that the caregivers of the pediatric patients experienced more support with the caregiving demands from a spouse or other family members. In contrast, the majority of caregivers in the present study were providing care for their spouse.
The second aim of this study was to identify predictors of diurnal cortisol rhythm in caregivers of patients with HNC. Results indicated that caregivers’ diurnal cortisol slope at T1 was predicted by caregivers’ schedule burden at T1, when adjusting for age and gender. Specifically, the more schedule burden a caregiver reported, the flatter (more blunted, abnormal) their cortisol slope. In addition to identifying a predictive relationship with diurnal cortisol slope at T1, results demonstrated relationships with diurnal cortisol slope at T2. When controlling for age and gender, both caregivers’ overall QOL at T1 and care recipient’s functional QOL at T1 individually predicted diurnal cortisol slope in caregivers at T2. In other words, higher (better) caregiver overall QOL and care recipient functional QOL were each associated with a steeper (more normal) diurnal cortisol slope at T2. These findings suggest that a mind–body interaction may be associated with the HNC caregiving experience. In contrast to our hypothesis, we found no relationship between diurnal cortisol slope and caregiver demographic characteristics, care recipient swallowing ability, disease and treatment-related variables, or care recipient and caregiver social support in this sample. Moreover, although care recipients’ emotional and overall QOL at T1 were correlated with caregivers’ diurnal cortisol slope at T2, these relationships did not remain within the regression analyses. Collectively, these findings suggest that the caregiver’s neuroendocrine system may respond more negatively toward the conclusion of the radiation treatment experience compared to at the initiation of the treatment, specifically by demonstrating a more dysregulated diurnal cortisol rhythm. In addition, these findings suggest that caregiver schedule burden and overall QOL as well as care recipient functional QOL may each have an important impact on the body’s neuroendocrine response to the subjective caregiving experience.
This study was the first to evaluate diurnal cortisol rhythm in caregivers of patients with HNC and one of only a few that have evaluated it in the broader population of cancer patient caregivers (Miller et al., 2002, 2014; Rohleder, Marin, Ma, & Miller, 2009; Thomas et al., 2012). Overall, investigations into HNC-patient caregiving have been restricted to psychosocial outcomes only and have neglected to explore how the body physiologically responds to the caregiving experience (Longacre, Ridge, Burtness, Galloway, & Fang, 2012). Researchers who have evaluated diurnal cortisol rhythm in the broader cancer caregiving population have predominantly utilized cross-sectional study designs without considering how cortisol slope may change across time points (Miller et al., 2002; Thomas et al., 2012). In contrast, in the present study, we prospectively evaluated cortisol rhythm and demonstrated a changing pattern for HNC caregivers over the radiation treatment experience. Another strength of the present study was the evaluation of both caregiver and care recipient factors that might contribute to the caregiver’s cortisol response. Prior research has demonstrated interdependence among HNC patient and caregiver psychosocial factors (Nightingale et al., 2014; Patterson, Rapley, Carding, Wilson, & McColl, 2013). The present study augments that literature by also demonstrating a relationship between the care recipient’s QOL and the caregiver’s cortisol response.
A noted limitation in the present study is the small sample size, which may have resulted in inadequate power to identify additional relationships within our data. However, despite the small sample size, this study yielded significant results and included a larger sample size of caregivers than many of the previous studies evaluating cortisol in cancer caregivers (Miller et al., 2002; Rohleder et al., 2009; Thomas et al., 2012). Due to the small sample size, we conducted separate linear regression analyses to test the predictor variables. This strategy resulted in a small amount of variance accounted for in the regression analyses and suggests there are more factors unaccounted for that play a role in HNC caregivers’ diurnal cortisol rhythm during radiation treatment. This study is also limited by the potential for testwise error, since we conducted these separate analyses without applying a Bonferroni correction. Further, although we accounted for some potential confounders including age and gender, we did not consider body mass index, depression, or pregnancy, which may have impacted cortisol response (Adam & Kumari, 2009). However, we intended this pilot study to be exploratory and it does provide preliminary evidence of factors associated with cortisol slope among HNC caregivers. Specifically, this is the first study to identify burden and QOL predictors of dysregulated diurnal cortisol rhythm in HNC caregivers, and consequently, it provides valuable information to augment the current literature. Another important limitation in this study is the lack of a control or comparison group. The inclusion of a control or comparison group may have helped to identify whether the findings were specific to HNC caregivers, as opposed to noncaregivers or other types of caregivers. A natural fluctuation in diurnal slope over time cannot be ruled out with certainty without the inclusion of a control or comparison group. However, a comparison group would have been limited to other types of cancer caregivers, given the use of cancer-specific burden and QOL instruments in this study. Consequently, this is a main limitation of the study, limiting the strength of our findings.
Finally, we collected cortisol 2 times each day (on waking and at 21:00 hr) for 3 consecutive days. Although prior research has demonstrated that this frequency of salivary cortisol collection provides an accurate measure of diurnal cortisol rhythm (Kraemer et al., 2006), it does not allow for the measurement of the CAR, which requires an additional collection at 30-min postwaking (Adam & Kumari, 2009). Given the financial and participant burden challenges associated with collecting salivary cortisol (Adam & Kumari, 2009; Kraemer et al., 2006), our goal was to restrict the number of samples collected to the minimal frequency needed to obtain an accurate diurnal cortisol rhythm, grounded in previously published evidence (Kraemer et al., 2006). Kraemer and colleagues (2006) recommend collecting samples for 3 consecutive days and suggest that the number of days of collection is more important than increasing the frequency to three samples a day. Consequently, their suggestion to analyzing a diurnal cortisol slope is to collect samples 2 times a day over 3 consecutive days, such as we did in this study.
Our findings demonstrate that caregiving schedule burden, caregiver QOL, and care recipient functional QOL are associated with a negative neuroendocrine response in HNC caregivers. However, additional research focused on the cortisol responses of HNC caregivers and other cancer caregivers is needed. Future studies should include larger sample sizes, employ a control or comparison group, and evaluate additional factors that may be associated with caregivers’ cortisol response. For example, coping style may serve to mediate the relationship between caregivers’ psychosocial functioning and their neuroendocrine response. Further investigation might also evaluate the value of alternative diurnal slope measures (e.g., the CAR) and consider the inclusion of additional biomarkers of stress such as α-amylase and proinflammatory cytokines (Nater, Skoluda, & Strahler, 2013).
Conclusions
Overall, our findings in the present study emphasize the potential negative impact of the caregiving experience on HNC caregivers, including dysregulation in diurnal cortisol rhythm during the radiation treatment period. Future interventions aimed at reducing caregivers’ schedule burden may be beneficial in reducing or preventing abnormal cortisol response in HNC caregivers. Strategies for such interventions may include teaching caregivers time management skills or strategies to cope with the unavoidable time demands associated with providing care. Additional interventions aimed at increasing both care recipients’ and caregivers’ QOL may also have a positive impact on caregivers’ cortisol response, but more research is needed to further understand these relationships. Further investigation into these relationships will provide key information that will allow nurses and the supportive-care team to target HNC caregivers most at risk for poor neuroendocrine outcomes. The ability to reduce or prevent a negative psychoneuroendocrine response may be an important contributory factor in preventing corollary health problems such as immunologic decline, cardiovascular disease, metabolic syndrome, and mortality among informal caregivers (Elenkov, 2004; McEwen, 1998; Rosmond, 2005; Sephton et al., 2000). Efforts to prevent comorbid diseases across caregivers are becoming more imperative, as caregiving responsibilities are increasingly shifted to family members.
Acknowledgments
The authors wish to acknowledge data entry support from Thomas Riherd, BS, and Gregory Riherd, MPH, at the University of Florida, College of Public Health and Health Professions.
Footnotes
Authors’ Note: Please contact the corresponding author to access any underlying research materials related to this article.
Author Contribution: Deidre B. Pereira contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Barbara A. Curbow contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. John R. Wingard contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Giselle D. Carnaby contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Chandylen L. Nightingale contributed to conception, design, acquisition, analysis, and interpretation; drafted and critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded through the Paul M., Laura L., and Amy L. Deutsch Endowment in Life Care Planning Dissertation Award. Dr. Nightingale’s work on this article was supported by a Cancer Control Traineeship, National Cancer Institute/National Institutes of Health (NCI/NIH; R25CA122061).
References
- Adam E. K., Kumari M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Aldwin C. (2007). Stress, coping, and development. New York, NY: Guilford Press. [Google Scholar]
- American Cancer Society. (2016). Cancer facts & figures 2016. Atlanta, GA: Author; Retrieved from http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/index [Google Scholar]
- Badr H., Gupta V., Sikora A., Posner M. (2014). Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncology, 50, 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belafsky P. C., Mouadeb D. A., Rees C. J., Pryor J. C., Postma G. N., Allen J., Leonard R. J. (2008). Validity and reliability of the eating assessment tool (EAT-10). Annals of Otology, Rhinology & Laryngology, 117, 919–924. [DOI] [PubMed] [Google Scholar]
- Capistrant B. D., Moon J. R., Glymour M. M. (2012). Spousal caregiving and incident hypertension. American Journal of Hypertension, 25, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaby-Mann G., Crary M., Amdur R. (2012). “Pharyngocise”: Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. International Journal of Radiation Oncology*Biology*Physics, 83, 210–219. [DOI] [PubMed] [Google Scholar]
- Cella D. F., Tulsky D. S., Gray G., Sarafian B., Linn E., Bonomi A.…Harris J. (1993). The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology, 11, 570–579. [DOI] [PubMed] [Google Scholar]
- Chen S. C., Tsai M. C., Liu C. L., Yu W. P., Liao C. T., Chang J. T. (2009). Support needs of patients with oral cancer and burden to their family caregivers. Cancer Nursing, 32, 473–481. [DOI] [PubMed] [Google Scholar]
- Clements A. D., Parker C. R. (1998). The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology, 23, 613–616. [DOI] [PubMed] [Google Scholar]
- Crary M. A., Mann G. D., Groher M. E. (2005). Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of Physical Medicine and Rehabilitation, 86, 1516–1520. [DOI] [PubMed] [Google Scholar]
- Cumming T., Cadilhac D., Rubin G., Crafti N., Pearce D. (2008). Psychological distress and social support in informal caregivers of stroke survivors. Brain Impairment, 9, 705–714. [Google Scholar]
- D’Antonio L. L., Zimmerman G. J., Cella D. F., Long S. A. (1996). Quality of life and functional status measures in patients with head and neck cancer. Archives of Otolaryngology—Head & Neck Surgery, 122, 482–487. [DOI] [PubMed] [Google Scholar]
- Deeken J. F., Taylor K. L., Mangan P., Yabroff K. R., Ingham J. M. (2003). Care for the caregivers: A review of self-report instruments developed to measure the burden, needs, and quality of life of informal caregivers. Journal of Pain & Symptom Management, 26, 922–953. [DOI] [PubMed] [Google Scholar]
- Dirix P., Nuyts S., Vander Poorten V., Delaere P., Van den Bogaert W. (2008). The influence of xerostomia after radiotherapy on quality of life: Results of a questionnaire in head and neck cancer. Supportive Care in Cancer, 16, 171–179. [DOI] [PubMed] [Google Scholar]
- Drabe N., Zwahlen D., Buchi S., Moergeli H., Zwahlen R. A., Jenewein J. (2008). Psychiatric morbidity and quality of life in wives of men with long-term head and neck cancer. Psychooncology, 17, 199–204. [DOI] [PubMed] [Google Scholar]
- Eades M., Chasen M., Bhargava R. (2009). Rehabilitation: Long-term physical and functional changes following treatment. Seminars in Oncology Nursing, 25, 222–230. [DOI] [PubMed] [Google Scholar]
- Elenkov I. J. (2004). Glucocorticoids and the Th1/Th2 balance. Annals of the New York Academy of Sciences, 1024, 138–146. [DOI] [PubMed] [Google Scholar]
- Epstein J. B., Wilkie D. J., Fischer D. J., Kim Y. O., Villines D. (2009). Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head & Neck Oncology, 1, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given C. W., Given B., Stommel M., Collins C., King S., Franklin S. (1992). The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Research in Nursing & Health, 15, 271–283. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Hellhammer D. H. (1994). Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology, 19, 313–333. [DOI] [PubMed] [Google Scholar]
- Kraemer H. C., Giese-Davis J., Yutsis M., O’Hara R., Neri E., Gallagher-Thompson D.…Spiegel D. (2006). Design decisions to optimize reliability of daytime cortisol slopes in an older population. American Journal of Geriatric Psychiatry, 14, 325–333. [DOI] [PubMed] [Google Scholar]
- Lee S., Colditz G. A., Berkman L. F., Kawachi I. (2003). Caregiving and risk of coronary heart disease in U.S. women: A prospective study. American Journal of Preventive Medicine, 24, 113–119. [DOI] [PubMed] [Google Scholar]
- List M. A., Siston A., Haraf D., Schumm P., Kies M., Stenson K., Vokes E. (1999). Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: A prospective examination. Journal of Clinical Oncology, 17, 1020–1028. [DOI] [PubMed] [Google Scholar]
- Longacre M. L., Ridge J. A., Burtness B. A., Galloway T. J., Fang C. Y. (2012). Psychological functioning of caregivers for head and neck cancer patients. Oral Oncology, 48, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayre-Chilton K. M., Talwar B. P., Goff L. M. (2011). Different experiences and perspectives between head and neck cancer patients and their care-givers on their daily impact of a gastrostomy tube. Journal of Human Nutrition & Dietetics, 24, 449–459. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (2015). Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism—Clinical and Experimental, 64, S2–S10. [DOI] [PubMed] [Google Scholar]
- Miller G. E., Cohen S., Ritchey A. K. (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 21, 531–541. [DOI] [PubMed] [Google Scholar]
- Miller G. E., Murphy M. L., Cashman R., Ma R., Ma J., Arevalo J. M.…Cole S. W. (2014). Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, Behavior, and Immunity, 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney E. C., Brunner M., Alexander A. J., Clark J. (2015). Quantifying fibrosis in head and neck cancer treatment: An overview. Head & Neck, 37, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Murphy B. A., Gilbert J. (2009). Dysphagia in head and neck cancer patients treated with radiation: Assessment, sequelae, and rehabilitation. Seminars in Radiation Oncology, 19, 35–42. [DOI] [PubMed] [Google Scholar]
- Nater U. M., Skoluda N., Strahler J. (2013). Biomarkers of stress in behavioural medicine. Current Opinions in Psychiatry, 26, 440–445. [DOI] [PubMed] [Google Scholar]
- Nicholson N. (2008). Measurement of cortisol In Luecken L., Gallo G. (Eds.), Handbook of physiological research methods in health psychology (pp. 37–75). Los Angeles, CA: Sage. [Google Scholar]
- Nightingale C. L., Lagorio L., Carnaby G. (2014). A prospective pilot study of psychosocial functioning in head and neck cancer patient-caregiver dyads. Journal of Psychosocial Oncology, 32, 477–492. [DOI] [PubMed] [Google Scholar]
- Patterson J. M., Rapley T., Carding P. N., Wilson J. A., McColl E. (2013). Head and neck cancer and dysphagia; caring for carers. Psychooncology, 22, 1815–1820. [DOI] [PubMed] [Google Scholar]
- Penner J. L. (2009). Psychosocial care of patients with head and neck cancer. Seminars in Oncology Nursing, 25, 231–241. [DOI] [PubMed] [Google Scholar]
- Penner J. L., McClement S., Lobchuk M., Daeninck P. (2012). Family members’ experiences caring for patients with advanced head and neck cancer receiving tube feeding: A descriptive phenomenological study. Journal of Pain & Symptom Management, 44, 563–571. [DOI] [PubMed] [Google Scholar]
- Rohleder N., Marin T. J., Ma R., Miller G. E. (2009). Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. Journal of Clinical Oncology, 27, 2909–2915. [DOI] [PubMed] [Google Scholar]
- Rosmond R. (2005). Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology, 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Ross S., Mosher C. E., Ronis-Tobin V., Hermele S., Ostroff J. S. (2010). Psychosocial adjustment of family caregivers of head and neck cancer survivors. Supportive Care in Cancer, 18, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Beach S. R. (1999). Caregiving as a risk factor for mortality: The caregiver health effects study. Journal of the American Medical Association, 282, 2215–2219. [DOI] [PubMed] [Google Scholar]
- Sephton S. E., Sapolsky R. M., Kraemer H. C., Spiegel D. (2000). Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute, 92, 994–1000. [DOI] [PubMed] [Google Scholar]
- Sherbourne C. D., Stewart A. L. (1991). The MOS social support survey. Social Science & Medicine, 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Sherwood P. R., Given B. A., Donovan H., Baum A., Given C. W., Bender C. M., Schulz R. (2008). Guiding research in family care: A new approach to oncology caregiving. Psychooncology, 17, 986–996. [DOI] [PubMed] [Google Scholar]
- Silvestre-Rangil J., Silvestre F. J. (2011). Clinico-therapeutic management of osteoradionecrosis: A literature review and update. Medicina Oral Patología Oral y Cirugía Bucal, 16, e900–e904. [DOI] [PubMed] [Google Scholar]
- Thomas K. S., Bower J. E., Williamson T. J., Hoyt M. A., Wellisch D., Stanton A. L., Irwin M. (2012). Post-traumatic disorder symptoms and blunted diurnal cortisol production in partners of prostate cancer patients. Psychoneuroendocrinology, 37, 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzner M. A., Jacobsen P. B., Wagner H., Jr, Friedland J., Cox C. (1999). The Caregiver Quality of Life Index-Cancer (CQOLC) scale: Development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Quality of Life Research, 8, 55–63. [DOI] [PubMed] [Google Scholar]