Abstract
Background
Both acute and chronic pain result in a number of behavioral symptoms in patients, including cognitive effects such as decreased attention and working memory. Intraperitoneal administration of dilute lactic acid in rodents has been used to induce abdominal inflammation and produce effects in behavioral assays of both sensory-discriminative and affective pain modalities.
Methods
Intraperitoneal injection of dilute lactic acid was used to study the impact of abdominal inflammation on an operant task requiring sustained visual attention in rats (N=7–15/group) that adapts dynamically to performance ability. The effects of ketoprofen and morphine on lactic acid induced impairment were compared to those on the disruptive effects of scopolamine.
Results
Lactic acid impaired performance in a concentration dependent manner, increasing the duration of cue presentation required to maintain optimal performance from 0.5±0.2 sec (mean±S.D.) to 17.2±11.4 sec following administration of 1.8% (v/v) (N=13). The latency to emit correct responses and to retrieve the food reward were both increased by lactic acid. All effects of lactic acid injection were reversed by both ketoprofen and morphine in a dose-dependent manner. Scopolamine however produced dose dependent, non-pain related disruption in sustained attention that was not altered by either ketoprofen or morphine.
Conclusions
These data demonstrate that abdominal inflammation induced by lactic acid produces robust disruption in a visual attention-based operant task and that this disruption is reversed by analgesics. Future studies will focus on pain-related circuitry and its impact on both limbic forebrain and frontal cortical mechanisms.
Keywords: attention, reinforcement, inflammation, cognitive, opioid, NSAID, rat
Introduction
Pain is a multisensory experience comprised of sensory/discriminative and affective/motivational components, which in turn influence cognitive ability1–4. Efforts have been directed in recent years to develop paradigms that complement sensory/discriminative measures of pain and include other behavioral domains, including cognition. To this end, a rodent gambling task has proven to be sensitive to knee pain induced by intra-articular injection of irritants in rats5,6. This task is a correlate of the Iowa Gambling Task used to assess executive function, decision-making, and risk-taking behavior in humans. Importantly, performance in the Iowa Gambling Task is disrupted by pain in humans7. Other cognitive domains are disrupted by pain in humans as well, including working memory and attention, and chronic pain is associated with loss in frontal cortical gray matter8–11. Several behavioral paradigms have been developed in rodents to assess attention and one commonly used paradigm is the 5 choice serial reaction time task12,13. This procedure requires the subject to identify which of 5 apertures located along one wall is briefly illuminated. If the animal correctly identifies the aperture by poking it’s nose into the opening, the subject is rewarded with food. In this manner the animal must sustain attention in discreet trials to obtain food reward. An intact prefrontal cortex is required for efficient performance of this task, and neuronal activity in this region is negatively influenced in both rats and humans by pain12,14–17.
We recently developed a titration variant of the classical 5 choice serial reaction time task procedure in which the duration of aperture illumination varies dynamically between trials based on performance18. With this paradigm, the aperture is illuminated (cue duration) for a relatively long duration of 30 sec in the first trial. If the animal responds correctly, then the cue duration decreases in the next trial. The cue duration continues to decrease with subsequent correct responses, following a preprogrammed time array. When the animal fails to respond to the visual cue (omission) or responds in the incorrect aperture, the cue duration increases. Advantages of this titration variant of the 5 choice serial reaction time task (5CTV) are the wide dynamic range of behavioral measurements and task difficulty, as well as the systematic and automated titration of task difficulty depending on individual performance capability. One other advantage is the relatively short amount of training time required for animals to achieve stable performance criteria compared to the classical method.
In this study we assessed the ability of acute abdominal inflammation to disrupt performance in the 5CTV assay, as well as the efficacy and potency of the analgesics morphine and ketoprofen. As a negative control for the relevance of pain, we assessed the disruptive effects of scopolamine in the 5CTV alone and in conjunction with these analgesics. These data demonstrate that acute abdominal inflammation disrupts performance in this operant model, that these effects are sensitive to clinically relevant opioid and anti-inflammatory analgesics, and these effects can be distinguished from generalized disruption in performance by scopolamine.
Methods
Subjects
Male Fisher 344 rats (N=60, Harlan Industries, Raleigh, NC) were used for all studies. Animals were acclimated to the laboratory for a minimum of 7 days or until they achieved a body weight of at least 275 g. Animals were then singly housed and reduced to approximately 90% of their free-feeding weight and fed sufficient standard rat laboratory chow (Lab Diet, St. Louis, MO) to maintain proper growth based on published growth curves from the vendor. Rats were kept on a reversed light:dark cycle (dark 05:00–17:00) in a room immediately adjacent to the behavioral laboratory. Water was available ad libitum at all times except during experimental sessions. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and guidelines adopted by the International Association for the Study of Pain, and were approved by the Wake Forest School of Medicine Animal Care and Use Committee.
Behavioral Procedures
Apparatus
Commercially available equipment was used for all studies (Med Associates Inc., St. Albans, VT) and has been described previously (Martin et al., 2015). Operant chambers were connected to a PC-compatible computer through a commercially available interface (Med Associates Inc.) and controlled by a program written in Med PC-IV (Med Associates Inc.).
Procedure
The cue titration variant of the 5 choice serial reaction time task (5CTV) has been described previously, including the 4 discreet phases of training (Martin et al., 2015). Briefly, rats were first trained to nose poke in the receptacle for food delivery to obtain a single reward (45 mg chocolate-flavored purified rat chow pellet, formula F0299, Bio-Serv, Flemington, NJ). Each successful nose poke was accompanied by a brief tone (0.5 sec) and rats were allowed to obtain a maximum of 100 pellets per 30 min session. Once each subject earned the maximum number of pellets for at least 2 consecutive days, the second phase of training was initiated. This phase consisted of training the subject to nose poke in the middle aperture located on the wall opposite of the food receptacle to obtain 2 pellets. Sessions consisted of 50 trials or 30 min. Each trial began with illumination of the LED stimulus at the rear of the aperture for 30 sec or until the animal made a nose poke response. Only nose pokes in the middle aperture were reinforced. If the animal did not respond within 30 sec or made a response in one of the other 4 apertures, the LED was turned off and a 5 sec time out period initiated, after which the next trial began. Once animals responded correctly for a minimum of 45 out of the 50 trials for 3 consecutive sessions, the next phase of the training was initiated. In the third phase of training, one of the 5 apertures was illuminated at random and the animal was required to respond in the illuminated aperture for food reinforcement. All other aspects of the procedure were the same as the second phase. Once subjects responded correctly for a minimum of 40 of 50 trials for 3 consecutive sessions, the final phase of training was initiated. In this phase, the duration that the LED cue was illuminated (cue duration, CD) was systematically varied according to the array (in sec): 30, 25, 20, 15, 10, 8, 6, 4, 2, 1, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, 0.1. The structure of each 5CTV session and of each individual trial is the same as that typically used for 5CSRT studies (see Figure 1 from Fizet et al., 2016 for visual depiction of typical trial and session structure)13. Briefly, the session began by illumination of the food receptacle light and delivery of two pellets into the food receptacle. The subject initiated trials by poking it’s head into the food receptacle, which began with illumination of the house light and turning off the food receptacle light after 2 sec. After the house light had been illuminated for 5 sec (intertrial interval), one of the aperture LEDs was illuminated at random for the cue duration, until the subject made a response, or within an imposed time limit (limited hold). The limited hold was set to the cue duration or to 5 sec if the cue duration was less than 5 sec (i.e., the subject always had a minimum of 5 sec to respond after illumination of the cue light). Correct responses during the cue duration or within the limited hold resulted in extinguishing the cue light, illumination of the food receptacle light, and delivery of two food pellets. There were no time requirements or limits for the subject to retrieve the food reward and the next trial was initiated 2 sec following detection of head entry into the food receptacle, signaled by extinguishing the food receptacle light. The next trial consisted of illumination of an aperture following the intertrial interval. If the subject made an incorrect response or failed to respond within the limited hold (omission) then both the cue and house light were turned off and a 2 sec time-out period was imposed. If a subject responded in any aperture during the time-out (time-out response), the time-out was reset to 2 sec. Additionally if a subject responded in an aperture during the intertrial interval (before illumination of the cue light, premature response), a time-out period was initiated. After the time-out the next trial was initiated, signaled by illumination of the house light, followed by illumination of the cue light following the intertrial interval of 5 sec. The cue duration was decreased according to the array if the subject made correct responses, or was increased with incorrect responses or omissions. In this manner the cue duration is titrated to within the limits of performance capabilities of each subject throughout each individual session18. Sessions consisted of 100 trials or 30 min, whichever occurred first. No animals were excluded from this study and the training period required was consistent with that published previously for the 5CTV procedure for all subjects18.
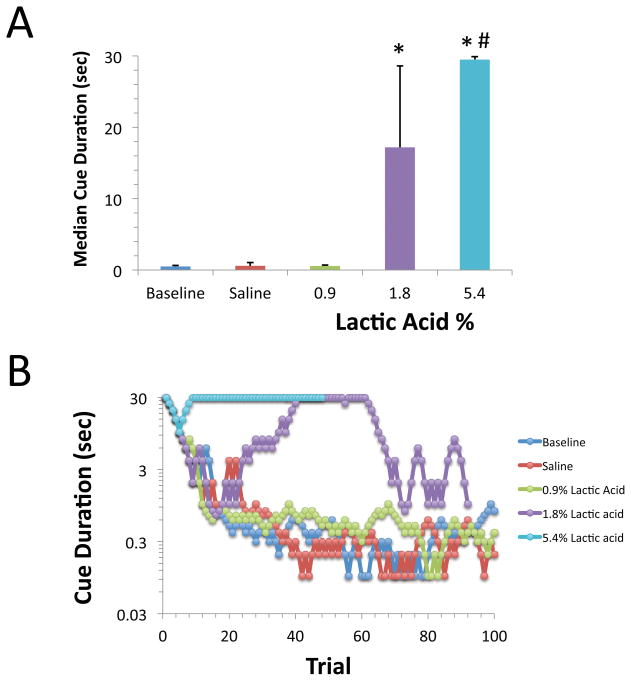
Figure 1. Effect of Lactic Acid concentration on visual attention threshold in the 5CTV.
A. Lactic acid increased the median cue duration in a concentration dependent manner following i.p. injection [F(4,50)=57.2, p<0.00001]. Saline and 0.9% lactic acid did not alter the median cue duration from baseline (BSL: Baseline, SAL: Saline) (*:p<0.05 compared to baseline, saline, and 0.9% lactic acid; #: p<0.05 compared to 1.8% lactic acid). Mean and S.D. are shown, N=7–13/group. B. Representative visual cue duration titration curves are shown. Each curve represents an individual session of 100 trials on a given day and treatment.
Behavioral endpoints
The behavioral endpoints collected were: cue duration for each trial, latency to each correct or incorrect response from initiation of the visual cue, latency to retrieve food reward following each correct response, number of correct responses, incorrect responses, omissions, premature responses (responses in aperture prior to illumination of cue), perseverative responses (multiple responses in aperture after a correct response in the same trial), time to completion of trials, and time out responses. The median cue duration was the primary behavioral endpoint and was calculated in Excel using the cue duration values from trials 15–100 or from 15 to the final trial completed. The %correct responses (total number of correct responses as a percentage of total number of trials completed), %incorrect responses, and %omissions were calculated as well and are presented as supplementary data.
Drug and lactic acid administration
Once the median cue duration did not vary by more than 15% from the mean for a minimum of 5 consecutive sessions for each subject, the effects of i.p. administration of lactic acid or 0.9% (w/v) saline were assessed in 22 animals. Lactic acid (0.9, 1.8, 5.4% v/v, 1 ml/kg) was administered on Tuesdays or Fridays immediately prior to 5CTV sessions, and only one injection of lactic acid was given per week consistent with other studies using this nociceptive stimulus.19 In a separate group of 24 animals, ketoprofen (0.01, 0.03, 0.1, or 0.3 mg/kg) was injected s.c. 30 min prior to injection of either saline or 1.8% lactic acid i.p., and 5CTV sessions were conducted immediately after the i.p. injection. These animals were also administered morphine (0.3, 1.0, or 3.0 mg/kg) s.c. 30 min prior to injection of saline or 1.8% lactic acid i.p. and 5CTV sessions were conducted in a similar manner. Animals received only one injection of lactic acid per week. In a separate group of 14 animals, scopolamine (0.03, 0.1, or 0.3 mg/kg) was administered s.c. 30 min prior to 5CTV sessions. These animals also received ketoprofen (0.3 mg/kg, s.c.) or morphine (3.0 mg/kg, s.c.) at the same time as injection of scopolamine (0.1 mg/kg, s.c.) 30 min prior to separate 5CTV sessions. None of the animals received all injection combinations from each group, however all animals received saline injections. All animals were individually randomized to treatment and treatment order, with the exception that each individual animal was limited to only one injection of lactic acid per week, and received i.p. saline injection on the other test day for that week, such that for each dose of either ketoprofen or morphine was given in the same week for each animal in combination with either saline or lactic acid, consistent with previous studies using lactic acid.19 The randomization for dose and drug order for each animal was obtained using the random number generator in Microsoft Excel and numerical coding for ketoprofen, morphine, or scopolamine dose or lactic acid concentration. Only animals that displayed stable performance in the 5CTV during Monday, Wednesday, or Thursday as defined above were administered drugs or lactic acid on the following Tuesday or Friday. The experimenter was not blinded to treatment.
Data Analysis
All data analyses were performed using Prism 6.0 (GraphPad Software Inc., San Diego, CA) for MacIntosh. A power analysis was performed using G*Power 3.1.9.2 for MacIntosh (http://www.gpower.hhu.de/en.html) with F-test and two-tailed one-way ANOVA parameters set to effect size of 0.7, alpha level at 0.05, power at 0.9, and number of groups set to 5. The estimated effect size was based on median cue duration estimates of 0.5 ± 0.2 sec (mean ± S.D.) at baseline and 20 ± 10 sec after 1.8% lactic acid administration. The primary outcome measure for all pharmacological manipulations was median cue duration, which was analyzed using two-tailed one-way ANOVA. ANOVAs were performed separately using data from animals that received i.p. lactic acid or i.p. saline in combination with morphine or ketoprofen, however the data from animals that received two saline injections were included in both analyses. Post-hoc analyses were performed using the Bonferroni t-test with correction for multiple comparisons. Secondary outcome measures analyzed were latency to correct responses and latency to retrieve food reward following correct responses, and these endpoints were analyzed similarly as median cue duration. The ROUT method to identify outliers was applied to the primary and secondary outcome measures for all groups, with false error rate Q set to 1%20. A two-tailed p-value of 0.05 or less was considered statistically significant. All other behavioral endpoints collected are summarized in the supplementary data section, but were not analyzed statistically.
Results
Effect of lactic acid concentration on performance in the 5CTV
Lactic acid increased the median cue duration in a concentration dependent manner following i.p. injection [F(4,50)=57.2, p<0.00001, Figure 1]. Neither i.p. administration of saline nor 0.9% lactic acid significantly altered the median cue duration compared to baseline, however both 1.8% and 5.4% lactic acid disrupted performance, increasing the median cue duration 34-fold and 60-fold compared to baseline, respectively. 5.4% lactic acid produced a significantly greater disruption in attention performance than 1.8% lactic acid (p<0.05), and none of the rats were able to complete all 100 trials within 30 min following administration of 5.4% lactic acid (data not shown). Representative visual cue duration titration curves are shown in the lower panel of Figure 1.
Lactic acid affected both the latency to emit a correct response from the initiation of the visual cue [F(4,44)=20.9, p<0.0001] and the latency to retrieve the food reward following a correct response [F(4,440=6.1, p<0.001] in a concentration dependent manner (Table 1). Both latency values were significantly increased following administration of 1.8% or 5.4% lactic acid compared to baseline values (p<0.05) (Table 1). The latency to respond correctly was increased 11.0±3.8 fold following administration of 5.4% lactic acid compared to an increase of 1.5±0.4 fold in the latency to retrieve the food reward.
Table 1. Effect of lactic acid on response latencies in 5CTV.
Latencies (sec, mean and S.D.) from presentation of the visual cue until a correct response is emitted (Latency to Correct) or from correct responses to retrieval of the food pellets (Latency to Reward) are shown.
| % Lactic Acid | Latency to Correct | Latency to Reward |
|---|---|---|
|
| ||
| Baseline | 1.15 (0.51) | 1.49 (0.15) |
| Saline | 0.96 (0.20) | 1.65 (0.37) |
| 0.9% | 1.14 (0.40) | 1.42 (0.24) |
| 1.8% | 3.96 (2.82) *,# | 2.12 (0.50) ** |
| 5.4% | 12.69 (7.56) **,## | 2.21 (1.08) |
p<0.05;
p<0.01 compared to baseline.
p<0.05;
p<0.01 compared to saline using Bonferroni correction for multiple pairwise comparisons.
Effects of ketoprofen and morphine on lactic acid-induced impairment of 5CTV performance
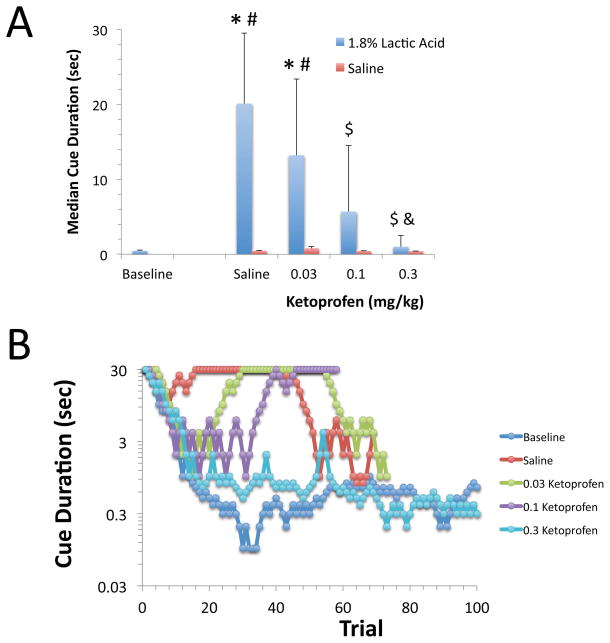
Median cue duration values at baseline or after pretreatment with saline, 0.03, 0.1, or 0.3 mg/kg of ketoprofen 30 min prior to administration of 1.8% lactic acid were compared. Ketoprofen reduced the effect of 1.8% lactic acid on median cue duration in a dose-dependent manner [F(4,67)=20.1, p<0.0001, Figure 2]. The effect of 1.8% lactic acid on median cue duration was significantly reduced by administration of either 0.1 or 0.3 mg/kg of ketoprofen, and the median cue duration was not significantly different from baseline values following pretreatment with either dose of ketoprofen in combination with 1.8% lactic acid (Figure 2). Administration of saline i.p. in combination with saline or any of the doses of ketoprofen s.c. did not result in any significant changes in median cue duration [F(4,61)=2.1, p=0.09, Figure 2]. Representative visual cue duration titration curves are presented for a single animal in the lower panel in Figure 2.
Figure 2. Effects of ketoprofen on lactic acid-induced impairment of visual attention performance.
A. Ketoprofen reduced the effect of 1.8% lactic acid on median cue duration in a dose-dependent manner [F(4,67)=20.1, p<0.0001]. Saline and ketoprofen at any dose had no effect on median cue duration in the absence of lactic acid administration. (BSL: Baseline, SAL: Saline) (*:p<0.05 compared to BSL, #: p<0.05 compared to SAL/SAL, $: p<0.05 compared to SAL/1.8% lactic acid, &: p<0.05 compared to 0.03Ket/1.8% lactic acid). Mean and S.D. are shown, N=10–15/group. B. Representative visual cue duration titration curves are shown. Each curve represents an individual session of 100 trials on a given day and treatment.
Ketoprofen produced a dose dependent inhibition of the effects of 1.8% lactic acid on both the latency to emit a correct response [F(5,78)=17.0, p<0.0001] and the latency to retrieve the food reward following a correct response [F(5,73)=13.2, p<0.0001]. Using the ROUT method in Prism 6.0, 3 outliers were identified within the latency to correct data set (1 from the 0.03 ketoprofen/lactic acid group and 2 from the 0.1 ketoprofen/lactic acid group) and 8 outliers were identified in the latency to reward data set (3 from the saline/lactic acid group, 3 from the 0.03 ketoprofen/lactic acid group, and 2 from the 0.1 ketoprofen/lactic acid group). In all circumstances, the values of outliers were significantly greater than the group mean. Administration of 1.8% lactic acid increased both the latency to correct and the latency to reward compared to baseline, and these effects were inhibited by pretreatment with either 0.1 or 0.3 mg/kg of ketoprofen (Table 2). Administration of saline or any of the doses of ketoprofen in combination with i.p. saline (in the absence of lactic acid) did not alter either of the latency measures (data not shown).
Table 2. Effect of ketoprofen and 1.8% lactic acid on response latencies in 5CTV.
Latencies (sec, mean and S.D.) from presentation of the visual cue until a correct response is emitted (Latency to Correct) or from correct responses to retrieval of the food pellets (Latency to Reward) are shown. BSL: baseline sessions, SAL: sessions with saline (i.p.) administration. SAL and ketoprofen were administered s.c. 30 min prior to administration of 1.8% lactic acid (i.p., 1 ml/kg).
| Ketoprofen (mg/kg) | Latency to Correct | Latency to Reward |
|---|---|---|
|
| ||
| Baseline | 1.03 (0.22) | 1.67 (0.33) |
| Saline/Saline | 0.99 (0.13) | 1.57 (0.15) |
| Saline/1.8% lactic acid | 5.06 (2.93) *,# | 2.39 (0.44)*,# |
| 0.03/1.8% lactic acid | 4.03 (2.29) *,# | 2.56 (0.66)*,# |
| 0.1/1.8% lactic acid | 1.73 (0.96)**,## | 1.78 (0.37)**,## |
| 0.3/1.8% lactic acid | 1.66 (0.65)**,## | 1.78 (0.34)**,## |
p<0.0001 compared to Baseline.
p<0.0001 compared to Saline/Saline.
p<0.01 compared to Saline/1.8% lactic acid.
p<0.01 compared to 0.03/1.8% lactic acid using Bonferroni correction for multiple comparisons.
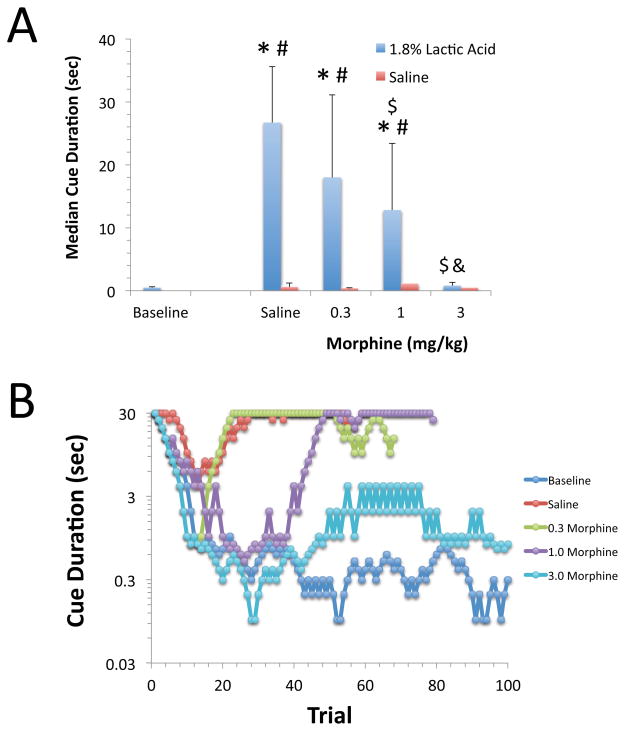
The effects of morphine on 5CTV performance were assessed and analyzed statistically as described for ketoprofen. Morphine reduced the effect of 1.8% lactic acid on performance as measured using the median cue duration in a dose-dependent manner [F(4,47)=19.4, p<0.0001, Figure 3]. The effect of 1.8% lactic acid on median cue duration was significantly reduced following pretreatment with 1.0 or 3.0 mg/kg of morphine, and the median cue duration was not significantly different from baseline values after pretreatment with 3.0 mg/kg of morphine in combination with i.p. treatment with 1.8% lactic acid (Figure 3). Administration of saline i.p. (in the absence of lactic acid) in combination with saline or any dose of morphine s.c. did not alter median cue duration values [F(4,45)=0.95, p=0.4, Figure 3]. Representative visual cue duration titration curves are presented for a single animal in the lower panel of Figure 3.
Figure 3. The effects of morphine on lactic acid-induced impairment of visual attention performance.
A. Morphine reduced the effect of 1.8% lactic acid on performance as measured using median cue duration in a dose-dependent manner [F(4,47)=19.4, p<0.0001]. Saline and morphine at any dose had no effect on median cue duration in the absence of lactic acid administration. (BSL: Baseline, SAL: Saline) (*:p<0.05 compared to BSL, #: p<0.05 compared to SAL/SAL, $: p<0.05 compared to SAL/1.8% lactic acid, &: p>0.05 compared to 0.3 morphine/1.8% lactic acid). Mean and S.D. are shown, N=8–14/group. B. Representative visual cue duration titration curves are shown. Each curve represents an individual session of 100 trials on a given day and treatment.
Morphine significantly reduced the effect of 1.8% lactic acid on both the latency to emit a correct response [F(5,57)=11.8, p<0.0001] and the latency to retrieve the food reward [F(5,51)=3.2, p=0.014] at all doses tested when compared to baseline (Table 3). Both the latency to emit a correct response and the latency to retrieve the food reward were significantly increased following administration of 1.8% lactic acid i.p. compared to baseline values. However, neither measure was significantly different from baseline when animals were pretreated with 0.3, 1.0 or 3.0 mg/kg of morphine s.c. prior to lactic acid injection (Table 3). Administration of saline or any of the doses of morphine in combination with i.p. saline (in the absence of lactic acid) did not alter either of the latency measures (data not shown).
Table 3. Effect of morphine and 1.8% lactic acid on response latencies in 5CTV.
Latencies (sec, mean and S.D.) from presentation of the visual cue until a correct response is emitted (Latency to Correct) or from correct responses to retrieval of the food pellets (Latency to Reward) are shown. Saline and morphine were administered s.c. 30 min prior to administration of 1.8% lactic acid (i.p., 1 ml/kg).
| Morphine (mg/kg) | Latency to Correct | Latency to Reward |
|---|---|---|
|
| ||
| Baseline | 1.08 (0.22) | 1.55 (0.21) |
| Saline/Saline | 1.11 (0.32) | 1.52 (0.24) |
| Saline/1.8% lactic acid | 6.77 (4.60)*,# | 2.07 (0.44)$,% |
| 0.3/1.8% lactic acid | 2.39 (1.24)** | 1.72 (0.64) |
| 1.0/1.8% lactic acid | 2.71 (1.82)** | 1.48 (0.26)$$ |
| 3.0/1.8% lactic acid | 1.54 (0.57) ** | 1.76 (0.51) |
p<0.0001 compared to Baseline.
p<0.0001 compared to Saline/Saline.
p<0.01 compared to Saline/1.8% lactic acid.
p<0.05 compared to Baseline. %, p<0.05 compared to Saline/Saline.
p<0.05 compared to Saline/1.8% lactic acid using Bonferroni correction for multiple comparisons.
Effects of scopolamine on 5CTV performance and lack of reversal by ketoprofen and morphine
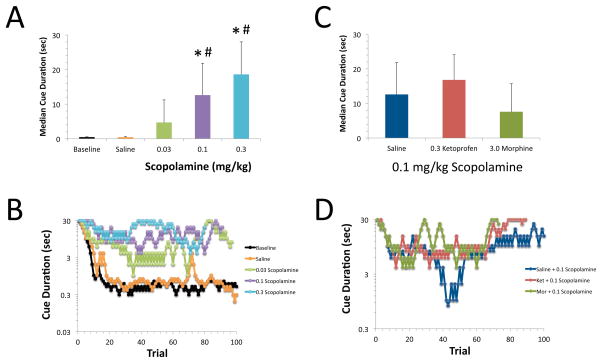
Scopolamine disrupted performance in the 5CTV as evidenced by a dose dependent increase in the median cue duration [F(4,66)=22.6, p<0.0001, Figure 4]. Administration of either saline or 0.03 mg/kg of scopolamine did not produce an increase in the median cue duration relative to baseline values, however both 0.1 and 0.3 mg/kg increased the median cue duration by 27- and 41-fold, respectively. Comparison of the effects of scopolamine in the presence or absence of 0.3 mg/kg of ketoprofen or 3 mg/kg of morphine found a significant effect of group [F(2,35)=3.5, p=0.04] however neither 0.3 mg/kg of ketoprofen nor 3 mg/kg of morphine altered the effect of 0.1 mg/kg of scopolamine on the median cue duration (p>0.05, Figure 4). There was a difference between the median cue duration following scopolamine treatment following administration of the two analgesics however, with the median cue duration following morphine treatment being less than following treatment with ketoprofen (Figure 4). Representative visual cue duration titration curves are shown in the lower panels of Figure 4.
Figure 4. Effects of scopolamine on visual attention performance and lack of reversal by ketoprofen and morphine.
A. Scopolamine disrupted performance in the 5CTV as evidenced by a dose dependent increase in the median cue duration [F(4,66)=22.6, p<0.0001]. Saline or 0.03 mg/kg of scopolamine did not produce a signficant increase in median cue duration relative to baseline values (BSL: Baseline, SAL: Saline) (*:p<0.05 compared to BSL, #: p<0.05 compared to SAL). Mean and S.D. are shown, N=13–15/group B. Representative visual cue duration titration curves are shown. Each curve represents an individual session of 100 trials on a given day and treatment. C. Neither 0.3 mg/kg of ketoprofen nor 3 mg/kg of morphine altered the effect of 0.1 mg/kg of scopolamine on the median cue duration (p>0.05). Mean and S.D. are shown, N=11–13/group. D. Representative visual cue duration titration curves are shown for scopolamine and either ketoprofen or morphine.
Scopolamine increased both the latency to emit a correct response [F(4,64)=39.5, p<0.0001] and the latency to retrieve the food reward [F(4,63)=9.6, p<0.0001] in a dose dependent manner (Table 4). Ketoprofen (0.3 mg/kg) did not inhibit the effects of scopolamine on latency to correct responses or latency to retrieve the food reward. Morphine (3.0 mg/kg) reduced the effect of scopolamine on latency to retrieve the food reward, but not on the latency to emit correct responses.
Table 4. Effect of scopolamine alone or with morphine or ketoprofen on response latencies in 5CTV.
Latencies (sec, mean and S.D.) from presentation of the visual cue until a correct response is emitted (Latency to Correct) or from correct responses to retrieval of the food pellets (Latency to Reward) are shown.
| Scopolamine (mg/kg) | Latency to Correct | Latency to Reward |
|---|---|---|
|
| ||
| Baseline | 1.03 (0.22) | 1.67 (0.33) |
| Saline | 0.99 (0.13) | 1.57 (0.15) |
| 0.03 | 2.23 (0.89) | 3.68 (2.16) |
| 0.10 | 5.80 (2.97)*,# | 6.27 (4.21) |
| 0.30 | 9.16 (3.67)*,# | 4.84 (3.04) |
| 0.3 ketoprofen + 0.10 | 7.10 (1.90)*,# | 4.97 (2.86)*,# |
| 3.0 morphine + 0.10 | 3.75 (1.56)*,# | 2.51 (1.41) |
p<0.01 compared to Baseline.
p<0.01 compared to Saline using Bonferroni correction for multiple comparisons.
Discussion
Pain is a multidimensional experience, with sensory-discriminative and affective-motivational components. The sensory-discriminative effects of a multitude of experimental pain states have been explored in rodents for several decades, and significant progress has been achieved in defining the neuronal pathways and correlates for this dimension of pain. Recently, several models have been explored that investigate affective-motivational aspects of experimental pain in rodents, including alteration of behavior in both reinforcement paradigms including drug self-administration, conditioned place preference, and intracranial self-stimulation, as well as in fear/avoidance paradigms such as conditioned place avoidance21–29. These behavioral models have likewise been used to explore how pain-related circuitry interacts with reinforcement and fear-related pathways 30–34. Similar progress in exploring the influence of experimental pain stimuli in rodents on cognitive functioning is needed, as cognitive impairment resulting from pain is an important consequence of pain, and treatment modalities for cognitive impairment may not coincide with those useful for other dimensions depending on the mechanisms involved. The present data demonstrate that acute abdominal inflammation in rats disrupts performance in an operant task requiring sustained visual attention, and that clinically useful analgesics are capable of restoring performance to baseline levels. Further, the effects of these analgesics on performance disruption induced by abdominal inflammation are distinct from those induced by scopolamine, a compound frequently used as a positive control for disruption in attention based operant assays in rodents. It should be noted that 3 mg/kg of morphine did attenuate the effects of scopolamine on latency to retrieve the food reward, and this may indicate a general excitatory effect at this high dose.
Abdominal inflammation induced by lactic acid administration in rodents produces a number of behavioral effects thought to be associated with pain, including abdominal writhing (sensory/discriminative) and suppression of intracranial self-stimulation (affective/motivational).29 A recent study failed to find a disruptive effect of lactic acid administration on attention using a signal detection task however, except at a relatively large concentrations (3.2 and 5.6%) which increased the latency to correct responses and the number of omitted trials with no change in accuracy, similar to the effects found at a lower concentration in the present study.35 The same range of doses of scopolamine used in the present study produced disruption in this signal detection task35. Induction of colitis with 2,4,6-trinitrobenzenesulfonic acid, a more chronic form of abdominal nociception, disrupts performance in a novel object recognition task in rats, however the effects of morphine in this assay were mixed with only a single dose producing a reversal of these effects36. Ligation of the L5 and L6 spinal nerves also impairs performance in the novel object recognition task in rats37. This task has been described as possessing non-sustained attentional components, as well as working memory components and as such the role of attention per se with these manipulations is not known. Previous studies examining the effects of abdominal nociception and analgesics in attention-based assays have been mixed, however the present study appears to be relatively more sensitive to both abdominal inflammation and reversal of these effects by analgesics.
Other pain manipulations have been found to decrease performance in behavioral assays thought to be related to attention processes in rodents. Spared nerve injury decreases performance in the novel object recognition task and in the classical 5 choice serial reaction time task38,39. The effects of analgesics on disruption of attention was not determined in these studies, however it was demonstrated that the deficits persisted for several weeks or months beyond the time of surgical injury. Induction of paw inflammation with Complete Freund’s adjuvant or formalin, respectively, diminishes attention performance in both the novel object recognition task and the 5 choice serial reaction time task and morphine reverses these effects in both assays at relatively high doses6,40. In the present study, application of an up-down method to determine the threshold of cue duration at which rats could maintain optimum sustained performance in a visual attention task provided a sensitive assay for inflammatory abdominal pain, as well as dose-dependent reversal by relevant analgesics. Application of this method to assess cognitive effects of chronic pain following nerve injury could provide a means for evaluating mechanisms of disruption of attention and efficacy of analgesics in a longitudinal manner, as the 5CTV procedure provides daily measures of ability within a wide range of performance capabilities.
The role of motivation for food reward in performance within the 5 choice serial reaction time task is thought to be dissociable from that of attention processes. Prefeeding food restricted rats prior to 5 choice serial reaction time task sessions produces a similar increase in latency to correct responses and latency to retrieve a food reward, an effect mimicked by the anorectic d-fenfluramine41. The dopamine antagonist haloperidol increases latency to correct responses without affecting latency to food reward41. Ketamine, an NMDA antagonist with dissociable anesthetic properties, increases the latency to correct responses and number of omissions without altering the latency to retrieve food reward42. In the study cited above examining the effects of formalin on performance in the 5 choice serial reaction time task, latency to retrieve food rewards was reported as not affected statistically, however data were not presented40. The study cited above examining the effect of spared nerve injury on performance in the 5 choice serial reaction time task demonstrated an increase in latency to retrieve the food reward with nerve injury, even in the absence of an effect on motivation as measured using a progressive-ratio schedule of food-maintained behavior. This suggests that the effect may be related to motor impairment rather than motivation for food reward per se39. However, pain with movement in this task is likely not easily dissociable from motor impairment per se resulting from nerve damage. Analgesics would, however, be expected to provide beneficial effects in treating pain with movement, but not improve motor impairment resulting from non-sensory nerve damage. Abdominal lactic acid is not likely to produce direct motor impairment in the manner that would be expected from nerve ligation, but rather to produce interference of movement due to inflammatory nociception. The effects of lactic acid on response and reward latencies may therefore reflect either decreased motivation to obtain the food reward, or interference with movement due to nociception. In either case, both morphine and ketoprofen reversed the effects of intraperitoneal lactic acid, and this is likely due to inhibition of the nociceptive state.
It is important to note that performance in the classical 5 choice serial reaction time task is dependent upon a number of behavioral processes including attention, impulse control, and motivation for reward, as well as intact motor function. In this regard, % accuracy, defined as the ratio of correct trials to the sum of correct and incorrect trials, is generally accepted as the measure most impacted by attentional processes in this paradigm.12,13 However, others have noted that determining the role of attention in performance capability is more complex than simply measuring accuracy. For example, it has been suggested that subjects may sacrifice speed in order to maintain accuracy in this operant paradigm when attentional demand is high or if attention is impaired.12 In the present study, accuracy was not affected by manipulations that elevated the median cue duration, including both lactic acid and scopolamine. The median cue duration is influenced by correct, incorrect, and omitted trials however and thus is likely a measure of overall performance capability, affected by both attention and other processes, and indicates the level of difficulty at which animals are capable of performing efficiently. It has been suggested that examining the overall pattern of behavioral effects is the most robust means to determine behavioral mechanisms related to alteration of performance within the 5 choice serial reaction time task, and this may prove to be the case with the 5CTV titration variant as well. The overall pattern of increased latency to correct responses, retrieval of the food reward, and number of omitted trials has been described as “loss of response vigour” by Robbins, and such effects have typically been associated with diminished dopaminergic drive in the ventral and dorsal striatum.12 These regions are typically thought to be involved in rewarding effects of food, and motor responses associated with reward. It is noteworthy that i.p. lactic acid is thought to suppress intracranial self-stimulation in rats by suppressing dopaminergic neurons within limbic forebrain, and this same mechanism may be involved in the effects of lactic acid seen in the present study.28 As such, the effects of lactic acid on performance in this visual attention based operant task may be indirectly related to the affective/motivational component necessary for behavior in this paradigm. Examination of specific brain regions and neurochemistry will be necessary to elucidate this role.
Supplementary Material
Summary statement.
Abdominal inflammation induced by i.p. lactic acid disrupted performance in a visual attention task in rats, which was reversed by morphine and ketoprofen. The disruptive effects of scopolamine in this task were not reversed by analgesics.
Acknowledgments
Funding: This work supported by grant P01 GM113852 (JCE) from the National Institute on General Medical Sciences of NIH. There are no financial conflicts to report.
Footnotes
Conflict of Interest: The authors have no conflicts to report.
References
- 1.Bertolucci PH, de Oliveira FF. Cognitive impairment in fibromyalgia. Curr Pain Headache Rep. 2013;17:344–9. doi: 10.1007/s11916-013-0344-9. [DOI] [PubMed] [Google Scholar]
- 2.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. The journal of pain : official journal of the American Pain Society. 2004;5:491–7. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10:131–149. doi: 10.1023/a:1009020914358. [DOI] [PubMed] [Google Scholar]
- 4.Melzack R, Wall PD. Evolution of pain theories. Int Anesthesiol Clin. 1970;8:3–34. doi: 10.1097/00004311-197000810-00003. [DOI] [PubMed] [Google Scholar]
- 5.Pais-Vieira M, Aguiar P, Lima D, Galhardo V. Inflammatory pain disrupts the orbitofrontal neuronal activity and risk-assessment performance in a rodent decision-making task. Pain. 2012;153:1625–35. doi: 10.1016/j.pain.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Pais-Vieira M, Lima D, Galhardo V. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett. 2009;463:98–102. doi: 10.1016/j.neulet.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Apkarian AV, YS, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–36. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Apkarian AV, YS, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriarty O, EMB, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–4904. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesthesia and analgesia. 2007;104:1223–9. doi: 10.1213/01.ane.0000263280.49786.f5. [DOI] [PubMed] [Google Scholar]
- 11.Dick BD, Verrier MJ, Harker KT, Rashiq S. Disruption of cognitive function in fibromyalgia syndrome. Pain. 2008;139:610–6. doi: 10.1016/j.pain.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 13.Fizet J, Cassel JC, Kelche C, Meunier H. A review of the 5-Choice Serial Reaction Time (5-CSRT) task in different vertebrate models. Neurosci Biobehav Rev. 2016;71:135–153. doi: 10.1016/j.neubiorev.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Maddux JM, Holland PC. Effects of dorsal or ventral medial prefrontal cortical lesions on five-choice serial reaction time performance in rats. Behav Brain Res. 2011;221:63–74. doi: 10.1016/j.bbr.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBlanc BW, Bowary PM, Chao Y-C, Lii TR, Saab CY. Electroencephalographic signatures of pain and analgesia in rats. Pain. 2016;157:2330–40. doi: 10.1097/j.pain.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CJ, Huang M, Meltzer H, Martina M. Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Front Cell Neurosci. 2016;10:133. doi: 10.3389/fncel.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loggia ML, Berna C, Kim J, Cahalan CM, Martel MO, Gollub RL, Wasan AD, Napadow V, Edwards RR. The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. J Pain. 2015;16:692–9. doi: 10.1016/j.jpain.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin TJ, Grigg A, Kim SA, Ririe DG, Eisenach JC. Assessment of attention threshold in rats by titration of visual cue duration during the five choice serial reaction time task. J Neurosci Methods. 2014;241C:37–43. doi: 10.1016/j.jneumeth.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altarifi AA, Rice KC, Negus SS. Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther. 352:208–217. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. The journal of pain : official journal of the American Pain Society. 2011;12:868–74. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–6. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–22. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Martin TJ, Ewan E. Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Exp Clin Psychopharmacol. 2008;16:357–66. doi: 10.1037/a0013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki S, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J Neurochem. 2002;82:1192–8. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 26.Hung CH, Wang JC, Strichartz GR. Spontaneous Chronic Pain After Experimental Thoracotomy Revealed by Conditioned Place Preference: Morphine Differentiates Tactile Evoked Pain From Spontaneous Pain. J Pain. 2015;16:903–12. doi: 10.1016/j.jpain.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs PN, McNabb CT. The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integr Neurosci. 2012;11:61–72. doi: 10.1142/S0219635212500045. [DOI] [PubMed] [Google Scholar]
- 28.Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS. Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain. 156:175–84. doi: 10.1016/j.pain.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–7. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin TJ, Buechler NL, Kim SA, Ewan EE, Xiao R, Childers SR. Involvement of the lateral amygdala in the antiallodynic and reinforcing effects of heroin in rats after peripheral nerve injury. Anesthesiology. 2011;114:633–42. doi: 10.1097/ALN.0b013e318209aba7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narita M, Ozaki S, Ise Y, Yajima Y, Suzuki T. Change in the expression of c-fos in the rat brain following sciatic nerve ligation. Neurosci Lett. 2003;352:231–3. doi: 10.1016/j.neulet.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Navratilova E, YXJ, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20709–13. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. Journal of Neuroscience. 2015;35:7264–71. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188:139–48. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Freitas KC, Hillhouse TM, Leitl MD, Negus SS. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev Res. 2015;76:194–203. doi: 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain. 2004;109:214–24. doi: 10.1016/j.pain.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Suto T, Eisenach JC, Hayashida K. Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. Pain. 2014;155:1935–42. doi: 10.1016/j.pain.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low LA, Millecamps M, Seminowicz DA, Naso L, Thompson SJ, Stone LS, Bushnell MC. Nerve injury causes long-term attentional deficits in rats. Neurosci Lett. 2012;529:103–7. doi: 10.1016/j.neulet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Higgins GA, Silenieks LB, Van Niekerk A, Desnoyer J, Patrick A, Lau W, Thevarkunnel S. Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav Brain Res. 2015;286:347–55. doi: 10.1016/j.bbr.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 40.Boyette-Davis JA, Thompson CD, Fuchs PN. Alterations in attentional mechanisms in response to acute inflammatory pain and morphine administration. Neuroscience. 2008;151:558–63. doi: 10.1016/j.neuroscience.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 1992;106:228–34. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- 42.Nikiforuk A, Popik P. The effects of acute and repeated administration of ketamine on attentional performance in the five-choice serial reaction time task in rats. Eur Neuropsychopharmacol. 2014;24:1381–93. doi: 10.1016/j.euroneuro.2014.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.