Abstract
A better understanding of the pathophysiology of HFpEF is important. Detailed phenotyping of pulsatile hemodynamics has provided important insights into the pathophysiology of left ventricular remodeling and fibrosis, diastolic dysfunction, microvascular disease and impaired oxygen delivery to peripheral skeletal muscle, all of which contribute to exercise intolerance, the cardinal feature of HFpEF. Furthermore, arterial pulsatile hemodynamic mechanisms likely contribute to the frequent presence of comorbidities, such as renal failure and dementia, in this population. Our therapeutic approach to HFpEF can be enhanced by clinical phenotyping tools with the potential to “segment” this population into relevant pathophysiologic categories, or to identify individuals exhibiting prominent specific abnormalities that can be targeted by pharmacologic interventions. This review describes relevant technical and physiologic aspects regarding the deep phenotyping of arterial hemodynamics in HFpEF. In an accompanying review, the potential of this approach to enhance our clinical and therapeutic approach to HFpEF is discussed.
Keywords: heart failure, heart failure with preserved ejection fraction, arterial hemodynamics, wave reflections, afterload, pulsatile load
Introduction
The burden of heart failure has increased dramatically over the last several years.1,2 Heart failure affects ~2% of the western population and is the most common cause of hospitalization in adults >65 years of age.1 Not only is heart failure already an epidemic, but with the aging of the population, a dramatic further increase in its prevalence is expected. Approximately half of patients with HF have heart failure with a preserved left ventricular (LV) ejection fraction (HFpEF). Furthermore, the relative prevalence of HFpEF (as a proportion of the total burden of heart failure) appears to be increasing as the population ages.1, 14 Therefore, an important further increase in the prevalence of HFpEF is expected.1 Patients with HFpEF demonstrate high annual mortality rates, ranging from ~3.5–6% in randomized trials3–5 to ~15% in community-based studies.6 Approximately 50% of such deaths occur from cardiovascular causes.7 In addition to its high morbidity and mortality, HFpEF has been shown to be associated with an impaired quality of life.8,9
Multiple therapies that provide substantial clinical benefit in heart failure with reduced ejection fraction are available. However, multiple phase III trials over the last few decades have failed to demonstrate a clear benefit of various candidate pharmacologic interventions in HFpEF. Therefore, a better understanding of the processes that contribute to the pathophysiology of HFpEF is important.
Detailed phenotyping of arterial hemodynamics has provided important insights into the pathophysiology of LV remodeling and fibrosis, diastolic dysfunction, microvascular disease, and oxygen delivery to peripheral skeletal muscle, all of which contribute to exercise intolerance, the cardinal feature of HFpEF. Furthermore, it has been proposed that HFpEF is a heterogeneous syndrome, with different degrees of contribution from various pathophysiological processes. This may unfavorably impact the average responses of this patient population to novel therapies tested in clinical trials. Better clinical phenotyping tools, which in turn may be used to “segment” the HFpEF population into relevant pathophysiologic categories, or simply to identify individuals with prominent specific abnormalities that are targeted by pharmacologic interventions, represent a promising approach to enhance our therapeutic approach to HFpEF.
This review deals with relevant technical and physiologic aspects regarding the deep phenotyping of systemic arterial hemodynamics in HFpEF. An accompanying review deals with the potential of this approach to enhance our translational, clinical and therapeutic approach to HFpEF. Given space limitations, this review does not address pulmonary arterial load and right ventricular-pulmonary vascular interactions. The reader is referred to a recent excellent review about this topic.10
The concept of arterial load and limitations of the pressure-volume plane
The LV ejects blood against the hydraulic load imposed by the systemic arterial tree. Given the pulsatile nature of the LV as a pump, arterial load is time-varying, complex and cannot, therefore, be expressed as a single number of parameter.11,12
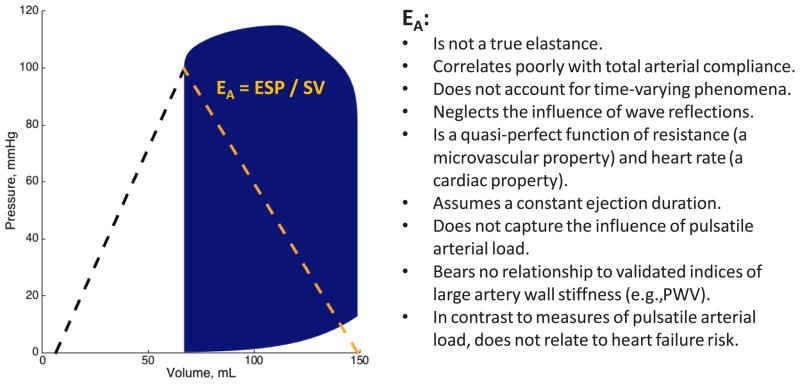
The pressure-volume plane has been a useful approach to study LV function and energetics. The LV end-systolic elastance, EES, is the slope of the end-systolic pressure-volume relation (Figure 1) and represents an informative load-independent measure of LV chamber pump performance and systolic stiffness. Although EES is ideally assessed invasively using data from a family of pressure-volume loops obtained during an acute preload or afterload alterations, “single-beat” methods have also been developed and have undergone limited validation,13,14 allowing for non-invasive EES estimations using simple echocardiographic measurements.
Figure 1.
Limitations of effective arterial elastance (EA) as an index of arterial load.
The concept of EES and its role in myocardial energetics was first formulated by Suga several decades ago.15,16 For a given beat, the pressure-volume area (PVA) is the sum of: (1) The stroke work (or external work), which is the area within the pressure-volume loop trajectory (blue area in Figure 1); (2) The potential energy, which is the approximately triangular area to the left of the single pressure-volume loop, enclosed by the end-systolic pressure-volume relation, the left border of the pressure-volume loop, and the end-diastolic pressure-volume relation. 16,17 According to the time varying-elastance paradigm, the PVA represents the total mechanical energy generated by LV contraction until the end of systole. In a single heart operating at a stable contractile state under various preload and afterload conditions, the PVA correlates strongly with myocardial oxygen consumption (MVO2) per beat. In such conditions, this approach (specifically, the ratio of stroke work to PVA) offers insights regarding the energetic efficiency of the system (when the latter is defined as the stroke work generated for any given MVO2). However, pressure-volume analyses are much less reliable to compare the energetic efficiency of the LV-arterial system between individuals or disease populations, because the function that relates the pressure-volume area to MVO2 is highly variable between individuals.12,16–19 This results in weak relationships between the PVA and MVO2 (and by extension, between PVA-derived efficiency the true underlying efficiency) between individuals.
After the role of the pressure-volume plane to assess LV function and energetics was established, an “extension” of this approach to assess arterial load and ventricular-arterial coupling was developed, primarily to understand the determinants of stroke volume.20–22 Analyses of ventricular-arterial coupling in the pressure-volume plane have been reviewed in detailed elsewhere.12,20–24 In this paradigm, arterial load is quantified as an “effective arterial elastance”, which is defined as the ratio of end-systolic pressure to stroke volume. When arterial load is defined is this manner, the EA/EES ratio can be computed as an index of ventricular-arterial coupling. Due to geometric considerations, the EA/EES ratio roughly correlates with the ratio of stroke work to PVA, and therefore, to the operating energetic and mechanical efficiency of the LV in single hearts operating at different loading conditions. However, given the limitations of the PVA as a surrogate of MVO2 when comparing different individuals or diseased populations, the same reservations apply to the EA/EES ratio.
Importantly, the EA/EES ratio is intimately related to EF (EF=1/[1+EA/EES]); consequently, markedly “inefficient” ratios are closely associated with the presence of important reductions in LV EF. In general, the EA/EES ratio is a marker of the energetic efficiency of the system in situations in which the LV ejection fraction (EF) is frankly abnormal.12,20–25 This approach is less useful to compare energetic ventricular-arterial coupling when the ejection fraction is preserved.
A small study including patients with HFpEF suggested an increased EA and EES beyond that associated with aging and/or hypertension.26 However, subsequent studies with larger sample sizes demonstrated that EA and EES were similarly increased in hypertensive controls and HFpEF patients.27,28 Furthermore, patients with HFpEF demonstrate normal “coupling” of the LV (EES) and the arterial load (EA), as assessed in the pressure-volume plane, suggesting that this approach fails to capture key features of the abnormal ventricular-arterial cross-talk in this condition. However, the pressure-volume plane helps us understand some aspects of the pathophysiology of HFpEF, including the limited stroke volume reserve, increased blood pressure lability, pre-load sensitivity,12,23,29,30 and the propensity of this population to experience side effects with interventions that cause important reductions in peripheral vascular resistance.
Despite its high popularity and the physiologic insights provided by analyses in the pressure-volume plane in HFpEF, this approach demonstrates serious limitations to characterize pulsatile arterial load and broader aspects of ventricular-arterial interactions in HFpEF. As stated previously, an attempt to represent the complex time-varying arterial hydraulic load with a single number is unrealistic. The EA/EES ratio does not account for time-varying phenomena during ejection.12 In particular, the LV loading sequence (late vs. early systolic load), which is intrinsically neglected by analyses in the pressure-volume plane, is an important determinant of maladaptive remodeling, hypertrophy, diastolic dysfunction and heart failure risk.30–37 In addition, the commonly made assumption that EA is a lumped parameter of resistive and pulsatile arterial load, is factually incorrect.12,38–40 Despite its name, EA is not a true elastance (i.e., the inverse of a compliance) and is mostly dependent on vascular resistance (a microvascular, rather than a conduit artery property).39,41 Furthermore, EA is highly sensitive to heart rate.39,41 In fact, EA has been shown to be minimally sensitive to changes in arterial compliance or to various specific parameters of pulsatile arterial load within physiologically/clinically relevant ranges.12,38–40 In a recent study40 EA and detailed specific indices of pulsatile arterial load were determined from time-resolved central pressure and flow measurements: (1) In a large community-based sample of middle-aged adults; (2) In a diverse clinical population of older adults, and; (3) In response to the handgrip maneuver, a physiologic intervention known to induce pronounced changes in pulsatile arterial load.42 This series of studies consistently demonstrated that EA is a quasi-perfect function of the product of systemic vascular resistance and heart rate, with weak, inconsistent and in some cases, erratic/paradoxical relationships with proper measures of pulsatile load, such as aortic root characteristic impedance (Zc), measures of wave reflections, and total arterial compliance. These findings are in full agreement with modeling studies,39 and demonstrate that EA depends almost entirely on SVR and heart rate, and has very poor sensitivity to the human pathophysiological ranges of pulsatile afterload observed in vivo. Finally, there was no correlation between EA and carotid-femoral pulse wave velocity, the current reference method to quantify large artery stiffness.43,44 Current American Heart Association guidelines specifically recommend against utilizing EA as a measure of arterial stiffness.43 Given the considerations above, it is incorrect to interpret EA as an index of arterial “stiffening” or, by extension, to equate a parallel increase in EA and EES as a state of “ventricular-arterial stiffening”. Similarly, it is important to recognize that the presence of similar EA values between 2 populations does not mean that arterial stiffness is similar, and that a change in EA in response to an intervention (or lack thereof) does not reflect changes in arterial stiffness.
The poor performance of EA to capture underlying pulsatile arterial load is readily explained by the various simplifying assumptions made during its original derivation, as previously discussed in detail.12,39,40 First, arterial pressure does not increase from zero to end-systolic pressure as the result of the stroke volume injection into the arterial tree, because arterial pressure hovers around a non-zero value (mean arterial pressure, which is in turn entirely determined by peripheral resistance, heart rate and stroke volume). Second, the derivation of EA assumed a “square-shaped” pressure curve in systole, with its upper side corresponding to end-systolic pressure, thus ignoring the contribution of pulsatile phenomena to the contour of the arterial pressure curve above end-systolic pressure. Sunagawa et al, in their original derivation of EA, appropriately raised caution regarding the fact that the contribution of these phenomena can become important, with a relatively large error in this coupling model, when the area under the systolic area of the curve becomes a large fraction of the overall pressure curve or when pulsatile phenomena lead to an increase in the area under the pressure curve above end-systolic pressure, which may occur for instance, in the presence of prominent pressure augmentation from wave reflections.30,45 Such conditions happen to be characteristic hemodynamic features among older people (particularly women)43,46–50 and patients with HFpEF.32,51
To the degree that EA does not properly characterize arterial pulsatile load, the EA/EES ratio is a very limited index of “ventricular-arterial coupling”, even if the important limitations of this approach to assess LV energetics are ignored. This issue is particularly problematic in HFpEF, because pulsatile load is highly relevant for ventricular-arterial interactions in this patient population. Ventricular-arterial coupling encompasses multiple different physiologic aspects that require characterization beyond the pressure-volume plane.
These considerations are not purely theoretical, but translate into important limitations to the application of this approach in clinical studies. For example, in a recent study, measures of wave reflection, but not EA or peripheral vascular resistance, were significantly correlated with the invasively measured time constant of isovolumic relaxation, the gold standard index of diastolic relaxation.52 Similarly, a recent study demonstrated that EA did not predict incident HF in the Multiethnic Study of Atherosclerosis (MESA) cohort,53 whereas indices of reflection magnitude and late systolic load were strong predictors of incident HF in this cohort.54,55 Finally, a single dose of inorganic nitrate, which has been recently shown to improve aerobic capacity in patients with HFpEF, did not reduce EA, but substantially reduced wave reflections,56 and this reduction significantly correlated with the degree of improvement in peak VO2 in these patients.57 These findings are consistent with the need to time-resolve arterial load in order to gain clinically-relevant insights into ventricular-arterial coupling and the effects of therapeutic interventions.
Wave reflections in the arterial tree
Every beat, the LV generates a pulse wave that travels forward in the arteries and gets partially reflected at sites of impedance mismatch, such as points of branching or change in wall diameter or material properties along the arterial tree. Innumerable tiny reflections merge into a net reflected wave, which travels back to the heart.30,46,47 This reflected wave is often seen as a single discrete wave, originating from an “effective” reflection site, but is actually the result of scattered reflections, originating from distributed reflection sites. Important sources of wave reflections include the microvasculature, the bifurcations in middle-sized muscular arterial segments, the tapering of the aorta, well as sites of tortuosity or focal wall stiffening and/or narrowing in conduit arteries, with the bulk of wave reflections arising distal to the aortic arch (i.e., from the lower body).30,46,47 The time of arrival of the reflected wave to the proximal aorta depends on the location of reflection sites and on the pulse wave velocity (PWV) of conduit vessels, particularly the aorta, which transmits both forward and backward traveling waves from and towards the LV, respectively.30,58–60 Aortic PWV is directly related to the stiffness of the aortic wall (square root of elastic modulus).30,46,61 Stiffer aortas thus conduct the forward and backward traveling waves at greater velocities and therefore promote shorter reflected wave transit times (i.e., earlier arrival of wave reflections to the LV).11,62
Due to the shorter wave transit time from the heart to reflection sites and back to the proximal aorta in the presence of increased pulse wave velocity among older adults,11,62 wave reflections arrive back at heart while the LV is still ejecting blood, with predominant effects during mid-to-late systole.30,63 Wave reflections thus increase the late systolic workload of the LV and profoundly impact its loading sequence (late relative to early systolic load). In the presence of preserved LV pump function (such as occurs in HFpEF), typical effects of the reflected wave on the aortic pressure waveform include a systolic shoulder followed by a second systolic peak that augments aortic pressure in mid-to-late systole.60 Such features are prominent in patients with HFpEF.32,51,56
Assessing arterial load and ventricular-arterial interactions via pressure-flow relations
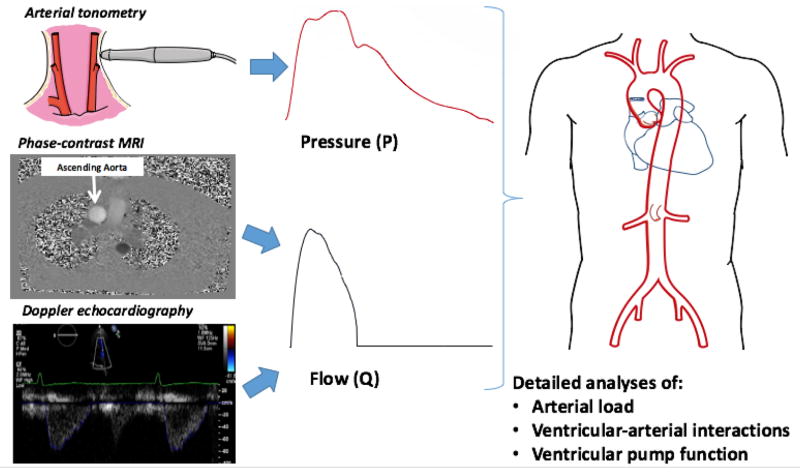
Although blood pressure is often taken as a useful surrogate of LV afterload (“pressure overload”), it should be recognized that afterload affects the pressure and flow generated by the LV in a reciprocal fashion. Furthermore, pressure is not only dependent on afterload, but is also strongly influenced by LV chamber pump function.23,47,60,64 Therefore, LV afterload cannot be described in terms of pressure alone, but should be assessed from pressure-flow relations. A detailed phenotypic characterization of pulsatile arterial load can be performed with non-invasive pressure and flow measurements using imaging techniques combined with arterial applanation tonometry (Figure 2).
Figure 2.
Primary measurements required for the assessment of arterial load via analyses of aortic pressure-flow relations. A pressure-flow pair can be acquired with a combination of arterial tonometry (which provides a pressure waveform) and either Doppler echocardiography or phase-contrast MRI of the ascending aorta (either of which can provide a flow waveform). Analyses of pressure-flow relations allow for a detailed assessment of arterial load, ventricular-arterial interactions and ventricular function.
Arterial tonometry can be used to obtain high-fidelity central pressure waveforms from the carotid artery, which is an adequate surrogate of the aortic pressure waveform.65 Alternatively, peripheral waveforms (brachial or radial) can be transformed with the use of generalized transfer functions in the frequency domain, to produce estimated central pressure waveforms. Generalized transfer functions take advantage of the relatively small variability in the relationship between the harmonics of pressure in the aorta and the respective harmonics of pressure in specific peripheral arteries. This variability is particularly small for low frequencies (first few harmonics of the pressure waveform) but can vary markedly between people at higher frequencies. “Low frequency” information in the central pressure waveform includes the peak pressure, or areas under the pressure curve (pressure-time integrals) in early systole, late systole, or diastole. Higher frequencies determine sharp inflections in the waveform and morphologic “details” (such as the inflection point and augmentation index); these features in the central pressure waveform may not be particularly reliable when synthesized with the use of transfer functions.
Aortic flow can be measured with through-plane phase-contrast MRI of the ascending aorta (Figure 2), which produces a flow velocity profile of the aortic cross-section in each phase of the cardiac cycle. Doppler echocardiography of the LV outflow tract can also be used, because LV systolic outflow through the LV outflow tract equals aortic inflow. With both techniques, temporal resolution should be maximized to obtain the details of the flow waveform. With phase-contrast MRI, a single cardiac cycle (and flow waveform) is typically reconstructed from information acquired over multiple cardiac cycles. For Doppler echocardiography and arterial tonometry, signal averaging of several cardiac cycles is typically used to obtain a pressure waveform and a flow waveform for analyses.
Once the central pressure-flow pair is available, central arterial pressure-flow relations can be studied in great detail, allowing for a comprehensive assessment of LV afterload, including wave reflections.11,46,47 Analyses of pressure-flow relations also provide indices of ventricular function and ventricular-arterial interactions. Various key phenotypes and their physiologic/clinical interpretations are summarized in Table 1.
Table 1.
Key physiologic parameters of arterial load and ventricular arterial cross-talk
| Parameter | Definition and interpretation | Determinants |
|---|---|---|
| Systemic vascular resistance | Ratio of mean pressure to mean flow. Represents the steady (non- pulsatile) LV load. Key determinant of mean arterial pressure. | Microvascular rarefaction and average arteriolar “tone” (diameter). |
| Aortic root characteristic impedance (Zc) | Zc is the ratio of pulsatile pressure to pulsatile flow in the absence of reflected waves. Aortic root Zc is the pulsatile impedance to LV ejection exerted by the aortic root. Aortic Zc governs the early systolic pulsatile pressure-flow relation (before wave reflections return to the LV), and thus, is the key determinant of early systolic pulsatile arterial load. It is also a key determinant of pulse pressure. | Aortic root size (inversely correlated) and stiffness (directly correlated) |
| Flow Zc product (QZc) | Pulsatile pressure required to “push” any given pulsatile flow through the aortic root in the absence of wave reflections. When wave reflections are absent, the systolic QZc product equals the systolic portion of the forward wave, which in turn equals the total pressure in systole. An excessive QZc product indicates a mismatch between flow needs and aortic root properties. | Aortic root Zc (size, stiffness) and flow rate. |
| Forward pressure wave (Pf) | Composite wave that includes: (1) The primary wave generated by the heart; (2) Peripheral wave reflections that are “rectified” (i.e., re- reflected) at the heart. It is a parameter of cross-talk between the LV, the aortic root and peripheral reflection sites. The systolic portion of Pf equals the QZc product only when reflections are absent in systole. When reflections are present, the systolic portion of Pf is equal to the QZc product plus the backward pressure wave (Pb). | Increases with: (1) An increased QZc product (and thus greater Zc or greater flow rate); (2) An increase in reflected wave amplitude. |
| Backward pressure wave (Pb) | Composite wave influenced by: (1) The forward wave; (2) Reflection coefficients at distributed sites; (3) Pulse wave velocity to and from reflection sites. These factors interact in complex ways to form a discrete net reflected wave measured at the aortic root. It is a parameter of ventricular-arterial cross-talk, rather than a pure arterial property. It does not characterize the contribution of the reflected wave to systolic vs. diastolic pressure. | All determinants of the forward wave and determinants of peripheral reflections (microvasculature, bifurcations in middle-sized muscular arterial segments, aortic tapering, tortuosity or focal wall stiffening and/or narrowing in conduit arteries). |
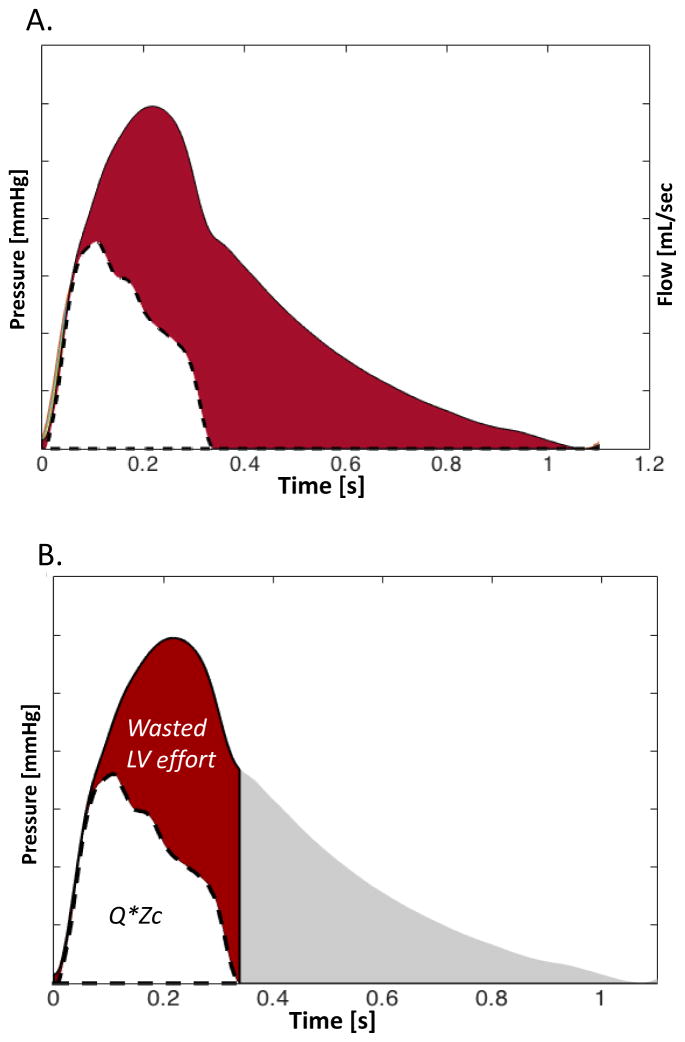
| Wasted LV effort | Pulsatile pressure that is not primarily required to promote pulsatile systolic flow through the aortic root Zc, but is necessary to overcome the effect of wave reflections. It pressure-flow analyses, can be computed as the difference between measured pulsatile pressure in systole and the QZc product (red area in Figure 4). This concept was originally proposed using pressure-only approaches.66,67 The concept presented here is an extension to the pressure-flow pair. It is a parameter of the contribution of wave reflections to systolic pressure. It is an index of ventricular-arterial cross-talk, rather than a pure arterial property. This area is equal to twice the systolic portion of the reflected wave. | Combination of ventricular and arterial properties that determine the amplitude and timing of Pf and Pb. It is also influenced by the temporal overlap between the reflected wave and systolic ejection |
| Total arterial compliance | Theoretical compliance of the entire arterial tree. Usually derived from Windkessel modeling, which does not implicitly account for wave propagation and reflection. It can be approximated as the ratio of stroke volume/pulse pressure. | Large arteries and to a lesser extent, muscular arteries and smaller vessels |
| Reflection magnitude | Ratio of backward/forward wave amplitude. It does not account for the shape of forward and backward waves, or the timing of the backward wave. Similarly, it does not characterize the contribution of the reflected wave to systole vs. diastole. | Microvasculature, bifurcations in middle-sized muscular arterial segments, aortic tapering, tortuosity or focal wall stiffening and/or narrowing in conduit arteries. |
| Effective arterial “elastance” (EA) | Ratio of end-systolic pressure/stroke volume. It is not a true elastance (i.e., inverse of a compliance). It correlates poorly with arterial compliance. EA is essentially a quasi-perfect function of resistance and heart rate (product of SVR x heart rate). Does not capture the influence of pulsatile arterial load and it not an index of arterial stiffness. | Resistance vessels (microvasculature) and heart rate. Does not capture conduit artery properties, such as stiffness or pulsatile impedance |
| LV End-systolic Elastance (EES) | Slope of the end-systolic pressure-volume relation (ESPVR), obtained by joining end-systolic pressure-volume points from a “family” of left ventricular (LV) pressure-volume loops obtained from the same subject during acute preload or afterload alterations at a constant inotropic state. It can be estimated using single-beat non- invasive methods. | LV contractility and stiffness. |
| Myocardial wall stress (MWS) | Time-varying mechanical load experienced by the contractile elements in the myocardium. It represents the amount of force and work the muscle generates during a contraction. Peak systolic MWS (an early systolic phenomenon) is closely and linearly related to myocardial oxygen consumption (MVO2), whereas the ratio of late/early MWS (i..e, myocardial loading sequence) is associated with abnormal diastolic relaxation and atrial dysfunction. | Complex interactions between myocardial contractile elements, instantaneous LV geometry and the time-varying hydraulic load imposed by the arterial tree. Integrates the influence of pre-load, arterial load, LV structure and function on myocardial load. |
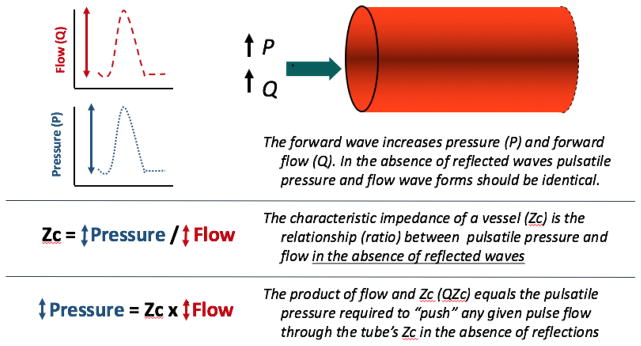
To better understand the effects of wave reflections on pressure and flow, it is useful to discuss the effects of intermittent flow injection into an elastic tube in which reflections are absent (Figure 3). Under such conditions, pulsatile energy imparted from one end of the tube, in the form of a compression wave, promotes forward flow and increases pressure within the tube (Figure 3). Measured flow and pressure signals demonstrate exactly the same shape. The amount of pressure rise (ΔP) versus flow change (ΔQ) (vertical scale in pressure and flow curves in Figure 3) is determined by the characteristic impedance (Zc) of the tube (therefore, Zc= ΔP/ΔQ). It follows that the product of pulsatile flow and Zc equals pulsatile pressure. In such a system, the QZc product represents the pulse flow required to “push” the pulse flow through the Zc of the tube.
Figure 3.
Analysis of pulsatile pressure and flow in a reflection-less elastic conduit vessel. The effects of a compression wave are shown. This model illustrates the concept of characteristic impedance (ZC) which governs the pulsatile pressure-flow relation in the absence of wave reflections.
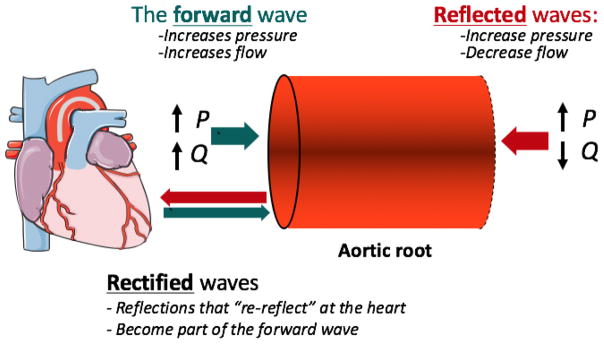
In the arterial tree, however, the pressure profile is determined by a combination of forward and backward waves at any given site. At the aortic root, measured pressure and flow waveforms result from the sum of a forward-traveling wave, which affects pressure and flow in the same direction, and a backward wave, which changes pressure and flow in opposite directions (Figure 4). The backward wave arises from the interaction of the forward wave with reflecting sites distal to the aortic root. A commonly unrecognized phenomenon is that the heart itself is also a reflector. Backward-traveling reflected waves can re-reflect at the heart, becoming part of the forward wave. The forward wave is therefore influenced by wave reflections.
Figure 4.
Effect of forward, backward and rectified compression waves on pulsatile pressure and flow in the aortic root.
In the arterial tree of older adults, a nearly reflection-less state occurs only in very early systole, before arrival of the bulk of backward-traveling waves. The slope of the pressure-flow relation during this period is governed by proximal aortic characteristic impedance (Zc). The slope of the pressure-flow relation in early systole, therefore, approximates aortic Zc and can be measured using a pressure-flow loop. Once this slope is defined we can represent the pressure and flow wave forms adequately “scaled” to each other to better assess departures from a “reflection-less” state (Figure 5). Soon after the onset of ejection, the linear relationship between pressure and flow (which is governed by the aortic root ZC) diverges, such that pressure increases relative to flow. This divergence is the consequence of wave reflections from the periphery arriving at the aortic root. Figure 4 demonstrates this phenomenon (red area). The pulsatile pressure-flow divergence generally becomes particularly prominent in mid-to-late systole (Figure 5A). We can assess the magnitude of this divergence as an index of the contribution of wave reflections to the systolic pressure profile. In order to do this, it is useful to compute the product of flow by aortic root Zc (QZc product), which is represented by the white area in Figure 5B. The QZc product can be interpreted as the minimum pressure that would be required to “push” the prevalent flow waveform through the aortic root Zc (i.e., in the complete absence of wave reflections). From a clinical and physiologic perspective, in the presence of a normal stroke volume, this product provides some quantification of the mismatch between aortic root properties (size and stiffness) and flow requirements, which contribute to increased pulse pressure with aging and various disease states. It is useful to note that, when wave reflections are absent, the systolic QZc product is equal to the systolic portion of the forward pressure wave, which is in turn equal to the systolic portion of the total pressure wave.
Figure 5.
(A) Pressure and flow pair scaled by aortic root characteristic impedance. In early systole, ZC governs the pulsatile pressure-flow relation. However, soon after ejection starts, the effects of wave reflection increase pressure relative to flow (red area): (B) Same signals, after flow has been multiplied by aortic root ZC and the minimum of each signal has been subtracted, for a more intuitive graphic representation. The product of QZC has units of pressure and can therefore be directly related to the pressure waveform. The difference between QZC and measured pressure can be easily quantified in systole. It is an index of pressure that the LV needs to generate to overcome the effects of wave reflections, in order to eject the prevalent net flow. We hereby refer to this area as wasted LV effort, analogous to the concept was originally proposed using pressure-only approaches.66,67
The difference between measured pressure and the QZc product (red area in Figure 5B) can be interpreted as the pulsatile pressure that is not primarily required to promote pulsatile systolic flow through the aortic root Zc, but is necessary to overcome the effect of wave reflections. An analogous concept was originally proposed by Hashimoto, Nichols and O'Rourke and called “wasted LV effort”, using pressure-only approaches.66,67 The concept presented in Figure 5 is an extension of this principle to the pressure-flow pair. Whereas intuitive and potentially useful, this time-domain index requires further study. It should be considered a parameter of ventricular-arterial cross-talk, rather than a pure arterial property. Interestingly, as will be discussed later, this area is equal to twice the systolic portion of the reflected wave.
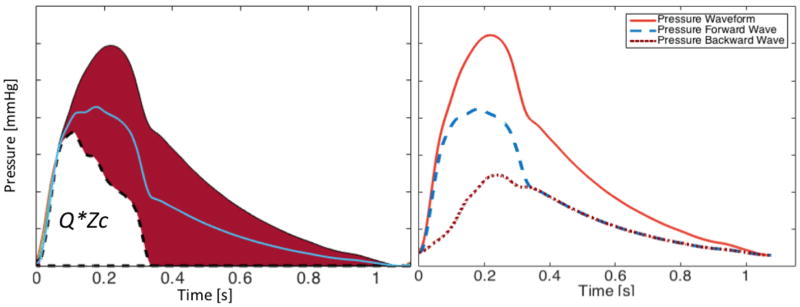
According to wave separation analysis, the measured flow waveform is the difference between forward and backward flow waves, whereas the measured pressure waveform is the sum of forward and backward pressure waves. This determines that the red area in Figure 5B (pulsatile pressure “above” QZc) gets “partitioned” exactly by half, with one half corresponding to the forward pressure wave and one half corresponding to the backward pressure wave (Figure 6). This leads to a few interesting observations: (1) The pulsatile pressure area above QZc is equal to twice the backward wave; (2) The pulsatile component of the forward wave is equal to the sum of the QZc product plus the backward wave. In other words, the forward wave exceeds the QZc product by an amount equal to the backward wave. Forward wave amplitude, therefore, cannot be interpreted purely as an index of mismatch flow needs and aortic root properties, because it contains important contributions from wave reflections.68
Figure 6.
Wave separation analysis. Because net flow is the difference between forward and backward flow, whereas net pressure is the sum of forward and backward pressure, the red area in the pressure waveform (pressure - QZc) gets “partitioned” exactly in half between the forward (Pf) and the backward (Pb) waves. Consequently: (1) The systolic portion of the forward wave does not equal QZc, unless reflections are absent in systole; (2). The amplitude of Pf at any given time exceeds QZc by an amount equal to the amplitude of Pb, and is thus significantly influenced by wave reflections; (3) At any given time, the difference between QZc and measured pulsatile pressure equals twice the amplitude of Pb.
A popular approach to characterize wave reflections is to compute reflection magnitude as the ratio of the amplitudes of the backward and forward waves (Pb/Pf). This computation, however, can underestimate the effects of reflections to pressure (and LV load), because peripheral reflections that re-reflect at the heart become part of the forward wave, and thus add to denominator, rather than solely the numerator of reflection magnitude. Similarly, reflection magnitude does not contain information about the timing of the reflected wave and its net effect on aortic (and LV) pressure in systole. Therefore, in many instances it becomes particularly important to pay attention to indices such as QZc, and the difference between total pressure and QZc during systole, in addition to more detailed analyses of pressure-flow relations in the frequency domain.
Pressure-flow analyses in the frequency domain
The amplitude of forward and backward waves are parameters of ventricular-arterial cross-talk, rather than measurements of arterial load. The most comprehensive approach to characterize the pattern of arterial load is based on decomposition of the pressure and flow waveforms in its harmonic components (analyses in the frequency domain). In analyses of a pressure-flow pair (spanning one complete cardiac cycle), the fundamental frequency, or 1st harmonic, is the heart rate, and higher harmonics are multiples of that frequency (for example, at a heart of 60 beats/minute, the fundamental harmonic has a frequency of 60 Hz, the 2nd harmonic has a frequency of 120 Hz, and so on). Each harmonic is a sine wave that (in addition to frequency), has 2 basic properties: (1) amplitude (or modulus, which is measured, for instance, in mmHg for pressure harmonics and mL/min for flow harmonics); (2) A phase angle, which in this case is simply a representation of the position of the specific pressure of flow harmonic sine wave in the time axis, relative to the cardiac cycle. Pressure or flow harmonics have such magnitude and phase that, when all harmonics are summed, the original waveform can be reconstructed (the sum of all pressure harmonics equals the measured pressure waveform, whereas the sum of all flow harmonics equals the measured flow waveform). Based on the principle that impedance equals the ratio of pressure over flow, in the frequency domain, each pressure harmonic can be divided over the corresponding flow harmonic to obtain a spectrum of impedances, which is collectively called input impedance ([ZIN]; note the difference between the input impedance spectrum, which is the summed load from all downstream of the proximal aorta, versus aortic root characteristic impedance [ZC], which is a “local” arterial property). Because harmonics of pressure and flow are sine waves (with magnitude and phase), the ZIN spectrum also has a magnitude and a phase value at each harmonic. The ZIN spectrum is the “gold standard” representation of arterial hydraulic load and is, in principle, a pure representation of arterial properties (its measurement being independent of LV function).
Whereas the ZIN pattern is fully informative about the arterial load, it is not particularly intuitive. However, some features of ZIN are worth mentioning. The mean pressure/mean flow (i.e., resistance) is represented as the modulus of the “0th” harmonic of ZIN. At high frequencies, the modulus of ZIN oscillates around the aortic root characteristic impedance (ZC). Resistance and aortic root ZC are real, rather than complex numbers. Therefore, the phase angle at 0th harmonic (0 Hz frequency), and the average phase angle of the higher harmonics of ZIN is zero. Some fluctuations of modulus and phase of ZIN are observed at higher frequencies, however, due largely to the effects of wave reflections. Wave reflections have a somewhat random impact at these high frequencies, such that their effects “cancel out” when values are averaged (thus leaving an average effective ZIN that is governed only by ZC at these higher frequencies). Wave reflection is a major determinant of the values of modulus and phase at low frequencies, which is the frequency range that contains the major harmonic components of ascending aortic and pressure. These low frequencies typically correspond to the first 3 harmonics of pressure and flow.
In older people, wave reflections are prominent and inadequately timed, imposing additional load during ventricular ejection. The lower harmonics thus demonstrate modulus values that are greater than ZC to the degree that wave reflections exert an additive effect to the modulus of impedance at these low frequencies.
Once aortic root ZC is known, each harmonic of pressure and flow can be separated into its forward and backward (reflected) components. A reflection coefficient can thus be computed for each harmonic.47,69 Each reflection coefficient is derived from sine waves, and therefore is a complex number with modulus (amplitude) and phase, which correspond to different degrees of destructive or constructive interference between forward and backward harmonics. In other words, the modulus of the reflection coefficient is not fully representative of the net pressure load induced by reflections at a specific frequency. Therefore, the net effect of reflections is best expressed as the real part of the reflection coefficient, which becomes increasingly positive as pressure from wave reflections increases (constructive interference), and negative when destructive interference leads to a net decrease in pressure by wave reflections at a given harmonic.70 Since the first few harmonics contain the vast majority of the pulsatile energy generated by the heart, reflection coefficients at these harmonics (such as the first 3 harmonics) are of particular interest.
An interesting consideration is that the reflection coefficients are different for each frequency, because the arterial tree reflects pulsatile energy back to the aortic root differentially, depending on the frequency. Furthermore, the phase of the reflection coefficients is not zero, such that there is a “phase shift” of the reflected wave components relative to the forward components. The complex nature of wave reflections in the arterial tree is well recognized and is key to our understanding of the effects of arterial dysfunction on central hemodynamics.11,62,71 First, due to the frequency-dependent complex nature of wave reflection, a reflected wave is not simply a “smaller” but otherwise identical “version” of the forward wave. Reflecting sites “reshape” the wave, partially because they shift its phase. Because of this phenomenon, reflected wave transit times (or the time of “arrival” of the composite reflected wave to the proximal aorta) cannot be inferred simply from the apparent observed delay between a characteristic point in the forward and backward waves (such as the foot of the wave). A distal shift of reflecting sites with aging72 has been proposed based on analyses that neglect the complex frequency-dependent nature of wave reflections in the arterial tree. Furthermore, these analyses were based on use of the pressure waveform inflection as a surrogate for reflected wave transit time. More recent analyses by Phan et al62 that utilized pressure and flow information, and more implicitly accounted for the complex nature of wave reflections, did not demonstrate such a distal shift and in fact suggested the opposite (a proximal shift of reflection sites with aging). These considerations have implications for our understanding of the role of wave reflections in microvascular disease, as discussed in more detail in the accompanying review. The complex nature of wave reflections can also aid in our understanding of the mechanism of action of therapeutic interventions that alter the phase of wave reflections (via effects on muscular conduit arteries), effectively reducing the contribution of wave reflections to LV load and the aortic pressure profile, without the need to affect resistance vessels (which are also an important source of wave reflections), or increase the total transmission of pulsatile energy to the peripheral capillary bed. As discussed in the second part of this review, this appears to be a promising therapeutic strategy in HFpEF.
Several additional physiologic insights can be provided by a direct assessment of the input impedance spectrum, but such details are beyond the scope of this review. The reader is referred to previous publications for a more detailed description of input impedance.11,47 Unfortunately, frequency domain analyses tend to be considered too complex for intuitive clinical assessments, but remain extremely valuable for mechanistic clinical research studies.
Myocardial wall stress
Although the terms “myocardial afterload” and “ventricular afterload” are often used interchangeably, the relationship between LV and myocardial afterload is influenced by the time-varying LV geometry during ejection, which in turn affects the myocardial wall stress (MWS) for any given LV chamber pressure. As discussed above, LV afterload is the hydraulic load imposed by the systemic circulation (aortic input impedance), which depends on the pressure required to generate flow (ejection) into the proximal aorta. In contrast, myocardial afterload is determined by the MWS required to generate fiber shortening. MWS thus reflects the time-varying mechanical load experienced by the contractile elements in the myocardium and the amount of force and work that the myocardial fibers generate during a contraction. Peak systolic MWS is closely and linearly related to myocardial oxygen consumption (MVO2),73 and therefore can be used to directly assess the mechanical efficiency of the cardiovascular system. Interestingly, the relationship between MVO2 and peak MWS when comparing different individuals appears has been reported to be much stronger73 (R2~0.61) than the relationship between the pressure-volume area and MVO2 (R2~0.35). This approach therefore is more appropriate to compare the mechanical efficiency of the cardiovascular system between individuals or disease populations. A comparison of the information provided by the pressure-volume plane vs. analyses of pressure-flow relations and MWS is presented in Table 2.
Table 2.
Comparison of the pressure-volume plane vs. analyses of pressure-flow relations and myocardial wall stress to assess various aspects of arterial load and ventricular-arterial coupling
| Pressure-volume Plane | Pressure-flow relations and Myocardial Wall Stress | |
|---|---|---|
| Able to assess LV pump function in a load-independent manner | Yes | Potentially, but not at present |
| Able to assess the energetic efficiency of a single heart at various loading conditions | Yes | Yes |
| Reliability to compare the energetic efficiency between individuals or disease populations | + | ++ |
| Characterizes and quantifies pulsatile arterial load | No | Yes |
| Accounts for the time-varying nature of afterload and the LV loading sequence | No | Yes |
| Relationship to diastolic dysfunction | +/− | ++ |
| Independently predictive of heart failure risk | No | Yes |
| Detects abnormalities in HFpEF compared to age-matched hypertensive controls | No | Yes |
| Equally useful for HFpEF and HFrEF | No | Yes |
| Reliable in the presence of segmental wall motion abnormalities | Yes | Pressure-Flow relations are reliable. Myocardial wall stress estimations are unreliable in this situation. |
In addition to the usefulness of MWS as a marker of MVO2, MWS has the key advantage of characterizing the time-varying mechanical load on the myocardium. Time-resolved MWS not only characterizes the systolic loading sequence, but also integrates the complex influence of arterial load and LV structure and function on myocardial load.36,43,44,47,74–77 MWS is determined by interactions between myocardial contractile elements, instantaneous LV geometry and the time-varying hydraulic load imposed by the arterial tree.74 Time-varying MWS therefore represents an integrated and highly informative index of myocardial-ventricular-arterial coupling.36,43,44,47,74–77
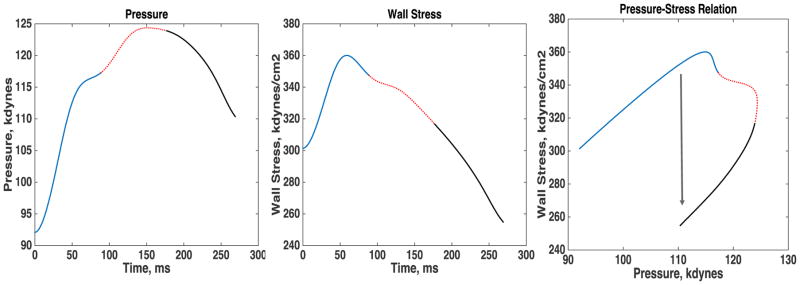
All key correlates of MWS (LV wall thickness, cavity size and pressure, which for any given flow ejected, depends on the aortic input impedance) exhibit marked variations during ejection. Therefore, time-resolving MWS during ejection (rather than using a single end-systolic estimation) is necessary to gain insights into the bidirectional dynamic interactions between the myocardium, the LV chamber properties, and the arterial tree. Previous studies have shown that, among normotensive and hypertensive adults with a normal LV ejection fraction, peak MWS typically occurs in early systole (Figure 7).36,74,75 This is followed by a marked change in the relationship between LV pressure and MWS during mid-systole, which determines a lower stress for any given LV (and aortic) pressure.74 This phenomenon, which appears ideal to protect cardiomyocytes against excessive load in mid-to-late systole,74,77–79 depends on the dynamic reduction of LV chamber size relative to wall volume.74 A lower LV EF (even within the “normal” EF range), more concentric LV geometry (as seen in concentric LV remodeling or hypertrophy)74 and reduced early-phase ejection (despite a preserved overall EF)80 are associated with a less efficient reduction in late systolic MWS for any given LV/aortic pressure.74 The implications of late systolic load for myocardial hypertrophy and diastolic relaxation are discussed in the second part of this review.
Figure 7.
Time course of ejection-phase pressure (A), myocardial wall stress (MWS, B) and the pressure-stress relation. Normally, brisk force development and fiber shortening occur in early systole, resulting in an early peak in MWS and shortening rate, followed by continued LV ejection and a dynamic reconfiguration of LV geometry that results in a mid-systolic reduction in MWS relative to LV pressure, thus protecting the cardiomyocytes against excessive load in mid-to-late systole (a period of increased vulnerability). The mid-systolic reduction in MWS, relative to pressure, is impaired in the presence of a lower LV EF (even within the “normal” EF range), concentric LV remodeling or hypertrophy, and/or reduced early systolic ejection (despite a preserved overall EF).
Other approaches
An additional approach to characterize LV-arterial interactions is wave intensity analysis. This is a time-domain method in which the intensity of waves (defined as the product of the time derivatives of pressure and flow velocity, [dP/dt] × [dU/dt]) is quantified.81,82 Simultaneous forward- and backward-traveling wave fronts of the compression or expansion (“suction”) types can be quantified. Typical wave intensity patterns in the proximal arterial system demonstrate a first positive peak of wave intensity from the forward compression wave generated by the LV, which accelerates the aortic flow and increases the pressure during LV contraction. A second peak is apparent in late systole, corresponding to a forward expansion wave (FEW) that decelerates flow and reduces pressure during relaxation. The characteristics of these forward waves may represent informative parameters of the ventricular-arterial cross-talk. However, wave intensity analysis underrepresents reflected waves, because it emphasizes high frequencies, whereas reflections exert their effects predominantly at low frequencies. This can lead to misinterpretations of underlying hemodynamics (such as assuming that diastole or mid-systole are “wave-free periods” simply because high-frequency wave fronts are small or absent). Further research is required to assess the potential clinical value of wave intensity analysis.
Conclusions
Comprehensive assessments of pulsatile arterial load and ventricular arterial interactions can be achieved with analyses of pressure-flow relations. Whereas the pressure-volume plane remains the gold-standard for LV chamber pump function, it has serious limitations to assess pulsatile arterial load and ventricular-arterial coupling. Despite the apparent complexity of pressure-flow analyses, this approach can be readily implemented with contemporary software from signals that can be acquired quickly and non-invasively at the bedside. As discussed in the second part of this review, analyses of prevalent pulsatile arterial hemodynamic abnormalities in HFpEF leads to a better understanding of the pathophysiology of the disease and can enhance our translational and therapeutic approach to this condition. Therapeutic approaches to improve arterial hemodynamic abnormalities that are prevalent in HFpEF are available and being tested in clinical trials.83 A comprehensive understanding of systemic arterial hemodynamics as well as the macrovascular-microvascular cross-talk, can provide important insights regarding: (1) the role of arterial load in LV remodeling and diastolic dysfunction; (2) the role of the arterial tree in abnormal peripheral oxygen extraction during exercise, and; (3) the high prevalence of certain comorbidities (cognitive dysfunction/dementia and, renal disease) in this patient population.
Acknowledgments
Sources of Funding: This work was supported by NIH grants R01 HL 121510-01A1 (J.A.C) and R56HL-124073-01A1 (J.A.C).
Footnotes
Compliance with Ethical Standards:
Disclosures: The author has received consulting honoraria from Bristol-Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Vital Labs and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, American Heart Association, Fukuda Denshi, Bristol-Myers Squibb, Microsoft and CVRx Inc., and device loans from AtCor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction.
References
- 1.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (pep-chf) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekstra T, Lesman-Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail. 2011;13:1013–1018. doi: 10.1093/eurjhf/hfr072. [DOI] [PubMed] [Google Scholar]
- 9.Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in charm. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Nichols WWORM, Vlachopoulos C. Mcdonald's blood flow in arteries: Theoretical, experimental and clinical principles. 6. Hodder Arnold; 2011. [Google Scholar]
- 12.Chirinos JA. Ventricular-arterial coupling: Invasive and non-invasive assessment. Artery Res. 2013:7. doi: 10.1016/j.artres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 14.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–2506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 15.Suga H. Theoretical analysis of a left-ventricular pumping model based on the systolic time-varying pressure-volume ratio. IEEE transactions on bio-medical engineering. 1971;18:47–55. doi: 10.1109/tbme.1971.4502789. [DOI] [PubMed] [Google Scholar]
- 16.Suga H. Total mechanical energy of a ventricle model and cardiac oxygen consumption. Am J Physiol. 1979;236:H498–505. doi: 10.1152/ajpheart.1979.236.3.H498. [DOI] [PubMed] [Google Scholar]
- 17.Khalafbeigui F, Suga H, Sagawa K. Left ventricular systolic pressure-volume area correlates with oxygen consumption. Am J Physiol. 1979;237:H566–569. doi: 10.1152/ajpheart.1979.237.5.H566. [DOI] [PubMed] [Google Scholar]
- 18.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka H, Takeuchi M, Odake M, Yokoyama M. Assessment of myocardial oxygen consumption (vo2) and systolic pressure-volume area (pva) in human hearts. Eur Heart J. 1992;13(Suppl E):85–90. doi: 10.1093/eurheartj/13.suppl_e.85. [DOI] [PubMed] [Google Scholar]
- 20.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 21.Sunagawa K, Maughan WL, Friesinger G, Guzman P, Chang MS, Sagawa K. Effects of coronary arterial pressure on left ventricular end-systolic pressure-volume relation of isolated canine heart. Circ Res. 1982;50:727–734. doi: 10.1161/01.res.50.5.727. [DOI] [PubMed] [Google Scholar]
- 22.Sunagawa K, Sagawa K, Maughan WL. Ventricular interaction with the loading system. Ann Biomed Eng. 1984;12:163–189. doi: 10.1007/BF02584229. [DOI] [PubMed] [Google Scholar]
- 23.Kass DA. Ventricular arterial stiffening: Integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 24.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: Mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264:H1817–1824. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 27.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 29.Ooi H, Chung W, Biolo A. Arterial stiffness and vascular load in heart failure. Congestive heart failure. 2008;14:31–36. doi: 10.1111/j.1751-7133.2008.07210.x. [DOI] [PubMed] [Google Scholar]
- 30.Nichols WWORM, Vlachopolous C. Theoretical, experimental and clinical principles. Hodder Arnold; 2011. Mcdonald’s blood flow in arteries. [Google Scholar]
- 31.Fukuta H, Ohte N, Wakami K, Asada K, Goto T, Mukai S, Tani T, Kimura G. Impact of arterial load on left ventricular diastolic function in patients undergoing cardiac catheterization for coronary artery disease. Circ J. 2010;74:1900–1905. doi: 10.1253/circj.cj-10-0283. [DOI] [PubMed] [Google Scholar]
- 32.Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 35.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–813. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 36.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: The asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 37.Quail MA, Short R, Pandya B, Steeden JA, Khushnood A, Taylor AM, Segers P, Muthurangu V. Abnormal wave reflections and left ventricular hypertrophy late after coarctation of the aorta repair. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08763. [DOI] [PMC free article] [PubMed]
- 38.Maughan WL, Sunagawa K, Burkhoff D, Sagawa K. Effect of arterial impedance changes on the end-systolic pressure-volume relation. Circ Res. 1984;54:595–602. doi: 10.1161/01.res.54.5.595. [DOI] [PubMed] [Google Scholar]
- 39.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Rietzschel ER, Shiva-Kumar P, De Buyzere ML, Zamani P, Claessens T, Geraci S, Konda P, De Bacquer D, Akers SR, Gillebert TC, Segers P. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension. 2014;64:1022–1031. doi: 10.1161/HYPERTENSIONAHA.114.03696. [DOI] [PubMed] [Google Scholar]
- 41.Chemla D, Antony I, Lecarpentier Y, Nitenberg A. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol. 2003;285:H614–620. doi: 10.1152/ajpheart.00823.2002. [DOI] [PubMed] [Google Scholar]
- 42.Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, Townsend R, Emmi AG, Jr, Kirkpatrick JN, Keane MG, Ferrari VA, Wiegers SE, St John Sutton MG. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H320–330. doi: 10.1152/ajpheart.00334.2009. [DOI] [PubMed] [Google Scholar]
- 43.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on H. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ, Zamorano JL. Recommendations on the use of echocardiography in adult hypertension: A report from the european association of cardiovascular imaging (eacvi) and the american society of echocardiography (ase)dagger. Eur Heart J Cardiovasc Imaging. 2015;16:577–605. doi: 10.1093/ehjci/jev076. [DOI] [PubMed] [Google Scholar]
- 45.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 46.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 1: Pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56:555–562. doi: 10.1161/HYPERTENSIONAHA.110.157321. [DOI] [PubMed] [Google Scholar]
- 47.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 48.Zamani P, Bluemke DA, Jacobs DR, Jr, Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: The multi-ethnic study of atherosclerosis. Hypertension. 2015;65:85–92. doi: 10.1161/HYPERTENSIONAHA.114.04333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamani P, Jacobs DR, Jr, Segers P, Duprez DA, Brumback L, Kronmal RA, Lilly SM, Townsend RR, Budoff M, Lima JA, Hannan P, Chirinos JA. Reflection magnitude as a predictor of mortality: The multi-ethnic study of atherosclerosis. Hypertension. 2014;64:958–964. doi: 10.1161/HYPERTENSIONAHA.114.03855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chirinos JA, Kips JG, Roman MJ, Medina-Lezama J, Li Y, Woodiwiss AJ, Norton GR, Yasmin, Van Bortel L, Wang JG, Cockcroft JR, Devereux RB, Wilkinson IB, Segers P, McEniery CM. Ethnic differences in arterial wave reflections and normative equations for augmentation index. Hypertension. 2011;57:1108–1116. doi: 10.1161/HYPERTENSIONAHA.110.166348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber T, Wassertheurer S, O'Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B. Pulsatile hemodynamics in patients with exertional dyspnea: Potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883. doi: 10.1016/j.jacc.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Goto T, Ohte N, Fukuta H, Wakami K, Tani T, Kimura G. Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women. Circ J. 2013;77:123–129. doi: 10.1253/circj.cj-12-0733. [DOI] [PubMed] [Google Scholar]
- 53.Zamani P, Lilly SM, Segers P, Jacobs DR, Jr, Bluemke DA, Duprez DA, Chirinos JA. Pulsatile load components, resistive load and incident heart failure: The multi-ethnic study of atherosclerosis (mesa) J Card Fail. 2016;22:988–995. doi: 10.1016/j.cardfail.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chirinos JA, Segers P, Duprez DA, Brumback L, Bluemke DA, Zamani P, Kronmal R, Vaidya D, Ouyang P, Townsend RR, Jacobs DR., Jr Late systolic central hypertension as a predictor of incident heart failure: The multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4:e001335. doi: 10.1161/JAHA.114.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: Mesa (multiethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chirinos JA, Beraun M, Townsend RR, Varakantam S, Zamani P. Effect of inorganic nitrate on wave reflections vs. Microvascular dilatory reserve: Role in improving aeobic capacity in heart failure with preserved ejection fraction. 2016;134:A15786. [Google Scholar]
- 58.Mitchell GF. Arterial stiffness and wave reflection in hypertension: Pathophysiologic and therapeutic implications. Curr Hypertens Rep. 2004;6:436–441. doi: 10.1007/s11906-004-0037-1. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell GF. Clinical achievements of impedance analysis. Med Biol Eng Comput. 2009;47:153–163. doi: 10.1007/s11517-008-0402-3. [DOI] [PubMed] [Google Scholar]
- 60.Nichols WW, O'Rourke MF. Theoretical, experimental and clinical principles. Oxford University Press; 2005. Mcdonald’s blood flow in arteries. [Google Scholar]
- 61.Chirinos JA. Arterial stiffness: Basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 62.Phan TS, Li JK, Segers P, Reddy-Koppula M, Akers SR, Kuna ST, Gislason T, Pack AI, Chirinos JA. Aging is associated with an earlier arrival of reflected waves without a distal shift in reflection sites. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003733. [DOI] [PMC free article] [PubMed]
- 63.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension. 56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 64.Segers P, Stergiopulos N, Westerhof N. Quantification of the contribution of cardiac and arterial remodeling to hypertension. Hypertension. 2000;36:760–765. doi: 10.1161/01.hyp.36.5.760. [DOI] [PubMed] [Google Scholar]
- 65.Colan SD, Borow KM, Neumann A. Use of the calibrated carotid pulse tracing for calculation of left ventricular pressure and wall stress throughout ejection. Am Heart J. 1985;109:1306–1310. doi: 10.1016/0002-8703(85)90356-4. [DOI] [PubMed] [Google Scholar]
- 66.Hashimoto J, Nichols WW, O'Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: Influence of arterial wave reflection. Am J Hypertens. 2008;21:329–333. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 67.Westerhof BE. Wave reflection: Wasted effort in left ventricular hypertrophy. Am J Hypertens. 2008;21:243. doi: 10.1038/ajh.2007.76. [DOI] [PubMed] [Google Scholar]
- 68.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovascular research. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 70.Swillens A, Segers P. Assessment of arterial pressure wave reflection: Methodological considerations. Artery Research. 2008;2:9. [Google Scholar]
- 71.Westerhof BE, Westerhof N. Magnitude and return time of the reflected wave: The effects of large artery stiffness and aortic geometry. J Hypertens. 2012;30:932–939. doi: 10.1097/HJH.0b013e3283524932. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 73.Strauer BE. Myocardial oxygen consumption in chronic heart disease: Role of wall stress, hypertrophy and coronary reserve. Am J Cardiol. 1979;44:730–740. doi: 10.1016/0002-9149(79)90295-9. [DOI] [PubMed] [Google Scholar]
- 74.Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, Townsend R, Ferrari VA, Wiegers SE, St John Sutton M. Time-varying myocardial stress and systolic pressure-stress relationship: Role in myocardial-arterial coupling in hypertension. Circulation. 2009;119:2798–2807. doi: 10.1161/CIRCULATIONAHA.108.829366. [DOI] [PubMed] [Google Scholar]
- 75.Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER, Asklepios I. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60:64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 76.Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ, Zamorano JL. Recommendations on the use of echocardiography in adult hypertension: A report from the european association of cardiovascular imaging (eacvi) and the american society of echocardiography (ase) J Am Soc Echocardiogr. 2015;28:727–754. doi: 10.1016/j.echo.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Chirinos JA. Deciphering systolic-diastolic coupling in the intact heart. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08849. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: A novel form of stage b heart failure? J Am Coll Cardiol. 2012;60:2178–2181. doi: 10.1016/j.jacc.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 79.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: A novel form of stage b heart failure? J Am Coll Cardiol. 2012;60:2178–2181. doi: 10.1016/j.jacc.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 80.Gu H, Li Y, Fiok H, Simpson J, Kentish JC, Shah A, Chowienczyk P. Reduced first-phase ejection fraction and sustained myocardial wall stress in hypertensive patients with diastolic dysfunction. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parker KH, Jones CJ. Forward and backward running waves in the arteries: Analysis using the method of characteristics. J Biomech Eng. 1990;112:322–326. doi: 10.1115/1.2891191. [DOI] [PubMed] [Google Scholar]
- 82.Westerhof N, Segers P, Westerhof BE. Wave separation, wave intensity, the reservoir-wave concept, and the instantaneous wave-free ratio: Presumptions and principles. Hypertension. 2015;66:e21. doi: 10.1161/HYP.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 83.Chirinos JA, Zamani P. The nitrate-nitrite-no pathway and its implications for heart failure and preserved ejection fraction. Curr Heart Fail Rep. 2016;13:47–59. doi: 10.1007/s11897-016-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]