Abstract
Background
Pre-transplant sensitization is a limiting factor in solid organ transplantation. In heart transplants, ventricular assist device (VAD) implantation has been associated with sensitization to human leukocyte antigens (HLA). The effect of VAD on non-HLA antibodies is unclear. We have previously shown that polyreactive natural antibodies (Nabs) contribute to pre-sensitization in kidney allograft recipients. Here we assessed the generation of Nabs following VAD implantation in pre-transplant sera and examined their contribution to the cardiac allograft outcome.
Methods
IgM and IgG Nabs were tested in pre-transplant serum samples collected from 206 orthotopic heart transplant (OHT) recipients, including 128 VAD and 78 no-VAD patients. Nabs were assessed by testing serum reactivity to apoptotic cells by flow cytometry and to the generic oxidized epitope, malondialdehyde, by ELISA.
Results
No difference was observed in serum levels of IgM Nabs between VAD and no-VAD patients. However, serum IgG Nabs levels were significantly increased in VAD compared to no-VAD patients. This increase was likely due to presence of VAD, as revealed by lower serum IgG Nabs levels before implantation. Lastly, elevated pre-transplant IgG Nabs level was associated with the development of primary graft dysfunction in this patient series.
Conclusions
Our study demonstrates that VAD support elicits IgG Nabs reactive to apoptotic cells and oxidized epitopes. These findings further support the idea of a broad and non-specific B-cell activation by VAD, resulting in IgG sensitization. Moreover, the association of serum IgG Nabs levels with the development of primary graft dysfunction suggests a possible role for these antibodies in the inflammatory reaction accompanying this complication.
INTRODUCTION
The development of antibodies reactive to human leukocyte antigens (HLA) in patients awaiting transplantation, a phenomenon known as sensitization, is an impediment for the timely identification of a suitable donor [1]. Immunizing events resulting in sensitization include pregnancies, blood transfusions or previous transplants. Mechanical circulatory support, in the form of ventricular assist device (VAD) implants, has also been associated with sensitization in multiple studies [2] [3] [4]. In some reports, sensitization consequent to VAD increased the incidence of antibody-mediated rejection (AMR), thereby impacting on the overall clinical outcome [5]. To date, the immune mechanism causing continuous flow devices to elicit anti-HLA antibodies is still elusive. The growing use of VAD as a bridge to heart transplant however, warrants a closer examination of this mode of sensitization.
Although the most recognized antibodies involved in graft destruction are IgG specific to HLA, other types of antibodies have also been described that display pathogenic potential, such as antibodies to vimentin [6], myosin [7], AT1R [8], or the perlecan fragment, LG3, [9]. In previous studies we reported the implication of antibodies reactive to apoptotic cells in AMR following kidney transplantation [10]. These latter antibodies are known as natural antibodies (Nabs) as they are presumed to develop spontaneously in healthy individuals without the need for prior immunization [11]. Their levels, however, can differ vastly between individuals especially in patients awaiting kidney transplants. In the present study, we investigated the levels of Nabs in pre-transplant serum collected from recipients of orthotopic heart transplantation (OHT). We more specifically examined whether VAD implantation could trigger the generation of Nabs. Lastly, we looked for an association between serum Nabs and post-transplant complications.
METHODS
Patient characteristics and sample collection
Study approval was obtained from the Columbia University Institutional Review Board. The patient group consisted of 206 transplant recipients who received OHT at NewYork-Presbyterian Hospital/Columbia University Medical Center between 2011 and 2014 and whose pre-transplant serum specimen was available. Samples collected from 20 healthy subjects were used as controls. The baseline characteristics of all study patients are summarized in Table 1. All episodes of rejection, i.e. acute cellular rejection (ACR) and antibody-mediated rejection (AMR), were graded according to the 1990/2004 and 2013 International Society of Heart and Lung Transplantation (ISHLT) consensus criteria, respectively, with ACR ≥ 1R/1B and AMR > 0 classified as significant rejection. Primary graft dysfunction (PGD) was defined as the need for mechanical circulatory support (VAD or extracorporeal membrane oxygenation) within 24 hours of transplant. Cardiac allograft vasculopathy (CAV) was diagnosed by angiography initially performed at one year post-OHT and then every other year thereafter. Additional angiograms were performed in the event of unexplained graft dysfunction. Angiograms were graded according to the 2010 ISHLT CAV recommended nomenclature. Dobutamine stress echocardiography was used to screen for CAV during non-angiogram years. Panel-reactive antibodies (PRA) to both class I and class II antigens were assessed in patient sera by complement-dependent cytotoxicity (CDC) using purified panel T and B lymphocytes. Pre-transplant donor-specific HLA antibodies (DSA) were tested by both solid-phase Luminex assay and cell-based CDC methods. Antibodies measured by Luminex with a mean fluorescent intensity (MFI) > 3000 were considered positive although not a contraindication for transplant if a prospective crossmatch was found negative. In contrast, MFI > 6000 was considered a contraindication for transplant. No patient was transplanted with a positive crossmatch in our cohort.
Table 1.
Baseline characteristics of VAD and no-VAD patients
| Characteristics | Overall (n = 206) | VAD (n = 128) | No-VAD (n = 78) | p-value |
|---|---|---|---|---|
| Age years, median (IQR) | 57.0 (46.0–64.0) | 57.0 (46.0–64.0) | 55.5 (45.3–61.8) | - |
| Male, n (%)* | 151 (73.3) | 101 (78.9) | 50 (64.1) | 0.023* |
| Follow up, median (IQR) | 841.5 (462.0–1198.0) | 788.5 (370.3–1130.3) | 943.0 (645.3–1212.5) | - |
| Donor age | 35.5 (23.0–44.8) | 30.0 (23.0–45.0) | 32.5 (24.0–42.0) | - |
| Donor male, n (%)* | 129 (62.1) | 88 (68.8) | 41 (52.6) | 0.026* |
| VAD duration days, median (IQR) | - | 272.5 (163.3–590.5) | - | - |
| Type of VAD, n (%) | ||||
| Heartmate II | - | 107 (83.6) | - | - |
| HVAD | - | 8 (6.3) | - | - |
| Centrimag | - | 7 (5.5) | - | - |
| Thoratec | - | 2 (1.6) | - | - |
| Other | - | 4 (3.1) | - | - |
| HLA (by Luminex), n (%)* | 73 (35.4) | 38 (29.7) | 35 (44.9) | 0.035* |
| PRA > 10 % at Tx, n (%) | 6 (2.9) | 5 (3.9) | 1 (1.3) | 0.412 |
| PRA > 0 % at Tx, n (%) | 38 (18.4) | 29 (22.7) | 9 (11.5) | 0.063 |
| Adverse outcomes n (%) | ||||
| PGD | 33 (16.0) | 25 (19.5) | 8 (10.3) | 0.116 |
| REJ | 87 (42.2) | 48 (37.5) | 39 (50) | 0.083 |
| CAV | 43 (20.9) | 28 (21.9) | 15 (19.2) | 0.305 |
| Death | 25 (12.1) | 20 (15.6) | 5 (6.4) | 0.077 |
CAV, cardiac allograft vasculopathy; IQR, interquartile range; PGD, primary graft dysfunction; PRA, panel-reactive antibodies; REJ, cellular or antibody-mediated rejection; Tx, transplant; VAD, ventricular assist device.
p < 0.05
Purification of serum IgG and immunoglobulin quantitation
IgG Nabs can be masked by serum proteins. Thus, IgG were purified from patient serum specimens using the Melon Gel IgG Purification Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Briefly, serum samples were diluted 1:10 in Melon purification buffer and incubated with the Melon resin to retain non-IgG immunoglobulin and other abundant serum proteins whilst permitting elution of IgG immunoglobulin. Cytometric bead array (BD Biosciences, San Jose, CA) was used to quantitate Melon Gel-purified IgG and IgM from patient sera. Sample acquisition was performed on a BD LSR Fortessa flow cytometer (BD Biosciences).
Assessment of reactivity to apoptotic cells
The Jurkat T-cell line was used as a source of apoptotic cells in experiments assessing the binding of patient serum IgM or purified IgG to apoptotic cells. Apoptosis was induced in Jurkat cells by exposure to UV light (240 mJ/cm2) using a UV stratalinker 2400 (Stratagene, Santa Clara, CA). Following UV treatment, cells were incubated at 37 °C for 20 h, then 0.5 × 106 apoptotic cells were incubated for 30 min at 37°C with either serum IgM diluted 1 in 5 in phosphate buffered saline (PBS) or purified IgG samples diluted 1 in 2 in PBS. Cells were washed in PBS and incubated with FITC-conjugated anti-human IgM F(ab’)2 (Jackson ImmunoResearch Labs, West Grove, PA) or FITC-conjugated anti-human IgG F(ab’)2 secondary antibodies as appropriate (Life Technologies, Carlsbad, CA) for 30min at 4°C. Prior to acquisition, samples were stained with APC-conjugated Annexin V and 7-AAD (Biolegend, San Diego, CA) according to the manufacturer’s instructions. Sample acquisition was performed on a BD LSR Fortessa flow cytometer with high throughput sampling capabilities (BD Biosciences) after gating on apoptotic cells. To control for inter-assay variability, all samples were assessed using the same instrument settings in the same experiment.
Assessment of reactivity to MDA
ELISA assays were used in the detection of antibodies to the oxidized lipid epitope malondialdehyde (MDA). MDA-modified BSA was generated by incubating acid-hydrolyzed 1,1,3,3-tetramethoxypropane (Sigma-Aldrich, St. Louis, MO) with BSA. Briefly, 1 M 1,1,3,3-tetramethoxypropane was hydrolyzed in 96 mM HCl for 10 min at 37°C. The resulting MDA solution was neutralized with NaOH and the modification of BSA with 0.2 M MDA was carried out for 3 h at 37°C. Extensive dialysis against PBS was then performed at 4°C for 36 h. High-binding 96-well plates (Corning, Kennebunk, ME) were coated with 12.5 µg/mL MDA-modified BSA overnight at 4°C. Plates were blocked with TBS supplemented with 0.5% non-fat dry milk (TBS/milk) for 1 h at RT. Serum samples diluted 1 in 10 in TBS/milk or purified IgG diluted 1 in 4 in TBS/milk for IgM or IgG testing, respectively, were added for 2 h at RT. Pooled healthy sera were included as a standard to control for intra-assay variability. HRP-conjugated donkey anti-human IgM (Jackson ImmunoResearch Labs) or donkey anti-human IgG (Jackson ImmunoResearch Labs) were then incubated for 1 h at RT. The ELISA was developed using 3,3’,5,5’-tetramethylbenzidine (Life Technologies) and the optical density was read at 450 nm.
Enzyme-linked immunospot (ELISpot) analyses
Patient peripheral blood mononuclear cells (PBMC) were isolated by ficoll-paque density gradient centrifugation. A stimulation cocktail containing 1 µg/mL R848 (Mabtech, Nacka Strand, Sweden) and 10 µg/mL IL-2 (Mabtech) was added for 72 h at 37°C/5% CO2 to activate antibody production by memory B cells. The frequency of total IgM and IgG-producing cells in PBMC was determined using IgM or IgG ELISpot assays. Briefly, anti-IgM or IgG capture antibodies (Mabtech) were incubated in high protein-binding PVDF membrane plates (Millipore, Billerica, MA) for 5 h at RT. Activated cell suspensions were seeded in duplicate at 5,000, 2,500 and 1,250 cells/well and incubated for 8 h at 37°C/5% CO2. Detection was performed by incubating biotinylated anti-IgM (Mabtech) or anti-IgG (Mabtech) detection antibodies for 2 h at RT. Streptavadin-conjugated alkaline phosphatase (R&D, Minneapolis, MN) was added for 2 h at RT followed by incubation with BCIP/NBT substrate (R&D) for 30 min at RT. The reaction was stopped by rinsing wells with ultrapure water. In parallel experiments to assess MDA-specific antibody-secreting cells, ELISpot plates were coated with 62.5 µg/ml MDA-modified BSA for 5 h RT. Cell suspensions were added in duplicate at 50,000, 25,000 and 12,500 cells/well and incubated for 8 h at 37°C/5% CO2. ELISpot detection was performed as for total IgM and IgG detection.
IgG subclass typing
IgG subclass reactivity to apoptotic cells was determined by incubating 0.5 × 106 apoptotic Jurkat cells with 50 µL of IgG purified from the 50 most reactive patient serum specimens diluted 1 in 2 at 37°C for 30 min. Cells were washed in PBS then incubated with FITC-conjugated anti-IgG1, IgG2, IgG3 or IgG4 secondary antibodies (Clone 4E3, HP6002, HP6050, HP6025; Southern Biotech, Birmingham, AL) at 4°C for 30 min. Cells were acquired using an BD LSRFortessa flow cytometer (BD Biosciences) after gating on apoptotic cells.
Statistical analysis
GraphPad Prism 5 was used for univariate statistical analyses and receiver-operator characteristic (ROC) curve analysis (GraphPad Software Inc., La Jolla, CA). Categorical variables were assessed with Fisher’s exact test. Mann–Whitney U-tests were used to compare the continuous data for IgM or IgG reactivity to apoptotic cells and MDA between all subgroups of patients and healthy subjects. Wilcoxon signed rank test for matched pairs was used to compare serum Nabs before and after VAD implant. Correlation analyses between VAD duration and IgG Nabs levels were performed with Spearman’s correlation coefficient. Multivariable Cox proportional regression modelling was used to determine the pre-transplant predictors of poor graft outcome and was performed using software written for the APL+Win v. 14.1 programming environment (©APLNow LLC, Rockville, MD). Variables were considered for inclusion in the regression model by backwards elimination at the 0.1 alpha level. Continuous data were log-transformed for the multivariable analyses. Statistical significance was declared at the 0.05 level.
RESULTS
Patients
A total of 206 heart transplant recipients were included in our study and baseline characteristics are outlined in Table 1. Of these patients, 128 were bridged with VAD and included 118 LVAD (92.2%) and 10 BiVAD (7.8%). The majority (107/128, 83.6%) of the VAD patients were implanted with the Heartmate II device. Other implanted devices included Heartware HVAD (8/128, 6.3%), Centrimag (7/128, 5.5%), Thoratec PVAD (2/128, 1.6%), and unknown/other (4/128, 3.1%). HLA sensitization at time of transplant as measured by panel-reactive antibodies (PRA) was not common in this cohort with PRA > 10% seen in only 3.9% (5/128) of VAD patients and 1.3% (1/78) of non-bridged patients. However, PRA scores > 0% were seen in 22.7% (29/128) VAD patients and 11.5% (9/78) non-bridged patients while anti-HLA antibodies as measured by single antigen beads on the Luminex platform (MFI > 3000) were detected in 29.7% of VAD (38/128) and 44.9% (35/78) of no-VAD patients.
Pre-transplant IgG Nabs are increased in patients bridged with VAD
We examined the level of IgM and IgG Nabs by testing the reactivity of pre-transplant patient sera to MDA and whole apoptotic cells by ELISA and flow cytometry respectively. MDA is an oxidization-specific epitope known to accumulate in apoptotic cell membranes and is recognized by serum Nabs in mice and humans [12]. No difference was found in IgM Nabs reactivity to MDA or apoptotic cells between the VAD, no-VAD and healthy control groups (Figure 1A). In contrast, IgG Nabs levels were markedly increased to both targets in VAD patients when compared to no-VAD patients or healthy controls (Figure 1B, p < 0.0001). Strong correlation between Nabs reactivity to MDA and apoptotic cells was observed by receiver-operator characteristic (ROC) analysis (Figure S1). To determine if differences in total antibody concentration accounted for the level of Nabs reactivity, total IgG and total IgM were measured in a randomly selected subset of our cohort (VAD n = 23, no-VAD n = 24). No differences were observed in total IgG and IgM concentrations (Figure S2) between the VAD and no-VAD groups.
Figure 1. Pre-transplant IgM and IgG serum reactivity to MDA and apoptotic cells in VAD and no-VAD patients.
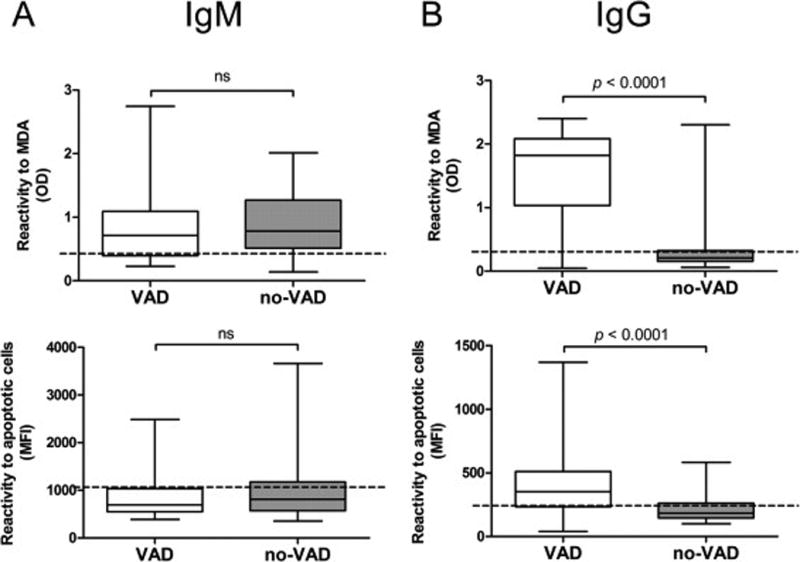
IgG and IgM reactivity to MDA and apoptotic cells was measured in pre-transplant VAD (n = 128) and no-VAD (n = 78) serum samples by ELISA and by flow cytometry respectively. (A) IgM Nabs binding to MDA (top) and apoptotic cells (bottom) are shown. (B) IgG Nabs binding to MDA (top) and apoptotic cells (bottom) (Mann-Whitney U-test, p < 0.0001). Dotted lines in all graphs represent the median levels of IgG and IgM Nabs measured using serum from healthy subjects (n = 20).
IgG Nabs increase in patients following VAD implantation
Since IgG Nabs were significantly increased in the VAD group, we next assessed whether the device implantation and the duration of support had an impact on the development of these antibodies. A comparison between IgG reactivity to MDA and apoptotic cells before and after VAD implantation was carried out for 73 patients for whom pre-VAD samples were available. IgG reactivity to both MDA (Figure 2A, p < 0.0001) and apoptotic cells (Figure 2B, p < 0.0001) distinctly increased following VAD implantation. Of the 73 paired sera tested, 65/73 (89.0%) and 58/73 (79.5%) exhibited at least a 10% increase in reactivity to MDA and apoptotic cells respectively, following VAD implant. However, no significant correlation was observed between the duration of VAD support and the change in level of IgG Nabs between pre- and post-VAD serum samples. (Figure 2A, 2B). We also examined the impact of VAD on PRA in 69 patients for whom pre- and post-VAD PRA values were available. In pre-VAD sera, 8/69 (11.6%) had PRA > 0% compared to 19/69 (27.5%) post-VAD (p = 0.0306, data not shown)
Figure 2. Impact of VAD implant on serum IgG reactivity to MDA and apoptotic cells.

(A) The left panel shows reactivity to MDA measured in pre-VAD and post-VAD (pre-transplant) serum samples collected from 73 VAD patients (Wilcoxon signed rank test for matched pairs, p < 0.0001). The right panel depicts the correlation between VAD support duration in days and percentage change in serum IgG reactivity to MDA between pre- and post-VAD samples (Spearman correlation, p = 0.427). (B) The left panel shows reactivity to apoptotic cells assessed by flow cytometry in pre-VAD and post-VAD serum samples collected from 73 VAD patients (Wilcoxon signed rank test for matched pairs, p < 0.0001). The right panel depicts the correlation between VAD support duration in days and percentage change in serum IgG reactivity to apoptotic cells between pre- and post-VAD samples (Spearman correlation, p = 0.393).
IgG Nabs are predominantly IgG3 and IgG1
We next used a series of IgG subclass-specific secondary antibodies in flow cytometry experiments to further characterize IgG Nabs that react to apoptotic cells. These experiments were performed using the serum of 50 patients with high IgG Nabs levels. As shown in Figure 3, IgG3 and, to a lesser extent, IgG1 were the most abundant IgG Nabs subclasses. These two subclasses are the most efficient complement-activating types of IgG.
Figure 3. Subclass determination of serum IgG Nabs.
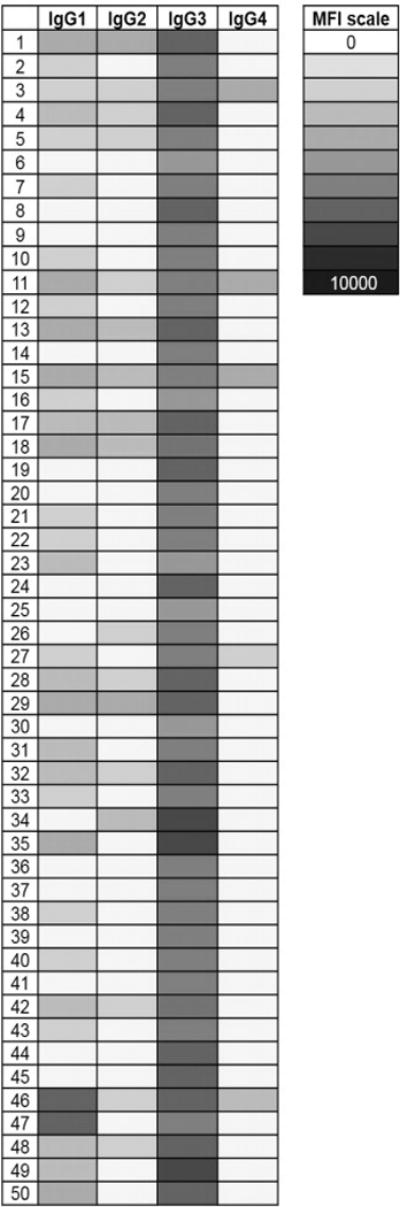
The subclass of serum IgG reactive to apoptotic cells was determined using secondary antibodies specific for IgG1-4 and measured by flow cytometry. Each row represents an individual subject in this heat-map representation. The intensity of the shade of gray corresponds to the median fluorescent intensity (MFI) of the serum IgG reactivity to apoptotic cells as indicated on the accompanying scale.
Serum Nabs increase is not associated with higher frequency of Nabs-producing blood cells
To investigate the source of serum Nabs, we assessed the frequency of anti-MDA-secreting cells using ELISpot in PBMC from VAD (n = 14) and no-VAD patients (n = 10) for whom cryopreserved cells were available as well as healthy controls (n = 8). The frequency of anti-MDA IgM-secreting cells was significantly higher in VAD patients compared to no-VAD patients and healthy controls (Figure 4A, p = 0.019) despite the lack of difference between serum IgM Nabs levels in all three groups (Figure 1A). In contrast, no significant difference was observed in the frequency of anti-MDA IgG-secreting cells between any of the groups (Figure 4B).
Figure 4. Frequency of MDA-specific IgM and IgG-producing cells in peripheral blood.
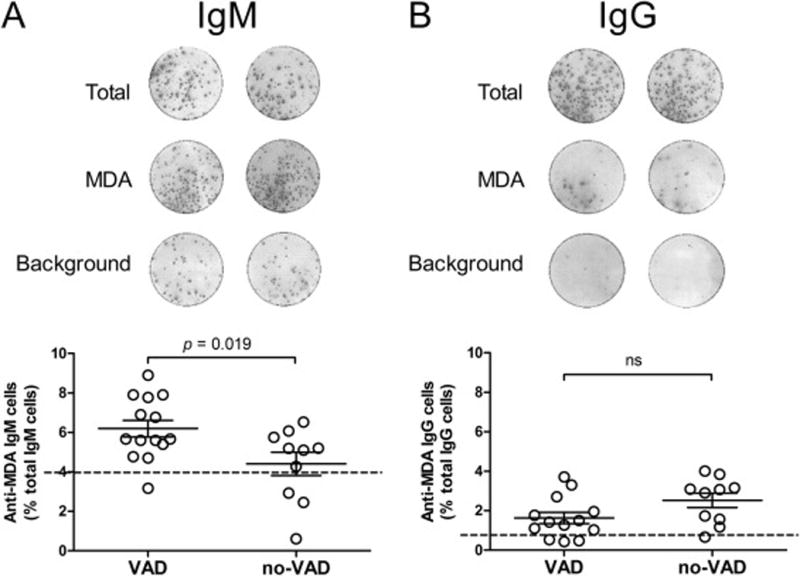
The frequency of MDA-specific IgM- and IgG-secreting cells was assessed by ELISpot in PBMCs collected from VAD patients (n = 14), no-VAD patients (n = 10) and healthy subjects (n = 8). (A) The top panel shows representative ELISpot results for total IgM secretion (2,500 cells/well), anti-MDA IgM (25,000 cells/well) and background reactivity to uncoated wells (25,000 cells/well). The bottom panel shows a comparison between the VAD and no-VAD group measurements (Student’s t-test, p = 0.019). (B) Representative ELISpot results are shown for total IgG secretion (2,500 cells/well), anti-MDA IgG (25,000 cells/well) and background reactivity (25,000 cells/well). The bottom panel shows a comparison between VAD and no-VAD groups. The dotted line shows the mean value obtained for healthy donors and results are expressed as mean ± SEM.
Post-transplant consequences of VAD implant and VAD-affected factors
We sought to determine if pre-transplant Nabs levels were associated with adverse events post-transplant including PGD, rejection (AMR grade > 0 or ACR grade ≥ 1R/1B), CAV and death. VAD implantation as well as factors potentially influenced by VAD (PRA, HLA antibodies) were also examined. Multivariable time-to-event analyses were performed using an adjusted Cox proportional hazards model. Of the variables included in the initial analyses, only IgG Nabs reactive to apoptotic cells, PRA > 0% and presence of pre-transplant HLA antibodies measured by Luminex were retained in the final model at alpha level 0.1. The variables VAD and IgG Nabs reactive to MDA were not entered in the model when IgG Nabs reactive to apoptotic cells were included. However, these variables were entered in the model when IgG Nabs reactive to apoptotic cells were excluded. In our final model, the presence of IgG Nabs reactive to apoptotic cells (HR 1.25, 95% CI 1.06 – 1.48, p = 0.008), HLA antibodies measured by Luminex (HR 1.51, 95% CI 1.11 – 2.07, p = 0.009 and PRA (HR 1.36, 95% CI 1.03 – 1.79, p = 0.031) were significant predictors of adverse post-transplant events (Table S1). When a cause-specific version of the model was fitted, IgG reactivity to apoptotic cells was shown to be significantly associated with PGD (HR 1.88, 95% CI 1.23 – 2.87, p = 0.003) while anti-HLA antibodies measured by Luminex (HR 1.62, 95% CI 1.15 – 2.29, p = 0.006) and PRA (HR 1.39, 95% CI 1.03 – 1.88, p = 0.032) were significant predictors for rejection (AMR or ACR, Table 2). IgG Nabs reactive to MDA were significant predictors of adverse post-transplant events (HR 1.12, 95% CI 1.01 – 1.24, p = 0.03) only when apoptotic cell Nabs were not included in the model (Table S2). When IgG Nabs reactive to either apoptotic cells or MDA were removed from consideration in the Cox model, VAD (HR 1.45, 95% CI 1.07 – 1.98, p = 0.016) and HLA antibodies measured by Luminex (HR 1.47, 95% CI 1.08 – 1.99, p = 0.014) were significant predictors of adverse post-transplant events (Table S3).
Table 2.
Cause-specific Cox proportional hazards modeling
| Variable | PGD | Rejection | CAV | Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| HR | 95% CI | p-value | H R | 95% CI | p-value | H R | 95% CI | p-value | H R | 95% CI | p-value | |
| PRA | 1.18 | 0.58–2.43 | 0.649 | 1.39 | 1.03–1.88 | 0.032* | - | - | - | 1.66 | 0.24–11.24 | 0.604 |
| HLA (Luminex) | 1.27 | 0.55–2.78 | 0.601 | 1.62 | 1.15–2.29 | 0.006* | 0.93 | 0.10–8.70 | 0.951 | - | - | - |
| IgG Nabs (apop. cells) | 1.88 | 1.23–2.87 | 0.003* | 1.13 | 0.94–1.36 | 0.206 | 2.04 | 0.62–6.76 | 0.242 | 2.59 | 0.74–9.02 | 0.135 |
CI, confidence interval; HR, hazard ratio
p < 0.05
- insufficient events available
DISCUSSION
Antibodies recognizing antigens expressed on donor grafts are responsible for acute and chronic forms of rejection following solid organ transplantation. While anti-HLA antibodies are generally considered the primary players, antibodies against self-antigens are increasingly being recognized as important contributors to AMR. Previous studies from our group have implicated IgG Nabs reactive to apoptotic cells in chronic AMR in kidney transplant recipients. Whether Nabs influence the outcome of other types of transplants however, is currently unknown. To our knowledge, this study is the first to investigate the role of Nabs in OHT. Our findings revealed that IgG Nabs were elevated in patients bridged with VAD. Pre-transplant IgG Nabs levels were also significantly associated with PGD, a significant cause of early mortality following OHT. As the numbers of OHT patients receiving VAD support continue to grow, evaluating the immunologic consequences of this type of devices is becoming increasingly pertinent.
VAD implants have previously been associated with presensitization to HLA in adults [2] [4] [13] [14] [15] and pediatric patients [16]. Presensitization to HLA is an important barrier to transplantation with sensitized patients experiencing longer wait times for suitable organs and higher waiting list mortality rates [15]. In addition, presensitized patients may exhibit reduced post-transplant survival [5] [17]. Recent studies have linked VAD-induced HLA-sensitization with an increased rate of PGD [4], increased risk of rejection [15] and mortality [5]. Much less is known about the impact of VAD on non-HLA antibodies and their influence on transplant outcomes. A recent study found that VAD implantation was not associated with sensitization to MICA [2]. On the other hand, patients bridged with LVAD appeared to have higher titers of anti-AT1R antibodies compared to unsupported patients [18] although this increase did not portend reduced graft survival. In the present study, VAD was associated with poorer outcomes (Table S3). Our statistical analysis supported the hypothesis that elevation of IgG Nabs caused by VAD was an explanatory factor linking VAD to decreased graft survival. Moreover, IgG Nabs were significant predictors of PGD, suggesting a direct effector function. Non-HLA antibodies, such as AT1R [19] have been shown to act synergistically with DSA to mediate graft rejection. Nabs may also synergize with DSA to contribute to PGD. Whether desensitization strategies can lessen the impact of pre-transplant Nabs on PGD remains to be determined. We cannot exclude the possibility that other underlying factors in VAD patients, unrelated to the IgG Nabs increase, may mediate PGD. Our findings appear to contrast with other studies reporting minimal, if any, effect of VAD on long-term outcomes [2] [14] [15] [16] [20].
Although IgG Nabs reactive to both the oxidized lipid epitope MDA and apoptotic cells were elevated in VAD, only IgG Nabs to apoptotic cells were associated with PGD in our final model. MDA reactivity was highly correlated with apoptotic cell reactivity (Figure S1), precluding the inclusion of both variables in the model at the same time. Accordingly, IgG Nabs tested by MDA ELISA were significantly associated with poorer survival (Table S2) when included in the model in the absence of IgG Nabs to apoptotic cells. The superiority of one variable over the other may be due to the nature of the assays used to measure IgG Nabs. The ELISA we used has a limited dynamic range compared to the flow cytometric apoptotic cell assay.
Nabs are present in normal human serum and are presumed to play a beneficial role in cell debris clearance [21] [22] and microbial defense [23] [24]. However, pathogenic Nabs are involved in various inflammatory conditions such as ischemia reperfusion injury [25] [26] and systemic inflammatory response syndrome [11]. The role of the IgG Nabs in the transplant setting is still emerging. We noted that IgG3 and, to a lesser extent, IgG1 were the most abundant IgG Nabs subclasses. These two subclasses are complement-fixing, pointing to a possible pathogenic role for these Nabs by complement-mediated graft damage. However, in contrast to our findings in renal transplant patients [10], we did not observe any association between pre-transplant IgG Nabs and rejection.
Nabs-producing cells are thought to be B1, a distinctive lineage of B cells. In humans, there is still no clearly defined phenotypic marker for B1 B cells. We thus assessed the frequency of these cells in the blood of VAD and no-VAD patients directly through their secretion of Nabs using functional ELISpot assays. Unexpectedly, the frequency of IgG Nabs-producing cells was comparable in VAD and no-VAD patients. This result contrasts with the level of IgG Nabs, which was sharply increased in VAD patients compared to patients who did not receive MCS. A possible explanation is that most Nabs-producing cells are located in secondary lymphoid organs such as the spleen, as previously reported in mouse models of cardiac transplants [27]. Conversely, the frequency of IgM Nabs-producing cells was elevated in the blood of VAD patients although serum IgM Nabs levels were not affected by VAD support and were not associated with adverse outcomes. This is in keeping with the general view of IgM as clinically inconsequential in the transplant setting [28]. Since the median VAD duration in our cohort was 272 days, and presuming Nabs elicited by VAD develop following typical humoral response kinetics, it is possible that IgM Nabs occured earlier than the IgG and only IgG Nabs were detectable at sampling time.
Uncertainty remains over the mechanism of sensitization due to VAD. Our results support the view of a broad B cell activation rather than stimulation through exposure to distinctive antigens. We propose that the humoral response induced by VAD support is a consequence of a systemic immune activation that leads to the generation of anti-HLA and non-HLA IgG including IgG Nabs along with anti-AT1R [18], - albumin [29], -phospholipids [30] and clinically irrelevant antibodies binding to HLA-coated beads [31]. In addition, the biomaterials used in the implanted devices are known to activate endothelial, coagulation and fibrinolytic pathways [32] and could plausibly contribute to sensitization in such a non-antigen specific manner [33]. It is possible that leukodepeleted blood transfusions received by VAD patients at our center contributed to sensitization. However, previous studies suggest that such sensitization is independent of transfusions [2] [5] [34].
In conclusion, our study is the first to report on Nabs in OHT. The results presented herein show unequivocally that VAD support triggers the generation of IgG Nabs in OHT patients. Higher Nabs levels are associated with PGD, suggesting a role in the pathophysiology of this life-threatening complication. Further studies are now necessary to confirm and extend our findings to larger OHT cohorts.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-AI116814. Flow cytometry instrumentation was purchased with NIH funding S10RR027050. KJC is supported by NIH Grant T32-HL-007854-21. The authors are grateful to Susan Sherlock for invaluable discussion regarding MDA ELISA development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
None of the authors have any conflicts of interest to disclose.
References
- 1.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–25. doi: 10.1016/j.healun.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askar M, Hsich E, Reville P, et al. HLA and MICA allosensitization patterns among patients supported by ventricular assist devices. J Heart Lung Transplant. 2013;32:1241–8. doi: 10.1016/j.healun.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Massad MG, Cook DJ, Schmitt SK, et al. Factors influencing HLA sensitization in implantable LVAD recipients. Ann Thorac Surg. 1997;64:1120–5. doi: 10.1016/s0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 4.Arnaoutakis GJ, George TJ, Kilic A, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg. 2011;142:1236–45. doi: 10.1016/j.jtcvs.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidambi S, Mohamedali B, Bhat G. Clinical outcomes in sensitized heart transplant patients bridged with ventricular assist devices. Clin Transplant. 2015;29:499–505. doi: 10.1111/ctr.12540. [DOI] [PubMed] [Google Scholar]
- 6.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 7.Morgun A, Shulzhenko N, Unterkircher CS, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23:204–9. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 8.Dragun D, Müller DN, Bräsen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 9.Soulez M, Pilon E, Dieudé M, et al. The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res. 2012;110:94–104. doi: 10.1161/CIRCRESAHA.111.250431. [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant. 2014;14:1581–91. doi: 10.1111/ajt.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu M, Fan P, Li W, et al. Identification of poly-reactive natural IgM antibody that recognizes late apoptotic cells and promotes phagocytosis of the cells. Apoptosis. 2007;12:355–62. doi: 10.1007/s10495-006-0581-z. [DOI] [PubMed] [Google Scholar]
- 12.Chou M, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu P, Schaffer JM, Oyer PE, et al. Influence of durable mechanical circulatory support and allosensitization on mortality after heart transplantation. J Heart Lung Transplant. 2016;35:731–42. doi: 10.1016/j.healun.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alba AC, Tinckam K, Foroutan F, et al. Factors associated with anti-human leukocyte antigen antibodies in patients supported with continuous-flow devices and effect on probability of transplant and post-transplant outcomes. J Heart Lung Transplant. 2015;34:685–92. doi: 10.1016/j.healun.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Ko B, Drakos S, Kfoury AG, et al. Immunologic effects of continuous-flow left ventricular assist devices before and after heart transplant. J Heart Lung Transplant. 2016;35:1024–30. doi: 10.1016/j.healun.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Castleberry C, Zafar F, Thomas T, et al. Allosensitization does not alter post-transplant outcomes in pediatric patients bridged to transplant with a ventricular assist device. Pediatr Transplant. 2016;20:559–64. doi: 10.1111/petr.12706. [DOI] [PubMed] [Google Scholar]
- 17.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–62. doi: 10.1016/j.athoracsur.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 18.Urban M, Slavcev A, Gazdic T, Ivak P, Besik J, Netuka I. The impact of angiotensin II type 1 receptor antibodies on post-heart transplantation outcome in Heart Mate II bridged recipients. Interact Cardiovasc Thorac Surg. 2016;22:292–7. doi: 10.1093/icvts/ivv344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinsmoen NL, Lai C, Mirocha J, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97:595–601. doi: 10.1097/01.TP.0000436927.08026.a8. [DOI] [PubMed] [Google Scholar]
- 20.Awad M, Czer LSC, De Robertis MA, et al. Adult heart transplantation following ventricular assist device implantation: early and late outcomes. Transplant Proc. 2016;48:158–66. doi: 10.1016/j.transproceed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IgM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–64. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 22.Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by, age, gender, and disease. PLoS One. 2013;8:e60726. doi: 10.1371/journal.pone.0060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–9. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 24.Panda S, Zhang J, Tan NS, Ho B, Ding JL. Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO J. 2013;32:2905–19. doi: 10.1038/emboj.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming SD, Egan RP, Chai C, et al. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–61. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 26.Kulik L, Fleming SD, Moratz C, et al. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–73. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicard A, Phares TW, Yu H, et al. The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients. Am J Transplant. 2012;12:1708–19. doi: 10.1111/j.1600-6143.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheinin SA, Radovancević B, Kimball P, et al. Effect of IgM-positive crossmatches on survival in heart transplant recipients. Tex Heart Inst J. 1995;22:67–71. [PMC free article] [PubMed] [Google Scholar]
- 29.Newell H, Smith JD, Rogers P, et al. Sensitization following LVAD implantation using leucodepleted blood is not due to HLA antibodies. Am J Transplant. 2006;6:1712–7. doi: 10.1111/j.1600-6143.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 30.Schuster M, Kocher A, John R, et al. B-cell activation and allosensitization after left ventricular assist device implantation is due to T-cell activation and CD40 ligand expression. Hum Immunol. 2002;63:211–20. doi: 10.1016/s0198-8859(01)00380-9. [DOI] [PubMed] [Google Scholar]
- 31.Nikaein A, El-Awar N, Hunt J, et al. Clinically irrelevant circulating human leukocyte antigen antibodies in the presence of ventricular assist devices. J Heart Lung Transplant. 2012;31:443–7. doi: 10.1016/j.healun.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 32.John R, Panch S, Hrabe J, et al. Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg. 2009;88:1171–9. doi: 10.1016/j.athoracsur.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 33.Kwon MH, Zhang JQ, Schaenman JM, et al. Characterization of ventricular assist device-mediated sensitization in the bridge-to-heart-transplantation patient. J Thorac Cardiovasc Surg. 2015;149:1161–6. doi: 10.1016/j.jtcvs.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar N, Daly R, Geske J, et al. LVAD implant as a bridge to heart transplantation is associated with allosensitization as measured by single antigen bead assay. Transplantation. 2013;96:324–30. doi: 10.1097/TP.0b013e3182985371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


