Abstract
Objectives
To assess prostate cancer (PCa) screening practices in primary care since the initial United States Preventive Services Task Force (USPSTF) recommendation against prostate specific antigen (PSA) testing for older men, and to assess primary provider variation associated with PCa screening.
Patients and Methods
Our study population included 160, 211 men age >= 40 with at least 1 visit in a primary care clinic in any of the study years in a large, integrated health system. We conducted a retrospective cohort study using electronic medical record data from January 2007 through December 2014. Yearly rates of screening PSA testing by primary care provider (PCP), rates of rescreening and rates of prostate biopsies were assessed.
Results
Annual PSA screening testing declined from 2007 to 2014 in all age groups as did biennial and quadrennial screening. Yearly rates declined for men >= age 70, from 22.8 % to 8.9 %, ages 50–69, from 39.2 % to 20 % and ages 40–49, from 11 % to 4.6 %. Overall rates were lower for African American men vs. non-African American men; for men with a family history of PCa, rates were similar or slightly higher than for those without a family history. PCP variation associated with ordering of PSA did not substantially change following the USPSTF recommendations. While the number of men screened and rates of follow-up PCa screening declined in 2011–2014 compared to 2007–2010, similar rescreening rates were noted for men age 45–75 with initial PSA levels < 1ng/ml or 1–3 ng/ml in both the earlier and later cohorts. For men age > 75 with initial PSA level < 3 ng/ml screened in both cohorts, follow-up screening rates were similar. Rates of prostate biopsy declined for men >=age 70 in 2014 compared to 2007. For men who had PSA screening, rates of first prostate biopsy increased in later years for African American men and men with a family history of PCa.
Conclusions
PCa screening declined from 2007 to 2014 even in higher risk groups and follow-up screening rates were not related to previous PSA level. However, rates of first prostate biopsy for men who had a PSA were higher for men with increased risk for PCa in later years. Variation in PSA testing was noted among PCPs. Future work should further explore sources of variation in screening practices and implementation of risk-based strategies for PCa screening in primary care.
Keywords: prostate cancer, primary care, cancer screening
Introduction
Prostate cancer (PCa) is common and may affect up to 14% of men in their lifetime.(1) Established risk factors for PCa include older age, African American race, and a family history of PCa. Compared to white men, African American men in the United States have a 58% greater incidence and 144% greater risk of mortality related to PCa. (2) Regarding family history, the relative risk of developing PCa is 2.48 with a first degree relative with PCa, and even higher if the affected family member is a brother.(2)
Approximately 80% of PCa are localized with 5 year relative survival rates of 100% (1) and there has been conflicting evidence for the mortality benefit of screening with the prostate specific antigen (PSA) test. (3,4) Thus the potential for overdiagnosis and harms of treatment including effects on sexual function and urinary symptoms,(5,6) even in men with known risk factors for PCa, have led to evolving practice guidelines regarding PCa screening over the past several years.(7–10) In 2008, the United States Preventive Services Task Force (USPSTF) recommended against PCa screening with the PSA test in men age > 75(7) and in 2012, extended this recommendation to men of any age; they did not recommend a risk based screening approach.(8) In contrast, both the American College of Physicians(ACP) and American Urologic Association(AUA) recommend a shared decision making approach to screening men under age 70 with consideration of individual risks.(9,10) The recent National Comprehensive Cancer Network (NCCN) Guidelines (11) suggest once the screening decision is made, follow-up screening should occur based upon PSA level. The differing recommendations for PCa screening have created confusion for primary care providers (PCPs). Previous studies reported that these guidelines reduced PSA screening rates in men >75 years(12–15), although studies of self-reported PSA testing (16–18) primary care PSA ordering have not.(19)
Variation in PCP ordering of PSA testing in men > age 75(20) and with limited life expectancy(21) has been described. Screening test ordering behavior may be associated with physician characteristics as well as beliefs.(22,23) It is not known, however, to what extent changes in PCa screening patterns since the initial USPSTF recommendations in 2008 are related to guideline recommendations, patient risk factors such as age, African American race or family history of PCa, or provider factors in routine primary care practice. We also do not understand current primary care practice patterns regarding follow-up screening. Thus we sought to 1) assess trends in PCa screening and rescreening rates in primary care practices in a large, integrated health system from 2007–2014 as related to age and risk factors for PCa and changing guideline recommendations for screening, 2) assess PCP provider variation in PSA testing, and 3) assess rates of prostate biopsies during this time period.
Patients and Methods
The Cleveland Clinic Health System (CCHS) provides outpatient primary care at 41 practices in diverse settings across Northeast Ohio. We conducted a retrospective cohort study using electronic medical record (EMR) data (EPIC system) from January 2007 through December 2014. We identified all men age 40 and above who had at least 1 visit in an Internal Medicine (IM) or Family Medicine (FM) clinic in the CCHS in any of the study years. We excluded men with a diagnosis of PCa (International Classification of Diseases, Ninth Revision, (ICD-9) codes 185, 233.4, 236.5 or V10.46) prior to the first PSA test in each study year. We obtained all visit dates for IM and FM visits, all Urology department visits in the CCHS, and all PSA test results in the study years. We used the reported PSA values in the structured laboratory results field in the EMR. To further focus our analysis on PSA tests performed for screening in primary care practices, we then excluded any PSA test result for a patient if he had a visit with a CCHS Urology department prior to the PSA test date each year and also excluded PSA tests ordered as “PSA DIAGNOSTIC” and “PSA FREE,” as well as any PSA test results associated with a specific symptom diagnosis code i.e., an ICD-9 code other than a V code.
For our analysis of primary provider variation in PSA testing, we used the group of IM or FM visit providers and assigned the primary care provider for each patient as the provider that the patient had seen most in the year the PSA blood test was drawn or the year prior. When there was an equal number of visits to more than one provider, the provider was assigned through random selection. For each year, we were able to choose the primary provider based upon greatest visit frequency 87% of the time, with the remaining providers assigned through random selection of providers with whom the patient had an equal number of visits. We limited all of our analyses to providers who had at least 100 unique patient visits per year in our dataset. Mixed-effects logistic regression models were used to adjust patient characteristics to analyze provider variation adjusted for patient age and race (African American vs. non-African-American).
We calculated rates of PSA screening tests in each study year stratified by the following age groups: >= 70, 50–69, and 40–49 based upon the recent ACP and AUA guideline recommendations against screening men >=age 70, and to use an individualized approach for younger men, starting at age 50 (ACP) or 55 (AUA) or earlier based upon risk factors. We then calculated rates of screening in men known to have higher risk for PCa and compared rates of screening in African American men vs. non-African American men and men with a family history of PCa vs. men without a family history in these age groups using the Chi-squared test.
To determine if screening rates in high risk groups were related to interaction with the health care system, we calculated and compared “ever-screened rates” for African American vs. non-African American men and for men with a family history of PCa vs. men without a family history, then calculated and compared number of visits per year with primary care (either IM or FM providers) as well as number of visits per year with a urology provider in these groups.
To assess frequency of follow-up PSA screening patterns for men who were screened in relation to timing of the USPSTF guidelines, we then created 2 cohorts of patients. The first cohort (2007–2010) had a PSA screening test in 2007–2008, to correspond with the timing of the initial USPSTF guidelines against screening men > age 75, and these men remained in the cohort in 2009–2010. The men in the second cohort (2011–2014) had a PSA screening test in 2011–2012, to correspond with the timing of the later USPSTF guidelines against PSA testing in any age group, and these men remained in the cohort in 2013–2014. Rescreening rates were assessed in this cohort by age and PSA level, to correspond with the recent NCCN guideline recommendations. We examined rescreening rates for men ages 45–75 with an initial PSA of less than 1 ng/ml and PSA between 1 to 3 ng/ml and also assessed rescreening rates for men > age 75 with a PSA level less than 3 ng/ml. We did not assess rescreening for men with PSA levels > 3 ng/ml as our dataset was created to assess primary care PCa screening patterns and excluded men who had a Urology department visit prior to the PSA each year. Our laboratory upper limit of normal was a PSA level of 4 ng/ml at the beginning of the study and was reduced to 2.59 ng/ml in the later years of the study, thus by design our dataset may have excluded most men with elevated PSA levels greater than 3 ng/ml who would have been referred to a urologist for an abnormal PSA level.
In addition, to determine if screening rates were related to a shift to increased screening intervals, we then analyzed biennial as well as quadrennial testing for men screened in 2008 vs. men first screened in 2010.
We calculated prostate biopsy rates by identifying prostate biopsies using procedure codes for prostate biopsy and resulted pathology reports in the EMR were also searched for “prostate.” Rates of prostate biopsy were determined for each year of the study by age group, African American race, and family history of PCa. These annual analyses were completed utilizing all men >=age 40 with a primary care visit each year as the denominator and using all prostate biopsy data. A second annual analysis was performed assessing rate of first prostate biopsy after a PSA test each year with only those men who had a screening PSA test in the denominator.
All analyses were conducted using R-Studio Version 3.0.2 (Boston, MA).
Results
Our study population included 160, 211 men age 40 years or older from January 2007 through December 2014. Rates of PSA screening tests declined from 2007 to 2014 in all age groups (Figure 1). For men >=age 70, rates declined from 22.8% to 8.9 % while for men age 40–49, rates declined from 11.0% to 4.6 %. In men ages 50–69, screening rates declined from 39.2 % to 20.0%. For African American men, the rates of screening were higher than for non-African American men in the 40–49 age group (Figure 2) but lower than non-African American men in the 50–69 age group (Figure 3). For men with a family history of PCa, the screening rates were also higher than those without a family history in the 40–49 age group (Figure 4) but not consistently higher in men age 50–69 (Figure 5). Throughout the study period, rates of PSA screening remained similar or lower for African American men than for non-African American men, decreasing from 27% in 2007 to 11% in 2014(Supplemental Table 1). For men with a family history of PCa, rates of PSA screening remained similar or slightly higher throughout the study period.
Figure 1.
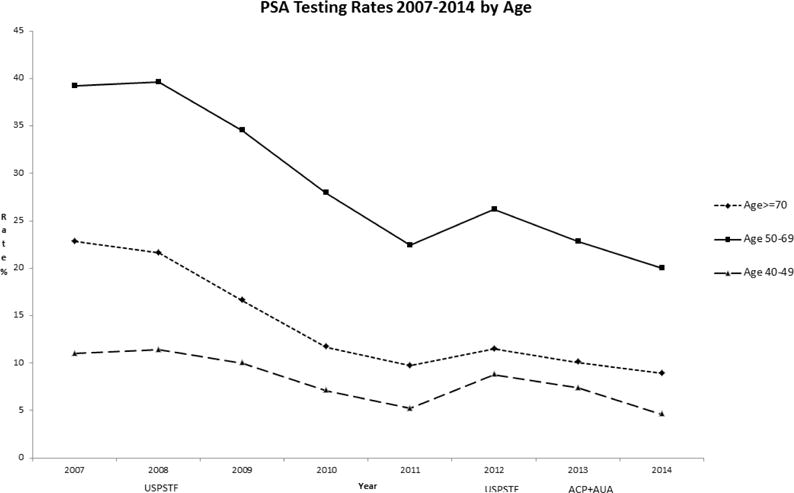
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
Figure 2.
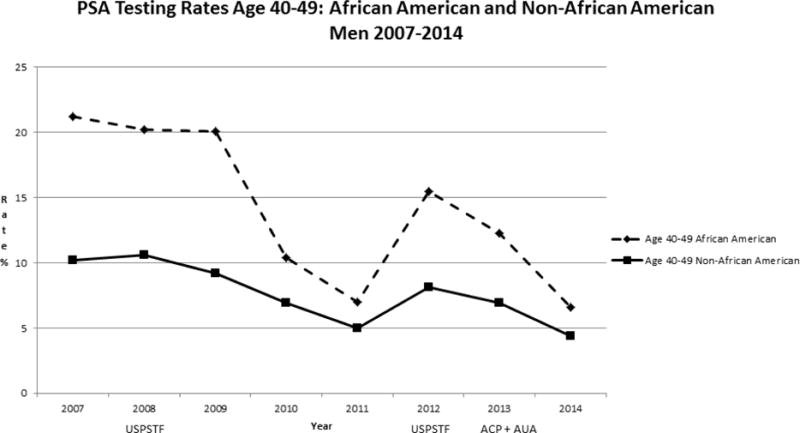
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
Figure 3.
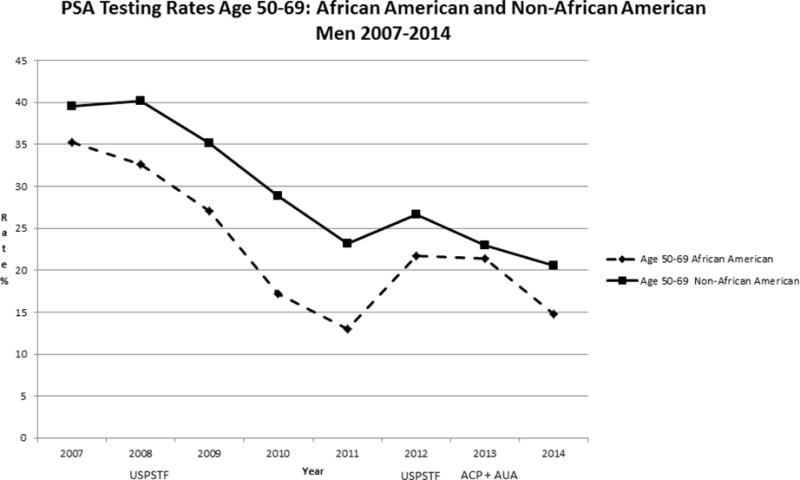
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
Figure 4.
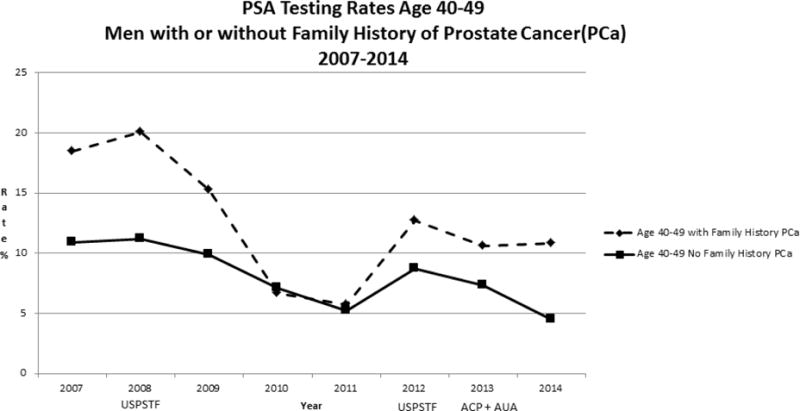
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
Figure 5.
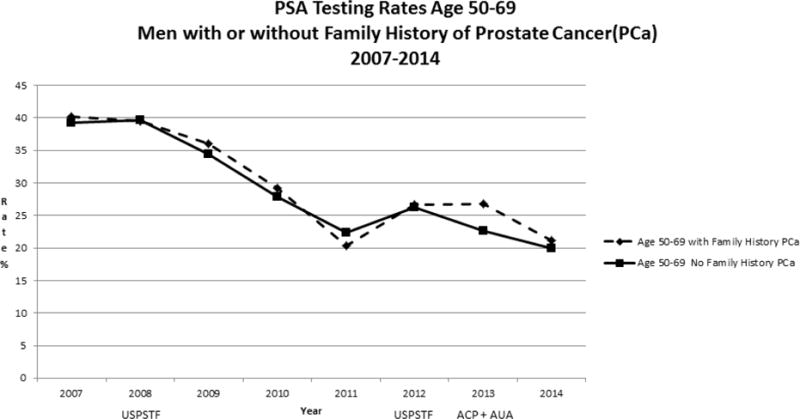
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
We also found that African American men were “ever-screened” for PCa less often: 26.1% (3909 of 14990) of African American men were screened in any of the study years vs. 28.8% (41798 of 145221) of Non-African American men, p<0.001. The mean number of primary care visits as well as urology visits per year for African American men, however, was greater than for non-African American men (2.11(Standard Deviation(SD)1.80) vs. 1.89(SD 1.57), respectively, p <0.001 for primary care visits and 0.29(SD 0.86) vs. 0.20(SD 0.63), respectively, p<0.001 for urology visits). For men with a family history of PCa, the “ever-screened” rate was higher than for men without a family history: 34.6% (1107/3202) men with a family history of PCa were screened in any of the study years vs. 28.4% (44600/157009) of men without a family history, p<0.001. The mean number of follow-up primary care visits per year was not significantly greater for men with a family history of PCa than for those without a family history (1.95(SD 1.46) vs. 1.91(SD 1.60), respectively, p 0.41) but the mean number of follow-up urology visits per year was greater (0.35(SD 0.92) vs. 0.21(SD 0.65, respectively, p <0.001).
Provider variation in ordering PSA screening tests remained large throughout our study period, ranging from 0% to a maximum of 51.9–62.2% depending on the year (Figure 6, Supplemental Table 2). The provider variation rates adjusted by patient age and race (African-American vs. non-African American) showed a similar pattern of variation (Supplemental Table 3).
Figure 6.
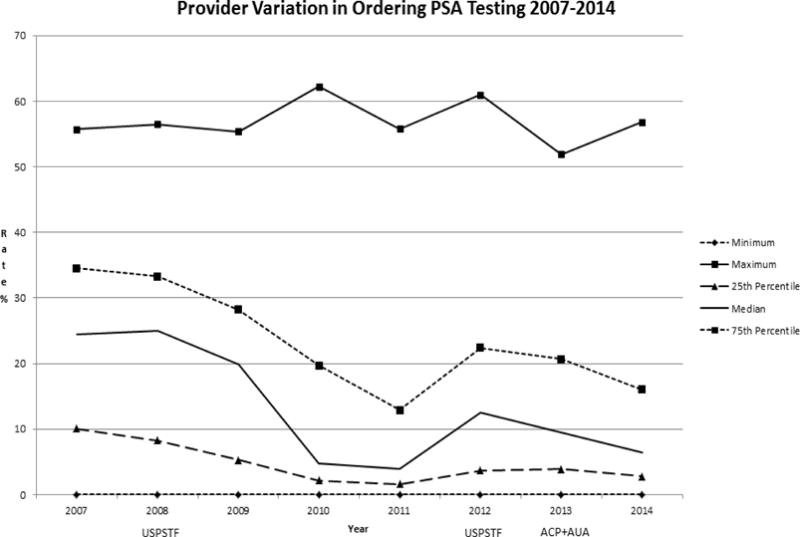
USPSTF = United States Preventive Services Task Force- Guidelines in 2008 and 2012
ACP = American College of Physicians - Guidelines in 2013
AUA = American Urologic Association - Guidelines in 2013
Analysis of 2 longitudinal cohorts between 2007–2014 showed that while the number of men screened declined in the in the 2011–2014 cohort compared to 2007–2010 cohort, and rates of follow-up PCa screening also declined, similar follow-up screening rates were noted for men age 45–75 with initial PSA levels < 1ng/ml or 1–3 ng/ml in both the earlier and later cohorts. Men with higher PSA levels were not more likely to be rescreened in either cohort, and men > age 75 with PSA less than 3ng/ml were rescreened at the same rate in both 2009–2010 and 2013–2014 (Tables 1 and 2).
Table 1.
Prostate Cancer Follow Up Screening Rates by PSA Level: 2007–2008 Cohort with Rescreening in 2009–2010
| Number of patients screened in 2007–2008 (N=17668) | Percent of patients rescreened in 2009–2010 | |
|---|---|---|
| Age 45–75, PSA <1 | 8298 | 44.4% |
| Age 45–75, PSA 1–3 | 6392 | 44.7% |
| Age > 75, PSA < 3 | 962 | 28.4% |
Table 2.
Prostate Cancer Follow Up Screening Rates by PSA Level: 2011–2012 Cohort with Rescreening in 2013–2014
| Number of patients screened in 2011–2012 (N=6881) | Percent of patients rescreened in 2013–2014 | |
|---|---|---|
| Age 45–75, PSA< 1 | 3352 | 31.9% |
| Age 45–75, PSA 1–3 | 2317 | 32.7% |
| Age >75, PSA< 3 | 214 | 27.6% |
For men screened in 2008, while the overall annual screening rates and by age group were lower in 2012, over half of men were rescreened once within a 4-year period, with lower rates for men >75 years. However, only 24% of men screened in 2008 were rescreened more than twice in 4 years (Table 3). For the much lower number of men first screened in 2010, 43% were rescreened within 4 years, with only 20% rescreened more than twice in 4 years. For men > age 75 the screening and rescreening rates were much lower in this later time period. (Table 4)
Table 3.
Annual, Biennial, and Quadrennial testing: Men screened in 2008 N=12305
| Years | Screening Rate All Age Groups |
Screening Rate Age 45–75 |
Screening Rate Age>75 |
|---|---|---|---|
| 2009 | 27% | 28% | 24% |
| 2010 | 21% | 22% | 12% |
| 2011 | 17% | 17% | 9% |
| 2012 | 22% | 23% | 8% |
| 2009–2010 | 39% | 40% | 30% |
| 2009–2012 | 51% | 53% | 34% |
| More than 2 rescreenings 2009–2012 | 24% | 25% | 12% |
Table 4.
Annual, Biennial, and Quadrennial testing: Men first screened in 2010 N=2782
| Screening Rate All Age Groups |
Screening Rate Age 45–75 |
Screening Rate Age >75 |
|
|---|---|---|---|
| 2011 | 16% | 17% | 11% |
| 2012 | 20% | 21% | 10% |
| 2013 | 18% | 19% | 4% |
| 2014 | 17% | 19% | 5% |
| 2011–2012 | 31% | 32% | 19% |
| 2011–2014 | 43% | 45% | 24% |
| More than 2 rescreenings 2011–2014 | 20% | 21% | 5% |
Utilizing all men >= age 40 with a primary care visit each year as the denominator, overall yearly rates of prostate biopsy were similar between 2007 and 2014. However, for men in the >=70 age group, biopsy rates decreased in 2014 compared to 2007 while for men with a family history of PCa, biopsy rates increased in 2014 compared to 2007(Table 5). When analyzing rates of first prostate biopsy after a PSA screening test, with all men who had a PSA test included in the denominator, the rates of prostate biopsies were increased for African American men and men with a family history of PCa in 2014 compared to 2007(Table 6).
Table 5.
Prostate Biopsy rates by year and subgroups
| Overall | Age 40–49 | Age 50–69 | Age>=70 | African American Men | Men with Family History of Prostate Cancer | |
|---|---|---|---|---|---|---|
| 2007 | 0.6 % | 0.1 % | 1.0 % | 0.6 % | 1.0 % | 0.6 % |
| 2008 | 0.5 % | < 0.1% | 0.8 % | 0.5 % | 0.8 % | 1.0 % |
| 2009 | 0.5 % | <0.1% | 0.8 % | 0.4 % | 1.0 % | 1.0 % |
| 2010 | 0.5 % | 0.1% | 0.7 % | 0.3 % | 0.4 % | 0.7 % |
| 2011 | 0.5 % | 0.1 % | 0.7 % | 0.4 % | 0.9 % | 0.4 % |
| 2012 | 0.6 % | 0.1 % | 0.9 % | 0.5% | 0.7 % | 1.0 % |
| 2013 | 0.5 % | 0.1 % | 0.8 % | 0.4 % | 0.9 % | 1.2 % |
| 2014 | 0.5 % | 0.1 % | 0.8 % | 0.3 % | 1.1 % | 1.8 % |
Table 6.
Prostate Biopsy rates for first biopsy after PSA test by year and subgroups for men who had a PSA test
| Overall | Age 40–49 | Age 50–69 | Age>=70 | African American Men | Men with Family History of Prostate Cancer | |
|---|---|---|---|---|---|---|
| 2007 | 1.6 % | 0.8 % | 1.6 % | 1.9 % | 2.4 % | 1.3 % |
| 2008 | 1.2 % | 0.2 % | 1.2 % | 1.6 % | 2.1 % | 2.0 % |
| 2009 | 1.3% | 0.3 % | 1.4 % | 1.5 % | 2.7 % | 1.7 % |
| 2010 | 1.4% | 0.7 % | 1.5 % | 1.8 % | 1.5 % | 2.9 % |
| 2011 | 1.4% | 0.8 % | 1.4 % | 1.9% | 3.2 % | 1.4 % |
| 2012 | 1.4% | 0.4 % | 1.4 % | 2.3 % | 1.5 % | 2.5 % |
| 2013 | 1.5% | 0.6 % | 1.6 % | 1.6 % | 2.0 % | 2.4 % |
| 2014 | 1.7% | 0.5 % | 1.9 % | 1.5 % | 4.8 % | 4.3 % |
PSA=prostate specific antigen
Discussion
In this retrospective study of primary care practices in a single health system, we found that yearly rates of PCa screening with the PSA test declined in all age groups after the initial USPSTF recommendation in 2008 against screening men over age 75. Biennial and quadrennial screening rates were also lower for men first screened in 2010 compared to those men screened in 2008. Our findings support a recent report that PSA testing was reduced among primary care physicians after 2010, (24) but differs from a report of recent trends in primary care PSA ordering patterns. (25) Interestingly, in all age groups, we saw a significant decrease in yearly screening rates until 2011, but noted an increase in rates in 2012, with continued decline after 2012. A possible explanation is that the 2011 decrease may have been related to the release of draft recommendations by the USPSTF in 2011 against screening in men of all ages, and that subsequent increased awareness of the topic of PCa screening at the time of the 2012 official publication of the USPSTF recommendation led to more frequent conversations with physicians and patients around PCa screening and thus an increase in ordering PSA testing in 2012.
Notably, we found that yearly PSA screening rates continued to decrease in younger men after the ACP and AUA recommendations in 2013 to use a shared decision making approach to screening in men under the age of 70, declining even in men with known risk factors for PCa. As discontinuing all PCa screening may lead to an increase in avoidable cancer deaths (26) strategies for risk based screening are currently advocated.(27–31) In men ages 40–49, the screening rates were higher in men with risk factors for PCa, including African American men and in those with a family history of PCa, suggesting that some individualized risk assessment or shared decision making may occur with early screening in these men, but screening rates still continued to decrease. However, men with a family history of PCa ages 50–69 were screened with the PSA at rates similar to men without a family history, and much lower than prior to the initial USPSTF recommendations in 2008.
Our finding of lower rates of screening among African American men compared to non-African American men in the 50–69 age group is concerning. African American race is associated with greater biochemical failure after radical prostatectomy for low grade PCa(32) and also predicted adverse pathologic features and pathologic upgrading in men with very low grade PCa who had radical prostatectomy.(33) Thus failure to diagnose even low risk PCa may adversely affect African American men disproportionately and actually shortening PSA screening intervals may lessen the number of African American men diagnosed with advanced disease.(34)
Regular access to a healthcare provider has been associated with PSA screening in African American men.(35,36) In our study, we found that despite having significantly greater number of visits per year with both primary care and urology providers African American men still had lower rates of screening, possibly related to the variation in rates of offering of screening by providers or decreased acceptance of screening by African American men, even with increased visits to a primary provider.
On the other hand, men with a family history of PCa did not have a significantly greater number of visits with primary care than those without a family history, but were generally screened equally or more often, perhaps related to the greater number of visits with a urologist.
The median rates of provider ordering of PSA screening as well as the 25th and 75th percentiles rates decreased in general after the 2008 USPSTF recommendation. While it is not possible in this study to determine the level of shared decision making taking place around PCa screening, the variation in provider ordering of PSA screening, with the minimal ordering rate of 0% in each study year, and maximum ordering rates which appear to be temporally unrelated to timing of guideline recommendations for screening, suggests that screening decisions may have been at least in part provider-driven throughout the study period.
Although total screening rates were lower, our findings suggest that the providers who continue to screen patients may not be following a risk based screening approach. The wide variation in provider ordering persisted even after adjustment for patient age and race (African American vs non-African American). Our findings of decreased annual screening as well as biennial and quadrennial rescreening rates seem to be temporally related to the USPSTF recommendations against PCa screening. We found similar rescreening rates in men with PSA levels less than 1ng/ml or 1–3 ng/ml. PSA retesting rates in men > age 75 with a PSA level less than 3ng/ml were similar in the earlier and later cohorts, again suggesting that risk-based screening is not occurring. Overall our findings suggest multiple factors other than PSA levels contributing to changing rates of PSA screening with evolving guidelines including age, risk factors (African American race, Family history of PCa), and provider ordering patterns. The more recent recommendations for intervals of rescreening based upon previous PSA level do not appear to be followed by primary care providers, likely related to lack of awareness of these guidelines; if followed, these guidelines could substantially change practice.
Our finding that rates of first prostate biopsy for men who were screened with a PSA test were higher in those men with known risks for PCa in 2014 compared to 2007 suggests that possibly risk-based biopsy practices are being followed. Nonetheless, the PSA screening rates in all men, even those with risk factors for PCa, decreased over the 2007–2014 time period suggesting that the intervention truly needed at this time would be to encourage initial risk-based screening practices.
Limitations
Our analysis represents PCa screening patterns in primary care practices in one health system and thus may not be generalizable to other geographic locations. In addition, our ability to capture family history of PCa, or PSA tests ordered for non-screening purposes is dependent on provider documentation practices, which may have evolved over the time period of our study. Our attribution of primary provider associated with PSA testing to the provider with whom the patient had the greatest frequency of visits in the year of and year prior to the PSA test may not be the provider whom the patient considers to be their PCP. However, our decision was based on the assumption that the primary provider who had the most contact with the patient around the timing of the PSA test may have been the most influential in the patient’s care. Finally, our analyses could not control for patients who were seen by a urologist not in our healthcare system, and are limited to men who had PSA test or prostate biopsy performed within our health system.
Conclusion
Rates of PCa screening declined from 2007 to 2014, even in higher risk groups, with large variation of PSA testing practices among primary providers, and without clear evidence for risk-based screening practices. However, rates of first prostate biopsy for men who were screened were higher in those men with known risks for PCa in 2014 compared to 2007. Future work should further explore reasons for variation in PCa screening, and encourage collaboration among primary care physicians and urologists, to implement shared decision making and appropriate risk-based strategies for PCa screening in primary care practices, especially in men with a family history of PCa and in African American men.
Supplementary Material
Acknowledgments
These results were presented in part as an oral presentation at the Society of General Internal Medicine National Meeting in Hollywood, FL on May 14, 2016.
This publication was made possible by the Clinical and Translational Science Collaborative (CTSC) of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glen B. Taksler, PhD is funded by the CTSC/NCATS grant KL2TR000440.
We would like to thank Paul McVey B. A. for his assistance with data extraction for this project.
Footnotes
The authors report no conflict of interest.
References
- 1.SEER Stat Fact Sheets: Prostate Cancer [Internet] Surveillance, Epidemiology, and End Results Program. Available from: http://seer.cancer.gov/statfacts/html/prost.html.
- 2.Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014 Oct;15(11):e484–92. doi: 10.1016/S1470-2045(14)70211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012 Jan 18;104(2):125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. The Lancet. 2014 Dec;384(9959):2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005 May;173(5):1701–5. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 6.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016 Sep 14;(0):0. doi: 10.1056/NEJMoa1606221. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008 Aug 5;149(3):185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 8.Moyer VA, U. S. Preventive Services Task Force Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jul 17;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P. Screening for Prostate Cancer: A Guidance Statement From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013 May 21;158(10):761–9. doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 10.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early Detection of Prostate Cancer: AUA Guideline. J Urol. 2013 Aug;190(2):419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guidelines in Oncology [Internet] 2016 Oct 6; Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection.
- 12.Ross JS, Wang R, Long JB, Gross CP, Ma X. Impact of the 2008 US Preventive Services Task Force Recommendation to Discontinue Prostate Cancer Screening Among Male Medicare Beneficiaries. Arch Intern Med. 2012 Nov 12;172(20):1601–3. doi: 10.1001/archinternmed.2012.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, Lin DW. Influence of Publication of US and European Prostate Cancer Screening Trials on PSA Testing Practices. J Natl Cancer Inst. 2011 Mar 16;103(6):520–3. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Friderici J, Stefan MS, Rothberg MB. Impact of the 2008 U.S. Preventative Services Task Force recommendation on frequency of prostate-specific antigen screening in older men. J Am Geriatr Soc. 2014 Oct;62(10):1912–5. doi: 10.1111/jgs.13061. [DOI] [PubMed] [Google Scholar]
- 15.Wallner LP, Hsu J-WY, Loo RK, Palmer-Toy DE, Schottinger JE, Jacobsen SJ. Trends in Prostate-specific Antigen Screening, Prostate Biopsies, Urology Visits, and Prostate Cancer Treatments From 2000 to 2012. Urology. 2015 Sep 1;86(3):498–505. doi: 10.1016/j.urology.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Scosyrev E, Wu G, Golijanin D, Messing E. Prostate-specific antigen testing in older men in the USA: data from the behavioral risk factor surveillance system. BJU Int. 2012 Nov 1;110(10):1485–90. doi: 10.1111/j.1464-410X.2012.11013.x. [DOI] [PubMed] [Google Scholar]
- 17.Prasad SM, Drazer MW, Huo D, Hu JC, Eggener SE. 2008 us preventive services task force recommendations and prostate cancer screening rates. JAMA. 2012 Apr 25;307(16):1692–4. doi: 10.1001/jama.2012.534. [DOI] [PubMed] [Google Scholar]
- 18.Sammon JD, Pucheril D, Diaz M, et al. COntemporary nationwide patterns of self-reported prostate-specific antigen screening. JAMA Intern Med. 2014 Nov 1;174(11):1839–41. doi: 10.1001/jamainternmed.2014.4117. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson R, Akhtar A, Haridas J, Bhat D, Roehrborn C, Lotan Y. Testing and referral patterns in the years surrounding the US Preventive Services Task Force recommendation against prostate-specific antigen screening. Cancer. 2016 Sep 22; doi: 10.1002/cncr.30330. [DOI] [PubMed] [Google Scholar]
- 20.Jaramillo E, Tan A, Yang L, Kuo Y, Goodwin JS. VAriation among primary care physicians in prostate-specific antigen screening of older men. JAMA. 2013 Oct 16;310(15):1622–4. doi: 10.1001/jama.2013.277514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang VL, Shi Y, Fung K, et al. CLinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med [Internet] 2016 Apr 4; doi: 10.1001/jamainternmed.2016.0695. [cited 2016 Apr 5]; Available from: http://dx.doi.org/10.1001/jamainternmed.2016.0695. [DOI] [PMC free article] [PubMed]
- 22.Philips GK, Reinier K, Ashikaga T, Luebbers RA. Attitudes and beliefs of primary care physicians regarding prostate and colorectal cancer screening in a rural state. J Cancer Educ Off J Am Assoc Cancer Educ. 2005;20(3):167–72. doi: 10.1207/s15430154jce2003_11. [DOI] [PubMed] [Google Scholar]
- 23.Tasian GE, Cooperberg MR, Cowan JE, Keyashian K, Greene KL, Daniels NA, et al. Prostate specific antigen screening for prostate cancer: Knowledge of, attitudes towards, and utilization among primary care physicians. Urol Oncol Semin Orig Investig. 2012 Mar;30(2):155–60. doi: 10.1016/j.urolonc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Zavaski ME, Meyer CP, Sammon JD, et al. DIfferences in prostate-specific antigen testing among urologists and primary care physicians following the 2012 uspstf recommendations. JAMA Intern Med [Internet] 2016 Feb 8; doi: 10.1001/jamainternmed.2015.7901. [cited 2016 Feb 10]; Available from: http://dx.doi.org/10.1001/jamainternmed.2015.7901. [DOI] [PubMed]
- 25.Hutchinson R, Akhtar A, Haridas J, Bhat D, Roehrborn C, Lotan Y. Testing and referral patterns in the years surrounding the US Preventive Services Task Force recommendation against prostate-specific antigen screening. Cancer. 2016 Sep 1; doi: 10.1002/cncr.30330. n/a – n/a. [DOI] [PubMed] [Google Scholar]
- 26.Gulati R, Tsodikov A, Etzioni R, Hunter-Merrill RA, Gore JL, Mariotto AB, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014 Nov 15;120(22):3519–26. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers AJ. Does prostate-specific antigen screening do more good than harm?: Depends on how you do it. JAMA Oncol [Internet] 2016 Mar 24; doi: 10.1001/jamaoncol.2015.6276. [cited 2016 Mar 26]; Available from: http://dx.doi.org/10.1001/jamaoncol.2015.6276. [DOI] [PubMed]
- 28.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016 May 1;91:12–8. doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talcott JA. Prostate Cancer. Ann Intern Med. 2015 Dec 1;163(11) doi: 10.7326/AITC201512010. ITC1. [DOI] [PubMed] [Google Scholar]
- 30.Preston MA, Batista JL, Wilson KM, Carlsson SV, Gerke T, Sjoberg DD, et al. Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. J Clin Oncol. 2016 Jun 13;:JCO667527. doi: 10.1200/JCO.2016.66.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson IM, Jr, Leach RJ, Ankerst DP. FOcusing psa testing on detection of high-risk prostate cancers by incorporating patient preferences into decision making. JAMA. 2014 Sep 10;312(10):995–6. doi: 10.1001/jama.2014.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamoah K, Deville C, Vapiwala N, Spangler E, Zeigler-Johnson CM, Malkowicz B, et al. African American men with low-grade prostate cancer have increased disease recurrence after prostatectomy compared with Caucasian men. Urol Oncol. 2015 Feb;33(2):70.e15–22. doi: 10.1016/j.urolonc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American Men With Very Low–Risk Prostate Cancer Exhibit Adverse Oncologic Outcomes After Radical Prostatectomy: Should Active Surveillance Still Be an Option for Them? J Clin Oncol. 2013 Aug 20;31(24):2991–7. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control. 2010 Jul 1;21(7):1071–80. doi: 10.1007/s10552-010-9535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sammon JD, Dalela D, Abdollah F, Choueiri TK, Han PK, Hansen M, et al. Determinants of Prostate Specific Antigen Screening among Black Men in the United States in the Contemporary Era. J Urol [Internet] 2015 Nov 17; doi: 10.1016/j.juro.2015.11.023. [cited 2016 Mar 8];0(0). Available from: http://www.jurology.com/article/S0022534715052374/abstract. [DOI] [PubMed]
- 36.Hosain GMM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in predictors of PSA screening in a tri-ethnic population. Cent Eur J Public Health. 2011 Mar;19(1):30–4. doi: 10.21101/cejph.a3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


