Abstract
Objective
To examine sites of initial recurrence in patients after resection of gastric and gastroesophageal junction Siewert II/III adenocarcinoma (GA).
Summary Background Data
There are few recent studies on recurrence for Western patients following potentially curative resection of GA.
Methods
A review of a prospectively maintained, single institution database was performed. Clinicopathologic factors, site(s) of initial recurrence, disease-free survival (DFS), and overall survival (OS) were examined.
Results
From January 2000–June 2010, 957 patients underwent potentially curative resection for GA, 435 patients (46%) had recurrent disease, and complete data on recurrence site(s) could be obtained in 386 patients. Tumors were Lauren intestinal type in 206 (53%) and diffuse or mixed-type in 180 (47%). Median time to recurrence was 12 months and 75% of recurrences occurred within 2 years. There was a significant difference in pattern of initial recurrence between the intestinal and diffuse/mixed cohorts (p = <0.001). For intestinal tumors, distant metastasis was the most common site (54%), followed by locoregional (20%), peritoneal (15%), and multifocal (11%). For diffuse/mixed tumors, peritoneal recurrence was the most common (37%), followed by distant (32%), locoregional (22%), and multifocal (9%). On multivariate analysis, Lauren histologic type was the only significant factor that was associated with both peritoneal recurrence (diffuse, HR 2.22, CI 1.38–3.94) and distant recurrence (intestinal, HR 1.888, CI 1.202–2.966). After recurrence, median OS was only 8.4 months.
Conclusion
In GA patients who recur after resection, patterns of recurrence vary significantly based on Lauren histologic type.
For patients with gastric adenocarcinoma, an improved ability to predict sites of recurrence after surgical resection may help determine adjuvant treatment and surveillance options. In our analysis of 386 patients with recurrence after potentially curative resection at a single Western institution, Lauren histologic type (intestinal vs. diffuse/mixed) was the most important factor in the pattern of initial recurrence.
INTRODUCTION
There are about one million cases of gastric cancer worldwide per year and over 700,000 deaths per year, making gastric cancer the fifth most common cancer and the third leading cause of cancer death.1 In the United States alone, there were an estimated 24,590 new cases and 10,720 deaths related to gastric cancer in 2015.2 Except in a few Asian countries such as Japan and South Korea where there is endoscopic screening for gastric adenocarcinoma, the majority of gastric adenocarcinoma patients present with locally advanced or metastatic disease. Survival rates after potentially curative surgery vary significantly between Asian and Western countries, with 3-year overall survival rates in prospective trials with surgery alone being about 70–80% in Asian countries and 30–40% in Western countries.3–6 Adjuvant chemotherapy or chemoradiation can improve absolute overall survival by 9–15%.
In Western countries, overall survival for patients with metastatic gastric adenocarcinoma is 3–5 months with best supportive care.7 The response rate to multi-agent chemotherapy is 50% or greater, but nearly all patients develop chemotherapy resistance, and median survival is extended only to 9–11 months.8 Patients who develop recurrence after potentially curative surgery also have limited survival. In a prior study from our institution, 77% patients with recurrence after potentially curative surgery were dead within one year.9
In 1965, Lauren described two distinct histological types of gastric adenocarcinomas: intestinal and diffuse.10 The intestinal type exhibits components of glandular, solid, or intestinal architecture as well as tubular structures. This type is more common in men and older patients, and is associated with environmental exposures such as Helicobacter pylori (H. pylori) infection. The diffuse type demonstrates single cells or poorly cohesive cells infiltrating the gastric wall, and progressive disease can ultimately lead to linitis plastica (a.k.a. leather bottle stomach). This type is more common in women and in younger patients and more associated with familial occurrence. Recent broad molecular analyses of gastric adenocarcinoma have discovered that intestinal and diffuse type tumors have quite different genomic profiles, with intestinal tumors often harboring chromosomal instability and diffuse tumor often being genomically stable.11
Prognostic factors for recurrence following potentially curative resection of gastric adenocarcinoma have been extensively investigated. The pattern of recurrence, especially in large series of Western patients, has been much less examined.12 Furthermore over the past 15 years, the increased use of neoadjuvant and adjuvant therapies, along with the development of higher resolution imaging technologies may have altered the site and detection of recurrences.13 We sought to examine recurrence patterns in patients at our institution following surgical resection in a contemporary cohort of patients. Because of the distinct histological, clinical, and genomic differences between intestinal and diffuse type tumors, we hypothesized that patterns of recurrence following potentially curative resection of gastric adenocarcinoma would vary significantly based on Lauren histologic type.
METHODS
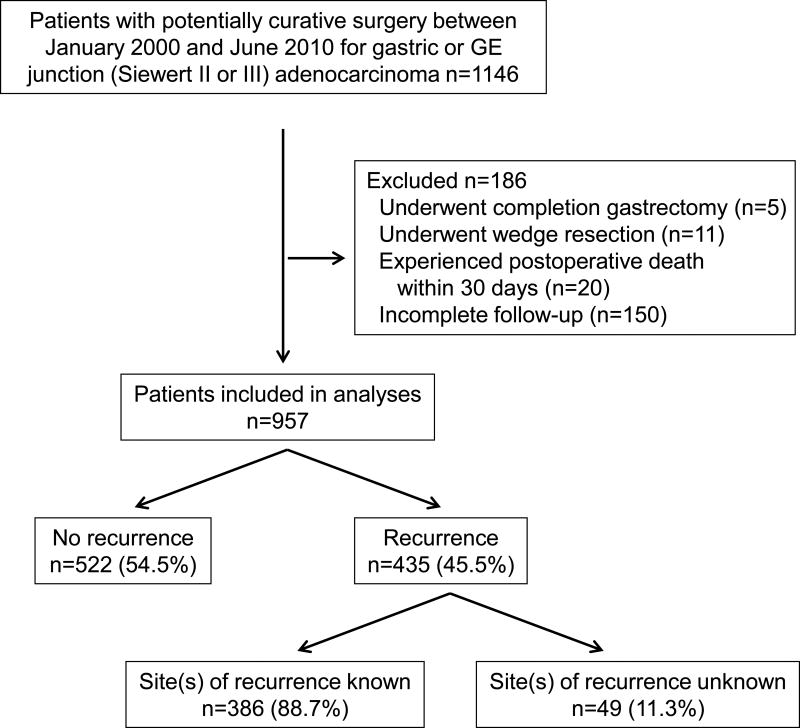
A retrospective review was performed of a prospectively maintained gastric cancer database at Memorial Sloan Kettering Cancer Center following Internal Review Board (IRB) approval. We initially examined 1,146 patients with gastric or gastroesophageal junction (Siewert type II or III) adenocarcinoma without metastatic disease who underwent potentially curative resection (i.e., R0 resection) between January 2000 and June 2010 (Fig. 1). Patients who underwent completion gastrectomy or wedge resection, who experienced postoperative death within 30 days, or had incomplete follow-up were also excluded. Of the remaining 957 patients, 522 (54.5%) had no recurrence and 435 (45.5%) had recurrence. The final study population included 386 patients who experienced recurrent disease and for whom the site(s) of recurrence was documented.
Figure 1.
CONSORT diagram of study population.
Patients who underwent surgery at MSKCC were from around the United States and also from abroad. For those patients who received followup at MSKCC, this generally included clinic visits every 3 months for two years and then every 6 months in year 3–5. Labs were obtained at each clinic visit and a chest/abdomen/pelvis CT scan was obtained every other visit. Followup was similar in patients with intestinal and diffuse/mixed tumors. For all such patients, the timing of recurrence and site or sites of recurrence were documented. Some patients received followup outside of MSKCC. Of patients followed outside MSKCC, 150 patients were excluded because we did not have any followup information on these patients. For another 49 patients followed outside of MSKCC, we received information that they suffered recurrence but we did not receive complete information regarding the site or sites of recurrence.
Patient characteristics and clinicopathologic data
Demographic and clinicopathologic characteristics and treatment of the study population were determined by review of the database and of the medical records. Tumor stage was determined according to the 7th edition of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) classification system.14 For patients with proximal gastric or gastroesophageal junction (Siewert type II or III) tumor, the AJCC gastric adenocarcinoma staging system was used rather than the esophageal adenocarcinoma staging system given several studies suggest the gastric system is more accurate.15 In patients with multiple synchronous gastric cancers (n=23), the lesion with the deepest infiltration of the gastric wall was considered to be the index tumor. Lymph node ratio was defined as the number of positive nodes divided by the number of examined nodes.
Perioperative treatment was defined as preoperative chemotherapy, preoperative chemoradiotherapy, postoperative chemotherapy, and/or postoperative chemoradiation. For the purpose of characterizing the extent of lymphadenectomy the 1998 Japanese Gastric Cancer Association definitions of D1 and D2 lymphadenectomy were used.16
The Laurén classification separates gastric adenocarcinomas into two primary subtypes, intestinal and diffuse, and tumors exhibiting features of both the intestinal and diffuse types (>25% of either component) are designated as mixed-type adenocarcinoma. The intestinal type is characterized by the formation of glands exhibiting various degrees of differentiation either with or without extracellular mucin production. The diffuse type is composed of poorly cohesive cells without gland formation. This type of tumor often may contain cells with or without intracytoplasmic mucin, known as “signet ring cells”.
Definition and categorization of recurrence
Recurrences were categorized by the site involved: locoregional, peritoneal, distant, or multiple. The presence of recurrent disease in two or more sites was defined as multiple. Multiple recurrences in the same site were not categorized as “multiple” sites of recurrence.
Locoregional recurrence included masses in the gastric bed, D2 lymphadenectomy nodal stations, or anastomotic recurrence. Peritoneal recurrence was documented by positive cytology in ascitic fluid or by convincing presence of peritoneal nodules on cross-sectional imaging as determined by the radiology report. Distant metastases were further defined according to the specific organ involved. Disease involving the cervical lymph nodes or abdominal nodes beyond the upper retroperitoneum was considered distant metastasis. Mediastinal lymph node recurrence was considered locoregional for gastroesophageal junction tumors and distant recurrence for all other tumors. Tumors involving the ovaries were considered peritoneal recurrence and classified as Krukenberg tumors.17 All recurrences were documented by pathologic diagnosis and/or radiologic imaging. Radiologic proof of recurrence was specifically reviewed in the context of the clinical situation and typically required sequential imaging demonstrating progression of metastatic lesions.
Outcome data and statistical analysis
The primary endpoint of the study was tumor recurrence pattern. Deaths from any cause and disease-related deaths (defined as death from recurrence) were analyzed. Overall survival (OS) was calculated from the date of operation to death from any cause. Disease-free survival (RFS) was calculated from the date of operation to the date of tumor recurrence or death with evidence of recurrence. For RFS, patients who died without known tumor recurrence were censored at the last documented evaluation. Patients were followed until death or the cut-off date of June 30, 2015. Patients with at least one followup visit/note and then subsequently lost to follow-up were treated as censored.
Descriptive statistical analysis was performed by IBM SPSS Statistics software, 64-bit version 22.0.0 (IBM Corp.). Continuous variables were compared using the Student’s t test, and categorical variables were analyzed using the Pearson’s chi square test. Logistic regression was used for multivariate analysis. Survival curves were generated by the Kaplan–Meier method and analyzed using the log rank test.15 Statistical significance was set at p<0.05.18
RESULTS
Clinicopathologic characteristics and treatment
In this study, we first reviewed 957 patients with gastric or gastroesophageal junction (Siewert II or III) adenocarcinoma who underwent potentially curative resection between January 2000 and June 2010 at our institution, met our inclusion criteria, and had follow-up information (Fig. 1). Five hundred twenty two patients (54.5%) had no evidence of recurrence at last follow-up and 435 patients (45.5%) developed recurrent disease. Among the patients with recurrent disease, complete data on site(s) of recurrence could be obtained for 386 subjects (89%). Demographic and clinicopathological characteristics for these 386 patients are outlined in Table 1. Median age was 66 years (range, 24–89 years), and 267 patients (69%) were male. Tumors were located in the mid or lower third of the stomach in 149 patients (38.6%) and the proximal stomach or GE junction (Siewert II or III) in 230 patients (59.6%). Seven patients (1.8%) had tumor diffusely involving the entire stomach.
TABLE 1.
Clinicopathologic Factors According to Lauren Classification
| All (N=386) |
Intestinal (N=206) |
Diffuse/Mixed (N=180) |
p value | |
|---|---|---|---|---|
| Age | 0.078 | |||
| Median (range) | 66 (24–89) | 67 (24–89) | 63 (30–88) | |
|
| ||||
| Gender | 0.001 | |||
| Male | 267 (69.3) | 158 (76.7) | 109 (60.6) | |
| Female | 119 (30.8) | 48 (23.3) | 71 (39.4) | |
|
| ||||
| Tumor size (cm, median, range) | 3.8 (0 – 23.0) | 3.6 (0 – 13.0) | 4.0 (0 – 23.0) | 0.001 |
|
| ||||
| Location | <0.001 | |||
| Lower 1/3 | 77 (19.9) | 35 (17.0) | 42 (23.3) | |
| Mid 1/3 | 72 (18.7) | 24 (11.7) | 48 (26.7) | |
| Upper 1/3 | 63 (16.3) | 26 (12.6) | 37 (20.6) | |
| GE junction* | 167 (43.3) | 121 (58.7) | 46 (25.6) | |
| Whole | 7 (1.8) | 0 | 7 (3.9) | |
|
| ||||
| Preoperative T stage† | 0.979 | |||
| T1 | 49 (12.7) | 24 (12.8) | 25 (14.3) | |
| T2 | 71 (18.4) | 37 (19.7) | 34 (19.4) | |
| T3 | 239 (61.9) | 125 (66.5) | 114 (65.1) | |
| T4 | 4 (1.0) | 2 (1.1) | 2 (1.1) | |
|
| ||||
| Differentiation | <0.001 | |||
| Well/Moderate | 120 (31.1) | 114 (55.3) | 6 (3.3) | |
| Poorly/Signet ring cell | 266 (68.9) | 92 (44.7) | 174 (96.7) | |
|
| ||||
| Vascular invasion | 0.004 | |||
| No | 154 (42.7) | 96 (46.6) | 58 (32.2) | |
| Yes | 232 (60.1) | 110 (53.4) | 122 (67.8) | |
|
| ||||
| Neural invasion | <0.001 | |||
| No | 165 (42.7) | 118 (57.3) | 47 (26.1) | |
| Yes | 221 (57.3) | 88 (42.7) | 133 (73.9) | |
|
| ||||
| Pathologic T stage | <0.001 | |||
| T1 | 55 (14.3) | 36 (17.5) | 19 (10.6) | |
| T2 | 49 (12.7) | 34 (16.5) | 15 (8.3) | |
| T3 | 151 (39.1) | 95 (46.1) | 56 (31.1) | |
| T4 | 131 (33.9) | 41 (19.9) | 90 (50.0) | |
|
| ||||
| Pathologic N stage | <0.001 | |||
| N0 | 123 (31.9) | 78 (37.9) | 45 (25.0) | |
| N1 | 80 (20.7) | 54 (26.2) | 26 (14.4) | |
| N2 | 74 (19.2) | 41 (19.9) | 33 (18.3) | |
| N3 | 109 (28.2) | 33 (16.0) | 76 (42.2) | |
|
| ||||
| Number of positive lymph nodes | <0.001 | |||
| Median (range) | 2 (0–63) | 1 (0–29) | 5 (0–63) | |
|
| ||||
| Number of examined lymph nodes | 0.011 | |||
| Median (range) | 20 (2–67) | 20 (2–58) | 21 (4–67) | |
|
| ||||
| Lymph node ratio | <0.001 | |||
| Median (range) | 0.1 (0–1.0) | 0.06 (0–0.9) | 0.2 (0–1.0) | |
|
| ||||
| Pathologic TNM Stage | 0.001 | |||
| I | 62 (16.1) | 44 (21.4) | 18 (10.0) | |
| II | 99 (25.6) | 59 (28.6) | 40 (22.2) | |
| III | 225 (58.3) | 103 (50.0) | 122 (67.8) | |
Significant factors in bold.
Gastroesophageal junction, Siewert II or III.
Preoperative T stage of 23 patients was unknown.
Of the 386 tumors, 206 (53.4%) were Lauren intestinal type and 180 (46.6%) tumors were Lauren diffuse or mixed type; clinicopathologic factors for patients with intestinal vs. diffuse/mixed tumors are shown in Table 1. Compared to the intestinal cohort, patients with diffuse/mixed-type GA were more commonly female and had tumors that were larger in size, primarily poorly differentiated or had signet ring cells, had more vascular and neural invasion, and had more advanced TNM stage. Patients with intestinal tumors also had less positive nodes and a lower lymph node ratio.
For surgical resection, patients underwent a distal gastrectomy (27.7%), total or proximal gastrectomy (26.9%), or esophagogastrectomy (45.4%) (Table 2). Ninety-three percent of patients had a D2 lymphadenectomy. About two-thirds of patients received some form of adjuvant treatment with 50.8% receiving preoperative treatment and 17.1% receiving postoperative treatment. Neoadjuvant or adjuvant therapy included chemotherapy only in 28.8% of patients and chemoradiation in 39.1% of patients. Intestinal-type tumors were more often in the GEJ, compared to diffuse/mixed-type tumors, which were more commonly gastric. As such, compared to patients with intestinal tumors, patients with diffuse/mixed tumors less commonly underwent esophagogastrectomy and more commonly received chemotherapy rather than chemoradiation.
TABLE 2.
Treatment Factors According to Lauren Classification
| All (N=386) |
Intestinal (N=206) |
Diffuse/Mixed (N=180) |
p value | |
|---|---|---|---|---|
| Type of Gastrectomy | <0.001 | |||
| Distal subtotal gastrectomy | 107 (27.7) | 49 (23.8) | 58 (32.2) | |
| Total or proximal gastrectomy | 104 (26.9) | 36 (17.5) | 68 (37.8) | |
| Esophagogastrectomy‡ | 175 (45.4) | 121 (58.7) | 54 (30.0) | |
|
| ||||
| Lymphadenectomy | 0.626 | |||
| D1 | 12 (3.1) | 8 (3.9) | 4 (2.0) | |
| D1+ | 16 (4.1) | 8 (3.9) | 8 (4.4) | |
| D2 | 358 (92.8) | 190 (92.2) | 168 (93.3) | |
|
| ||||
| Perioperative Treatment | 0.062 | |||
| None | 124 (32.1) | 66 (32.0) | 58 (32.2) | |
| Preoperative | 196 (50.8) | 113 (54.9) | 83 (46.1) | |
| Postoperative | 66 (17.1) | 27 (13.1) | 39 (21.7) | |
|
| ||||
| Perioperative Treatment | <0.001 | |||
| None | 124 (32.1) | 66 (32.0) | 58 (32.2) | |
| Chemotherapy | 111 (28.8) | 39 (18.9) | 72 (40.0) | |
| Chemoradiotherapy | 151 (39.1) | 101 (49.0) | 50 (27.8) | |
Significant factors in bold.
Pattern of recurrence for intestinal and diffuse type tumors
We initially performed an initial analysis of recurrence patterns for patients with intestinal, diffuse, and mixed tumors, and found that the recurrence pattern for patients with mixed tumors was similar to that of patients with diffuse tumors. Thus patients with mixed and diffuse tumors were combined for subsequent analyses.
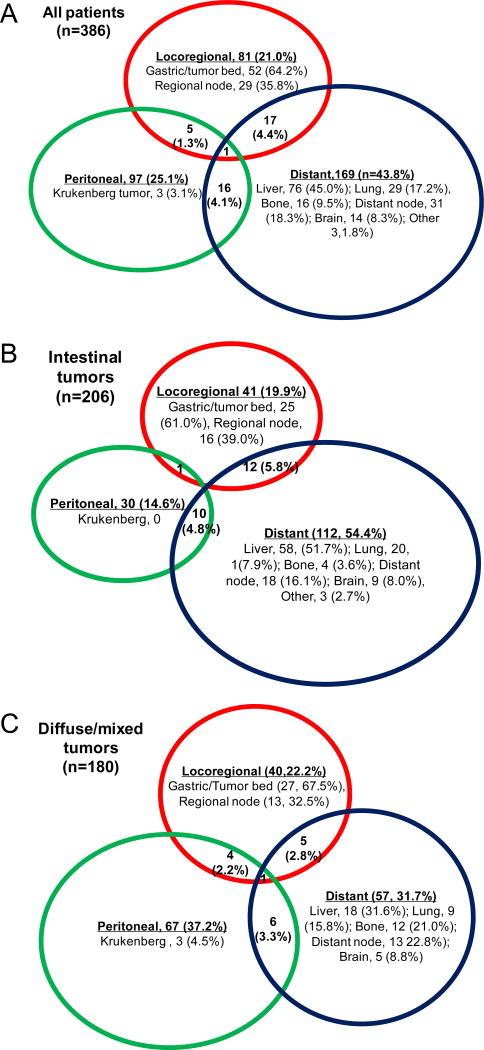
Figure 2A illustrates the distribution of initial recurrence sites for all patients. Most patients (89.9%) had initial recurrence involving only a single site; 38 patients (9.8%) had initial recurrence involving two sites, and one patient (0.3%) had initial recurrence involving all three sites. There was a significant difference in the pattern of recurrence between the intestinal and diffuse/mixed cohorts (Fig. 2B, C). Distant metastasis was the most common site of initial recurrence in patients with intestinal-type tumors (54.4%), followed by locoregional (19.9%) and peritoneal (14.6%). Recurrence was multifocal in 11.1%. For diffuse/mixed-type tumors, peritoneal recurrence the most common (37.2%), followed by distant recurrence (31.7%), locoregional recurrence (22.2%), and multifocal recurrence (8.9%). In patients with intestinal-type tumors, the most common distant recurrence site by far was the liver (61.0%) followed by the lung (17.9%) and distant lymph nodes (16.1%) (Fig. 2B). In contrast for diffuse/mixed-type tumors, sites of distant recurrence were more evenly distributed and included the liver (31.6%), distant lymph nodes (22.8%), and bone (21.1%) (Fig. 2C).
Figure 2.
Venn diagram of recurrence patterns in all 386 patients (A), 206 patients with intestinal tumors (B), and 180 patients with diffuse/mixed tumors (C). Sizes of circles are proportional to number of patients.
Disease-free and overall survival
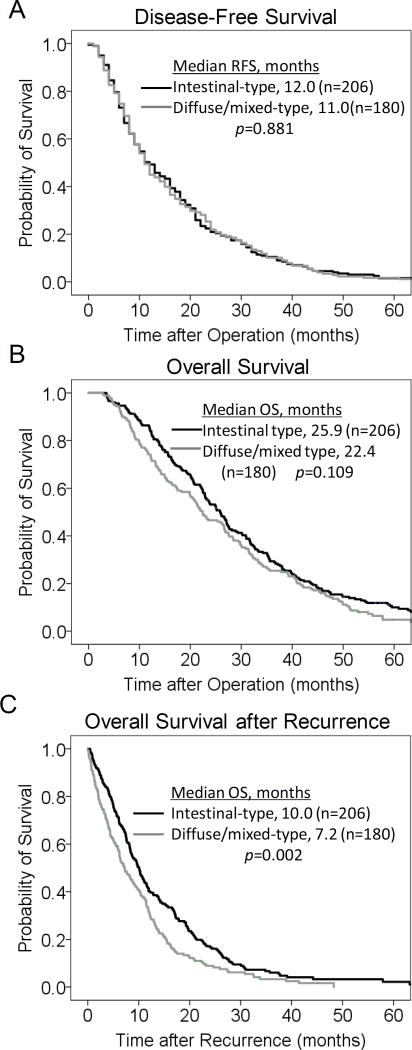
The median duration of follow-up for the 386 patients was 23.6 months (range, 2.8–106.3 months). The median time to recurrence from the time of operation was 12 months (Fig. 3A). 74.9% of recurrence occurred by two years, 88.1% by three years, 94.3% by four years, and 96.6% by five years. Only 3.4% of recurrences occurred beyond 5 years. There was no difference in disease-free survival between intestinal and diffuse/mixed tumors (Fig. 3B).
Figure 3.
Kaplan-Meier curves for disease-free survival (A), overall survival (B) and overall survival after recurrence (C) for intestinal vs. diffuse/mixed patients.
The median overall survival of these patients from the time of operation was 24.9 months (Fig. 3C). The 2 and 5 year overall survivals were 50.1% and 7.7%, respectively. In comparing patients with intestinal-type and diffuse/mixed-type tumors, there was no difference overall survival (25.9 vs. 22.4 months, p=0.11, Fig. 3D). Among the 386 patients diagnosed with recurrence, the median post-recurrence survival was only 8.4 months; 63.5% of patients had died by 1 year, and 87.0% of patients had died by 2 years (Fig. 3E). Post-recurrence survival was significantly better for patients with intestinal-type tumors compared to diffuse type tumors (Fig. 3F).
Univariate and multivariate analysis of factors associated with sites of initial recurrence
We next analyzed clinicopathologic factors associated with specific sites of initial recurrence (Table 3). Interestingly, advanced N stage was associated with a decreased risk of locoregional recurrence. Patient with advanced N stage likely had a D2 lymphadenectomy and nodal recurrence outside the D2 node stations was considered distant disease. Several clinicopathologic factors were associated with an increased risk of peritoneal recurrence including female gender, distal location, diffuse/mixed-type, neural invasion, and more advanced T stage. Prognostic factors for distant recurrence included male gender, intestinal-type, lack of neural invasion, and earlier T stage. On multivariate analysis, female gender, distal tumor location, diffuse/mixed type, and more advance pathologic T stage were significantly associated with peritoneal recurrence. Factors associated with distant recurrence included male gender, intestinal type, and earlier T stage. On multivariate analysis, intestinal type was the only factor associated with distant recurrence. Thus Lauren histologic type was the most important factor associated with site of initial recurrence on multivariate analysis.
TABLE 3.
Univariate Analysis of Factors Potentially Associated with Recurrence
| Locoregional Odds Ratio (C.I.) |
Peritoneal Odds Ratio (C.I.) |
Distant Odds Ratio(C.I.) |
||
|---|---|---|---|---|
| Age <66 | 1 | 1 | 1 | |
| ≥66 | 1.229 (0.752–2.009) | 0.959 (0.606–1.520) | 1.028 (0.681–1.551) | |
|
| ||||
| Gender Male | 1 | 1 | 1 | |
| Female | 0.739 (0.426–1.284) | 3.008 (1.862–4.860) | 0.586(0.3368–0.931) | |
|
| ||||
| Tumor size (cm) < 3.8 | 1 | 1 | 1 | |
| ≥ 3.8 | 1.031 (0.630–2.018) | 1.199 (0.756–1.901) | 0.882 (0.583–1.335) | |
|
| ||||
| Location Distal | 1 | 1 | 1 | |
| Proximal | 1.430 (0.850–2.404) | 0.328 (0.204–0.426) | 1.170 (0.765–1.789) | |
|
| ||||
| Lauren Intestinal | 1 | 1 | 1 | |
| Diffuse or mixed | 1.150 (0.704–1.878) | 3.478 (2.129–5.684) | 0.462 (0.302–0.706) | |
|
| ||||
| Preoperative T stage* T1–2 | 1 | 1 | 1 | |
| T3–4 | 0.984 (0.574–1.688) | 0.862 (0.523–1.422) | 0.502 (0.312–0.808) | |
|
| ||||
| Differentiation Well/Moderately | 1 | 1 | 1 | |
| Poorly/Signet ring | 1.176 (0.685–2.018) | 2.205 (1.264–3.848) | 0.584 (0.377–0.907) | |
|
| ||||
| Vascular Invasion No | 1 | 1 | 1 | |
| Yes | 1.427 (0.853–2.388) | 1.578 (0.970–2.566) | 0.702 (0.462–1.066) | |
|
| ||||
| Neural Invasion No | 1 | 1 | 1 | |
| Yes | 0.916 (0.559–1.501) | 2.531 (1.527–4.193) | 0.474 (0.311–0.720) | |
|
| ||||
| Pathologic T stage T1–2 | 1 | 1 | 1 | |
| T3–4 | 0.783 (0.458–1.339) | 2.970 (1.574–5.602) | 0.528 (0.334–0.834) | |
|
| ||||
| Pathologic N stage N0 | 1 | 1 | 1 | |
| N1–3 | 0.533 (0.322–0.883) | 1.207 (0.730–1.996) | 1.261 (0.807–0.973) | |
|
| ||||
| Number of positive lymph nodes | 1 | 1 | 1 | |
| 0.965 (0.925–1.008) | 0.930 (0.841–1.028) | 0.968 (0.922–1.016) | ||
|
| ||||
| Number of retrieved lymph nodes | 1 | 1 | 1 | |
| 0.977 (0.952–1.002) | 0.967 (0.927–1.009) | 0.981 (0.949–1.014) | ||
|
| ||||
| Lymph node ratio | 1 | 1 | 1 | |
| 0.537 (0.192–1.504) | 0.467 (0.077–2.815) | 0.479 (0.127–1.807) | ||
|
| ||||
| Type of Gastrectomy Distal | 1 | 1 | 1 | |
| Total or proximal | 1.235 (0.631–2.417) | 1.045 (0.596–1.834) | 0.674 (0.377–1.206) | |
| Esophagogastrectomy | 1.207 (0.659–2.208) | 0.219 (0.135–0.435) | 1.402 (0.856–2.297) | |
|
| ||||
| Perioperative Treatment None | 1 | 1 | 1 | |
| Preoperative | 1.249 (0.722–2.160) | 0.531 (0.312–0.905) | 1.003 (0.632–1.591) | |
| Postoperative | 0.625 (0.273–1.432) | 1.589 (0.848–2.977) | 0.792 (0.423–1.481) | |
|
| ||||
| Perioperative Treatment None | 1 | 1 | 1 | |
| Chemotherapy | 1.572 (0.905–2.732) | 1.421 (0.877–2.277) | 0.960 (0.613–1.502) | |
| Chemoradiation | 1.311 (0.755–2.276) | 0.873 (0.523–1.457) | 1.487 (1.012–2.183) | |
Significant factors in bold.
Preoperative T stage of 23 patients was unknown.
Peritoneal recurrence is often associated with advanced T stage. We analyzed the incidence of peritoneal recurrence for intestinal-type tumors and diffuse/mixed-type tumors by T stage. For the 206 intestinal-type tumors, 61 were T1/2 and 127 were T3/4. The rate of peritoneal recurrence was 10.0% and 16.9%, respectively (p=0.18). For the 180 diffuse/mixed-type tumors, 59 were T1/2 and 116 were T3/4 and the rate of peritoneal recurrence was 17.6% and 41.8%, respectively (p=0.009). Thus among patients that recurred, those with diffuse/mixed tumor penetrating into the subserosa or serosa had the highest risk of peritoneal recurrence.
Four treatment related variables were examined in this study: type of operation, extent of lymphadenectomy, timing of adjuvant treatment, and type of adjuvant treatment. On univariate analysis, esophagogastrectomy was associated with less peritoneal recurrence, and this is likely because the majority of these tumors were GE junction tumors, which have a lower peritoneal recurrence rate compared to gastric tumors. The vast majority of patients underwent a D2 lymphadenectomy so this variable was not included in the univariate analysis. Also on univariate analysis, preoperative adjuvant therapy was associated with a decreased risk of peritoneal recurrence compared to no adjuvant therapy and chemoradiation was associated with an increased risk of distant metastasis compared to no adjuvant therapy. On multivariate analysis, treatment-related variables were not independently associated with any site of recurrence (data not shown).
DISCUSSION
In this study, we examined patients gastric and GE junction Siewert II or III adenocarcinoma who underwent potentially curative resection at a single institution. Of 957 patients analyzed, 435 patients (43.5%) suffered a recurrence. For the 386 patients in whom complete location of recurrence information was available, 44% of recurrences occurred at distant sites such as the liver, 25% occurred in the peritoneal cavity, and 21% occurred locoregionally. The median time to recurrence was 12 months, and 75%% of recurrences occurred by two years. When examining clinicopathologic factors that were associated with recurrence, Lauren histologic type was the most important independent factor. Patients with intestinal type tumors had distant recurrence in 54% of cases, locoregional recurrence in 20%, and peritoneal recurrence in only 15%. In contrast, patients with diffuse type tumors had peritoneal recurrence in 37% of cases followed by distant in 32% and locoregional in 22%.
There is some indirect evidence that more extensive lymphadenectomies result in lower rates of locoregional recurrence. Locoregional recurrence after potentially curative surgery for gastric adenocarcinoma can be quite high. In a 1982 series from the University of Minnesota, 107 patients with gastric adenocarcinoma underwent second look laparotomy, and 80% had a recurrence.19 Of these recurrences, 88% were loco-regional, 54% were peritoneal, and 29% were distant. More recently in United States Intergroup 0116 trial, 177 of 275 patients (64%) in the surgery only group developed recurrent disease.5 In terms of the site of first relapse, 29% had local recurrence, 72% had regional recurrence, and only 18% had distant recurrence. Rates of locoregional recurrence are generally lower in reports from both Western and Asian institutions that perform more extensive (e.g. D2) lymphadenectomies. In this series, 93% of patients had a D2 lymphadenectomy and the median number of lymph nodes removed was 21 (range 2–67). Of patients that recurred, loco-regional recurrence was the initial and only site of recurrence in 21% of patients. Yoo et al examined 508 patients who developed recurrent disease after curative gastrectomy at Yonsei University in South Korea. Nineteen percent of patients had locoregional recurrence only as the first site of recurrence. In the Japanese prospective randomized trial of adjuvant S-1 chemotherapy, 188 (35.5%) of 530 patients treated with surgery suffered a recurrence.6 The site of first recurrence in these 188 patients was local in 7.9% and in lymph nodes in 24.5%. In the Dutch randomized trial of D1 vs. D2 lymphadenectomy, the 15-year followup found that more extensive lymphadenectomy was not only associated with decreased locoregional recurrence but also lower gastric cancer-related death.20
The results of this study may have been influenced by the delivery of adjuvant therapy. The Intergroup 0116 trial was the first prospective, randomized trial to demonstrate a survival benefit of chemoradiation over surgery alone. As one would expect, the chemoradiation appeared to primarily reduce locoregional recurrence (24% vs. 47%) rather than distant recurrence (16% vs. 18%).21 This study did not perform subgroup analysis based on Lauren histologic type. Given that 54% of patients received less than a D1 lymphadenectomy and only 10% of patients received a D2 lymphadenectomy, some have argued that the chemoradiation likely improved survival by making up for inadequate surgery. An observational study from Samsung Medical Center (Seoul, Korea), of 990 patients who underwent surgical resection along with D2 lymphadenectomy, found that the median survival time was significantly longer in the 544 patients who received chemoradiation than in the 446 patients who received no adjuvant therapy.22 The ARTIST trial also from Korea randomized 458 patients following D2 lymphadenectomy to postoperative chemotherapy or chemotherapy combined with chemoradiation.23 Overall there was no difference in disease-free survival, although subgroup analysis identified node positive patients as having improved disease-free survival following the combination of chemotherapy and chemoradiation. Overall locoregional recurrence was 8.3% in the chemotherapy arm and 4.8% in the combination arm.4 The MAGIC trial is the only adjuvant chemotherapy trail done in Western patients that demonstrated an improvement overall survival with perioperative chemotherapy. In this trial, 453 patients were randomized to surgery alone or surgery plus preoperative and postoperative chemotherapy. There was an improvement in five-year overall survival from 23 to 36 percent with perioperative chemotherapy. Local failure occurred in 14 percent of the chemotherapy-treated patients compared to 21 percent of those undergoing surgery alone. Distant metastases developed in 24 and 37 percent of patients, respectively. Finally, the CROSS trial randomized 366 patients with esophageal and GE junction cancer to surgery alone or preoperative chemoradiation followed by surgery.24 Median overall survival was 49.4 months in the chemoradiation group compared to 24.0 months in the surgery alone group. Better understanding of what sites are at highest risk of recurrence may help determine whether adjuvant chemoradiation or chemotherapy should be used.
For patients at risk of peritoneal recurrence, there may be some rationale for adding intraperitoneal chemotherapy to systemic intravenous chemotherapy. Despite the attractive rationale for this approach, studies examining this treatment strategy in gastric adenocarcinoma are limited. Cocolini et al. reviewed 20 randomized trials of intraperitoneal chemotherapy for gastric adenocarcinoma.25 The studies had between 46 and 269 patients and there was tremendous heterogeneity in the studies in terms of patients entered, method of delivering intraperitoneal chemotherapy, drugs used. In the largest trial performed by the Japan Clinical Oncology Group, 268 patients with serosa-positive tumors were randomized to surgery alone or surgery plus intraperitoneal cisplatin, systemic cisplatin and 5-fluorouracil, and oral 5-fluorouracil.26 This study found no difference in overall or relapse-free survival. It’s possible that chemotherapy directed to the peritoneum may be more effective than systemic chemotherapy for microscopic residual gastric adenocarcinoma and that the optimal drug(s) and method of delivery have not been discovered. If effective intraperitoneal treatment is developed, it should be directed at those with the highest risk of peritoneal recurrence.
There is a relative paucity of data on the effectiveness of intensive follow-up of gastric adenocarcinoma patients following potentially curative resection, and there are significant differences in the recommendations of various groups. The European Society for Medical Oncology (ESMO) clinical recommendations for the follow-up of gastric cancer state ”there is no evidence that regular intensive follow-up improves patient outcomes, [and] symptom-driven visits are recommended for most cases”.27 However, many patients are uncomfortable with minimal or no follow-up. The National Comprehensive Cancer Network practice guidelines for gastric cancer recommend a history and physical examination every 3–6 months for 1–3 years, every 6 months for 3–5 years, and then annually. CBC, chemistry profile, radiologic imaging and endoscopy are recommended as clinical indicated.28 Ultimately, the decision regarding the intensiveness of follow-up is left to the treating oncologist after discussion with the patient. The findings from this study have not changed the follow-up strategy at our institution. However given peritoneal recurrence is difficult to detect, we have a significantly higher index of suspicion for peritoneal recurrence in patients with diffuse/mixed tumors who develop new gastrointestinal symptoms.
Contemporary studies utilizing next generation sequencing and comprehensive molecular profiling have demonstrated distinct molecular subtypes within gastric cancer.11,29,30 The Cancer Genome Atlas (TCGA) Research Network proposed a molecular classification for gastric cancer into four subtypes: Epstein-Barr virus (EBV)-positive, microsatellite instability (MSI), genomically stable (GS), and chromosomal instability (CIN).11 Notably, the GS subtype contained the vast majority of diffuse-type gastric carcinomas as well as hotspot mutations in RHOA. The CIN subtype contained most of the intestinal type tumors and w characterized by mutations in TP53 and activation of the receptor-tyrosine kinase (RTK)-RAS pathway. Further studies may in the future delineate the molecular prognostic and predictive biomarkers that can better determine risk and sites of recurrence.
Survival after recurrence of surgically resected gastric adenocarcinoma remains poor in Western countries. The median overall survival after diagnosis of recurrence was 8.4 months. A recent multi-institutional study of United States institutions found an overall survival after recurrence of only 5.0 months.12 The reason for this difference may involve a number of factors including characteristics of enrolled patients (e.g. selection bias), more intensive strategies used to detect recurrence (e.g. lead time bias), and varying treatment of established recurrences. We found in this study that overall survival after detection of recurrence was worse in patients with diffuse/mixed tumors compared to intestinal tumors. Clearly better therapies are needed to either prevent or treat recurrent gastric adenocarcinoma following potentially curative surgical resection.
One primary finding of this study is that diffuse/mixed tumors tend to recur in the peritoneal cavity and there are several biological reasons that this may be true. First, diffuse tumors are more discohesive than intestinal tumors which may allow for great ability to migrate beyond the primary tumor site.10 Second, diffuse gastric cancer cells appear to possess an epithelial-to-mesenchymal (EMT) program which may further promote the ability to form peritoneal metastases.31 Third, diffuse gastric cancer cells may be able to interact with the microenvironment present on the peritoneal surface to form metastatic deposits more readily than intestinal gastric cancer cells.32
There are several limitations to this study. One major limitation of this study is that the analysis was performed on only patient with recurrent disease. Thus one cannot make apply our findings to all patients with resected gastric adenocarcinoma but only to the subset of patients that recur. Another limitation is that the follow-up strategy varied by surgeon. In addition, 11.3% of patients with recurrence did not have a recurrence pattern documented, and these patients had to be excluded from the analysis leading possibly to selection bias. Nevertheless, this study represents the largest single institution study to date of recurrence patterns in Western patients with gastric adenocarcinoma following surgical resection.
In conclusion, there may be distinct patterns of recurrence following potentially curative resection of gastric adenocarcinoma based on the biology of individual tumors. For those patients with recurrent disease, Lauren histologic subtype stratifies patients into two cohorts with the intestinal cohort recurring preferentially at distant sites and the intestinal/mixed cohort recurring preferentially in the peritoneal cavity. Further molecular and genomic studies that include all patients, not just those that recur, may help in predicting risk and site of recurrence. Survival following recurrence is quite poor and more effective adjuvant and metastatic therapies are needed, perhaps with immunotherapy or new targeted agents.
TABLE 4.
Multivariate Analysis of Clinicopathologic Factors Potentially Associated with Recurrence
| Locoregional Odds Ratio (C.I.) |
Peritoneal Odds Ratio (C.I.) |
Distant Odds Ratio (C.I.) |
||
|---|---|---|---|---|
| Gender Male | - | 1 | 1 | |
| Female | 2.250 (1.334–3.793) | 0.657 (0.407–1.060) | ||
|
| ||||
| Location Distal | - | 1 | - | |
| Proximal | 0.480 (0.287–0.803) | |||
|
| ||||
| Lauren Intestinal | - | 1 | 1 | |
| Diffuse or Mixed | 2.331 (1.361–3.992) | 0.584 (0.372–0.918) | ||
|
| ||||
| Neural Invasion No | - | 1 | 1 | |
| Yes | 1.381 (0.759–2.512) | 0.643 (0.396–1.043) | ||
|
| ||||
| Pathologic T stage T1–2 | - | 1 | 1 | |
| T3–4 | 2.219 (1.077–4.573) | 0.717 (0.428–1.201) | ||
|
| ||||
| Pathologic N stage N0 | 1 | - | - | |
| N1–3 | 0.533 (0.322–0.883) | |||
Significant factors in bold.
Footnotes
Conflict of interest: The authors declare they have no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 6.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma—an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg. 2014;219:664–675. doi: 10.1016/j.jamcollsurg.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Baiocchi GL, Kodera Y, Marrelli D, et al. Follow-up after gastrectomy for cancer: results of an international web round table. World J Gastroenterol. 2014;20:11966–11971. doi: 10.3748/wjg.v20.i34.11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [Google Scholar]
- 15.Suh YS, Han DS, Kong SH, et al. Should adenocarcinoma of the esophagogastric junction be classified as esophageal cancer? A comparative analysis according to the seventh AJCC TNM classification. Ann Surg. 2012;255:908–915. doi: 10.1097/SLA.0b013e31824beb95. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 17.Rosa F, Marrelli D, Morgagni P, et al. Krukenberg Tumors of Gastric Origin: The Rationale of Surgical Resection and Perioperative Treatments in a Multicenter Western Experience. World J Surg. 2015 doi: 10.1007/s00268-015-3326-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958:457–481. [Google Scholar]
- 19.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 20.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 21.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279–1285. doi: 10.1016/j.ijrobp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Lim dH, Kim S, et al. Phase III Trial Comparing Capecitabine Plus Cisplatin Versus Capecitabine Plus Cisplatin With Concurrent Capecitabine Radiotherapy in Completely Resected Gastric Cancer With D2 Lymph Node Dissection: The ARTIST Trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 24.van HP, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 25.Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12–26. doi: 10.1016/j.ejso.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Miyashiro I, Furukawa H, Sasako M, et al. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer. 2011;14:212–218. doi: 10.1007/s10120-011-0027-3. [DOI] [PubMed] [Google Scholar]
- 27.Jackson C, Cunningham D, Oliveira J. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):34–36. doi: 10.1093/annonc/mdp122. [DOI] [PubMed] [Google Scholar]
- 28.NCCN Clinical Practice Guidelines in Oncology - v.2.2020: Gastric Cancer. 2010 Ref Type: Online Source. [Google Scholar]
- 29.Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 30.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 31.Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130:2912–21. doi: 10.1002/ijc.26330. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita H, Yashiro M, Fukuoka T, Hasegawa T, Morisaki T, Kasashima H, et al. Diffuse-type gastric cancer cells switch their driver pathways from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor microenvironments. Carcinogenesis. 2015;36:1511–20. doi: 10.1093/carcin/bgv134. [DOI] [PubMed] [Google Scholar]