Abstract
Sublethal hypoxic or ischemic events can improve the tolerance of tissues, organs, and even organisms from subsequent lethal injury caused by hypoxia or ischemia. This phenomenon has been termed hypoxic or ischemic preconditioning (HPC or IPC) and is well established in the heart and the brain. This review aims to discuss HPC and IPC with respect to their historical development and advancements in our understanding of the neurochemical basis for their neuroprotective role. Through decades of collaborative research and studies of HPC and IPC in other organ systems, our understanding of HPC and IPC-induced neuroprotection has expanded to include: early- (phosphorylation targets, transporter regulation, interfering RNA) and late- (regulation of genes like EPO, VEGF, and iNOS) phase changes, regulators of programmed cell death, members of metabolic pathways, receptor modulators, and many other novel targets. The rapid acceleration in our understanding of HPC and IPC will help facilitate transition into the clinical setting.
Keywords: Preconditioning, Tissue tolerance, Endogenous protection, Molecular mechanisms, Stroke
1. Introduction
A decrease in oxygen concentration that comprises the cell’s intrinsic ability to sustain life is termed hypoxia. In comparison, scarcity of oxygen due to restriction of the blood supply to tissue is designated ischemia. Although the terms are often used interchangeably, ischemia is characterized not only by hypoxia, but also by insufficient nutrient supply due to decreased perfusion. Nevertheless, both of these are important problems that arise commonly in clinical settings.
Sublethal hypoxic or ischemic events can improve the tolerance of not only cells or tissues, but also entire organs and even the organism itself, to subsequent hypoxia or ischemia. This phenomenon is referred to as hypoxic or ischemic preconditioning (HPC or IPC respectively). The terms preconditioning (PC) and tolerance were first used in this context in the 1960s (Janoff, 1964). Current understanding of PC and tolerance were built throughout subsequent investigations and follow this basic premise: the stress induced by HPC triggers an adaptive response involving multiple genes, which ultimately counteracts the effects of pathways that cause cell death (Feng and Bhatt, 2015). With respect to nomenclature, both HPC and IPC are often referred to collectively under the heading of HPC, since both include hypoxia. In all, HPC and IPC have been well documented in relation to the heart and brain by a plethora of studies, as discussed below.
The basic physiology of oxygen delivery forms the framework of our discussion. To begin, a continuous oxygen and glucose supply is necessary to maintain the viability and function of the brain. Most notably, the entire central nervous system (CNS) is highly sensitive to changes in oxygen concentration due to a high intrinsic oxygen consumption rate (Luo et al., 2011). Therefore, during hypoxic episodes, the brain utilizes key adaptive mechanisms that allow it to survive and maintain homeostasis. In addition, with systemic hypoxia, other organ systems (such as the skin) conserve their use of the scarce oxygen supply to allow for the demands of life-supporting organs, such as brain and heart.
A key focus of research on HPC is the plasticity of the brain, which confers a lifelong ability to modify function and organization according to challenges posed by the external or internal environment. Hypoxic tolerance is though to stimulate brain plasticity through a combination of energy conservation and enhanced homeostatic control directed at subsequent hypoxic insults. This latter phenomenon is referred to as modulation. During HPC, modulation works hand in hand with plasticity and functions to sustain it. This occurs primarily at the cellular level and is discussed in detail below. Of clinical significance, the mechanisms underlying plasticity and modulation can point to novel strategies for the prevention and treatment of hypoxic and ischemic injury (Lu et al., 1999, 2005; Wang et al., 2007).
The body’s ability to adapt to hypoxia has been rigorously investigated. For example, many studies have been done on acclimation to high altitude and the effects of chronic hypoxia. This has led to a systems-based level of understanding of the physiological responses to hypoxia. However, the time course and severity of hypoxia in these settings differs from that in pathological processes, so the adaptative response may not be identical. Therefore, understanding the cellular mechanisms of hypoxic tolerance requires more than extrapolating from more physiological conditions, and may yield unique therapeutic targets.
In myocardial ischemia, the ischemic insult triggers biochemical alterations involving the release of molecules that stimulate a signaling cascade allowing the ischemic myocyte to withstand a subsequent episode of ischemia more robustly and for longer than normal myocytes. This scenario provides a rationale for pharmacological preconditioning, in which a drug that activates the same signaling pathways, rather than ischemia itself, induces preconditioning (Kloner and Jennings, 2001).
This progress-review article aims to succinctly integrate the significant research over the past decade our colleagues have done in laboratories around the world. Our goal is to not include all that is known regarding HPC since many excellent and comprehensive reviews are already available (Lutz, 1992; Perez-Pinzon et al., 1993; Hochachka et al., 1999; Mortola, 1999, 2004; Singer, 1999, 2004; Mitchell and Johnson, 2003; Lutz and Nilsson, 2004; Gidday, 2006; Luh and Yang, 2006; Ramirez et al., 2007; Storey, 2007; Hyder et al., 2010; Nayak et al., 2011; Vande Loock et al., 2012; Zhao et al., 2013a, 2013b; Hess et al., 2015a, 2015b). Rather we seek to provide a conceptual framework that will help to identify promising avenues of research that may yield therapeutic advances.
2. Hypoxic preconditioning (HPC): history and neuroprotective role
Traditional knowledge of systemic respiratory and cardiovascular responses does not fully explain adaptation to hypoxia at the level of tissues and cells. Long before the term HPC was introduced, Haldane noted this difficulty in relation to what he called the physicochemical brain (Haldane, 1927). An “acquired tolerance of tissue-cells to hypoxia” was thought to have developed through evolution. Later, this was termed a “tissue-cell adaptation to hypoxia” (Lu, 1963). Subsequently, animal models of hypoxia were developed to explore HPC from the vantage point of behavior, neurophysiology, neurochemistry, neuromorphology, and molecular biology.
Studies on HPC’s neuroprotective role began as early as 1986 when HPC was identified in the central nervous system (Schurr et al., 1986). Early human studies on hypoxia were not too much, and focused primarily on highland natives exposed to a low oxygen atmosphere and demonstrated no increased capacity for their homeostasis beyond their successfully adaptation to atmospheric hypoxia (Clinton et al.,1946; Houston and Riley, 1947). Early animal studies showed similar adaptations to low ambient oxygen levels. In one such study, day-3 newborn animals showed a minimal blood pressure decrease (dropping to only 67% the normal value) and no apparent physiological response for up to 17 min, using tracheal occlusion as the hypoxic trigger. This is in stark comparison to adult animals, whose blood pressure plummeted to zero within only 5 min of tracheal occlusion and exhibited a prominent reflexive physiological response (Lu, 1963). Another study on newborn animals demonstrated a continued heartbeat post-decapitation, as compared to adult animals, whose heartbeat abruptly stopped (Selle and Witten, 1941). These studies suggest that newborns exposed to relatively low oxygen levels have a more tolerant or adaptive response to hypoxia. Another animal study examined cat embryos following maternal carbon monoxide or cynanide intoxication (Fazekas et al., 1941). The embryos remained alive when removed at laparotomy, although their mothers had perished. These findings suggest that a robust adaptive response occurs that is not explained by the acute physiologic reflexive response.
As research accrued, it became clear that HPC serves as a protective mechanism for multiple organs against various kinds of injuries. For example in our laboratory, we utilized a whole body HPC (wb-HPC) model, for the first time to our knowledge, to assess an animal’s tolerance to hypoxia (Lu et al., 1999). Mice were placed in a sealed jar that created a hypoxic environment. This induced “auto-hypoxia”, as the animal’s own oxygen consumption in the air-tight environment depleted oxygen. The appearance of gasping was used as an indication of an animal’s tolerance. Once the animal began to gasp, it was removed and placed into another sealed jar. This procedure was repeated for 2, 3, 4, or 5 runs. Animals that were subjected to hypoxia for up to 4 or 5 runs were said to be hypoxically preconditioned. With each additional run of hypoxia we noted that the animal’s tolerance increased. Tolerance time in runs 2, 3, 4, and 5 was approximately 2, 4, 6, and 8 times greater, respectively, than times observed in the first run (Lu et al., 1999, 2005). This demonstrates HPC’s protective role.
Our study also showed similar results after inducing hypoxia through other mechanisms. Animals inside a hypobaric chamber with PO2 set at 2.7 kPa or those pretreated with cyanide demonstrated survival times 10 and 4 times longer, respectively, than normal controls (Lu et al., 1999). When animals were randomly paired based on sex and weight, control animals survived only for 1.7 min on average in the chamber while the animals subject to preconditioning remained alive for 146 min on average, or 86 times longer.
HPC’s role in relation to the brain has also been studied with animal models in our lab. Mice were decapitated and the length of time of residual activity in the isolated medulla oblongata and spinal cord were measured. Hypoxia-tolerant mice showed residual activity of isolated medulla oblongata and spinal cord as long as 124 and 66 s on average, respectively, which was 5 and 3 times greater than in control mice with no prior exposure to hypoxia (Lu et al., 1999, 2005).
This remarkable effect of HPC as a method of neuroprotection has been confirmed by many studies (Perez-Pinzon et al., 1996; Zhan et al., 2010; Thompsonet al., 2013; Pan et al., 2014; Stetler et al., 2014; Yun et al., 2014). For example, survival time in a hypobaric chamber of normal mice injected intraperitoneally with brain homogenate supernatant taken from hypoxically preconditioned mice was significantly increased compared to a control group injected with saline. Furthermore, cells co-cultured under hypoxia with brain extract from hypoxically preconditioned animals were substantially more viable than cells from the control group. The release of lactate dehydrogenase (LDH), an indicator of cell death, in cortical synaptosomes co-cultured with hypoxic preconditioned brain extract was progressively reduced, indicating protection by the extract. A recent study found that histone acetylation is vital in conferring tolerance to brain hypoxia in rats exposed to hypobaric hypoxia (Samoilov et al., 2016). In a study that examined the effects of HPC on white matter damage following a hypoxic-ischemic insult in neonatal rats, HPC protected myelin, either directly or by enhancing oligodendrocyte progenitor maturation to replace damaged myelin (Suryana and Jones, 2014). These neurochemical effects of preconditioned brain extract suggest there are adaptive changes at the neuromolecular level that confer tolerance to hypoxic stress.
HPC can be further understood in relation to the time between stimulus and adaptation. Rapid HPC, a protective phenotype lasting for a shorter duration, is induced in an early phase of minutes to several hours after sublethal hypoxic exposure (Schurr et al., 1986; Perez-Pinzon et al.,1997; Stagliano et al.,1999; Lu et al., 2005). This early adaptation is thought to be a consequence of alterations in of ion channel permeability, protein phosphorylation, and other post-translational modifications.
In contrast, delayed HPC is best understood as ‘classical preconditioning’ requiring gene activation and de novo protein synthesis. This is first manifested only hours to days following PC. In delayed PC, a diverse family of pro-survival genes, which codes for proteins that improve the brain’s tolerance to ischemic insults, is activated. This involves both inhibition injury mechanisms and an increase in mechanisms underlying survival and repair (Gidday, 2006).
3. Ischemic preconditioning (IPC): history and neuroprotective role
The phenomenon of IPC was first depicted as early as 1986 (Murry et al., 1986). Anesthetized dogs that received 40 min of circumflex coronary artery occlusion and subsequent reperfusion showed a significant decrease in myocardial infarct size if they were given four separate five-minute episodes of ischemia immediately before the 40-min occlusion. The net effect was a 75% decrease in infarct size compared to the control group. Further analysis in dogs with low coronary collateral flow showed that within the infarct at-risk zone, there was a markedly lower potential for necrosis (Miyazaki and Zipes, 1989). This reduction of infarct size following repetitive ischemic episodes was called ischemic preconditioning. The definition, however, was limited, and would later be extended to explain the overall effect of protection by brief ischemia against arrhythmias and cardiovascular dysfunction, not just infarct size (Ovize et al., 1994). A similar model of ischemic tolerance was later described in rat, rabbit, and pig hearts (Kloner and Jennings, 2001). In 1994, Kloner et al. described four types of studies that supported the concept of preconditioning in humans: angioplasty, pre-infarction angina, adaptation to angina, and preconditioning in human myocardial samples studies (Kloner and Yellon, 1994).
Although early studies of IPC focused on the heart, IPC’s effects on other organs, such as the lungs, was also explored. Studies on ischemic/reperfusion (I/R) pulmonary injuries have been conducted in guinea pig (Sonculet al., 1999), canine (Li et al., 1998; Friedrichet al., 2001), and rabbit (Gasparri et al., 1999; Wiegand et al.,1999; Zhang et al., 2003) models. These studies demonstrated that blockade of pulmonary hilar blood flow was able to normalize pulmonary dysfunction with subsequent exposure to I/R. Similarly findings were obtained in a rat model (Featherstone et al., 2000).
Numerous studies have shown that IPC can reduce liver and lung damage after liver transplantation (Peralta et al., 2001; Fernandez et al., 2002). A similar study demonstrated the reno-protective mechanisms of IPC. In this model, IPC was able to protect against I/R injury after renal arterial occlusion during transplantation (Fuller et al., 2005). In the intestine, IPC can also decrease bacterial translocation and slow the development of tissue necrosis when from subsequent intestinal I/R (Aksoyek et al., 2002; McCormick et al., 2003). In a very recent study, IPC reduced mortality in rats subjected to intestinal ischemia. Additionally, IPC results in a reduction of the inflammatory process and oxidative stress (Pinheiro et al., 2016). From these studies, we can understand that IPC has been demonstrated in multiple organ systems and shown to be effective.
Tolerance has been speculated to play a protectiverole in cerebral ischemia (Hakim, 1994). The first cerebral preconditioning study in vivo demonstrated that the capacity of the rat brain for anaerobic glycolysis increased after brief anoxia, and extended survival time following a subsequent exposure to more prolonged anoxia (Dahl and Balfour, 1964). A notable study demonstrated that if carotid blood flow was briefly disturbed, the delayed neuronal death of gerbil hippocampal CA1 pyramidal cells caused by global ischemia two days later could be completely prevented (Kitagawa et al., 1991). This finding was pivotal in launching research on ischemic tolerance in the brain as we know it today. With time, many reliable and reproducible experimental models of cerebral ischemic tolerance became widely accepted (Dawson and Dawson, 2000; Kirino, 2002; Dirnagl et al., 2003; Hagberget al., 2004; Sharp et al., 2004). Although investigations in this field have centered primarily on rodent and neuronal cell culture models, there is now evidence that ischemic tolerance is a form of evolutionarily preserved cerebral plasticity and can occur in both invertebrates and vertebrates(Gidday, 2015).
A special subtype of IPC was demonstrated in the early 1990s with the concept of remote ischemic preconditioning (Przyklenk et al., 1993). This is in contrast to traditional HPC-induced tolerance to hypoxia, which focuses on a local in-situ organ, namely the tissue exposed directly to hypoxia (local in-situ HPC, li-HPC). The pioneering study of remote ischemic preconditioning induced brief ischemia of the circumflex artery. This procedure then provided protection to a region of myocardium that is supplied by a different artery–the left anterior descending. Hence, the circumflex artery served as a remote preconditioning stimulus.
The remote preconditioning concept was extended further as it became obvious that the size of a myocardial infarct could be remarkably decreased by short prior episodes of ischemia and reperfusion in an organ or tissue far from heart. For example, studies using rodent models showed that ischemia of the kidney or intestine may play a protective role in myocardium (Gho et al.,1996; Pell et al., 1998). Such remote-ectopic IPC(re-IPC) appearsto induce a systemic mechanism to control ischemic damage(Ren et al., 2015). Accordingly, remote IPC confers neuroprotection against stroke by priming the peripheral immune system prior to a subsequent ischemic attack by enhancing host defenses (Liu et al., 2016). Emerging clinical trials are also demonstrating that remote preconditioning may be cardioprotective (Hausenloy and Yellon, 2011) and neuroprotective (Meng et al., 2012; Hess et al., 2015a, 2015b).
With regard to the remote preconditioning stimulus, it is important to identify the most effective remote tissue. Skeletal muscle ischemia has been shown to be a powerful remote preconditioning stimulus in larger animals and humans (Kharbanda et al., 2002). For example, inflating a blood-pressure cuff to induce four episodes of 5-min limb ischemia prevented ischemic endothelial dysfunction in the forearm of normal volunteers and decreased infarct size in an animal model of myocardial infarction. This same stimulus, applied to the recipient in a cardiac transplant model, prevented I/R injury to the donor heart (Konstantinov et al., 2005) and regulated expression of pro-inflammatory genes in circulating human neutrophils (Konstantinov et al., 2004). In a porcine model of cardiopulmonary bypass (CPB), re-IPC provided myocardial and pulmonary protection (Kharbandaet al., 2006). Therefore, skeletal muscle provides an effective target for preconditioning.
Obvious advantages of remote preconditioning include its non-traumatic nature and ease of implementation. This makes it an attractive technique that can be used efficiently in clinical settings. For example, a recent clinical study utilized prehospital, paramedic-administered remote PC on patients with suspected acute stroke. Although their results were inconclusive, tissue analysis suggested that prehospital remote PC may have immediate neuroprotective effects (Hougaard et al., 2014). Concerning the potential risks of remote preconditioning, it is important to note that the effects of transient skeletal muscle ischemia are relatively benign as compared to local ischemic preconditioning. This “nonlocal” effect of remote IPC may provide broader protection against ischemia-reperfusion injury and the CPB-induced systemic inflammatory reaction (Luh and Yang, 2006). Furthermore, there is no low cardiac output, and no risk of arrhythmia, myocardial dysfunction, or secondary organ injury with remote preconditioning. For example, I/R damage and CPB during cardiac surgery are related to a predictable systemic inflammation, myocardial dysfunction, and/or pulmonary endothelial dysfunction. In humans, following four 5-min cycles of upper-limb ischemic-preconditioning, regulation of genes coding for key proteins in leukocyte chemotaxis, adhesion, migration, cytokine synthesis, exocytosis, innate immunity signaling, and apoptosis was observed (Konstantinov et al., 2004). Although local ischemic preconditioning, induced by brief non-lethal ischemia in the target tissue, has beneficial effects following coronary angioplasty and surgical revascularization in some studies (Teoh et al., 2002; Laskey and Beach, 2003), its use has limitations in the clinical setting (Leesar et al., 2003; Lindhardt et al., 2004). Thus, remote preconditioning maybe more promising clinically.
Along with the limited side effects of remote preconditioning, ischemic preconditioning in remote organs or tissues produces greater tolerance than that seen in the local in situ organ-tissues themselves. For example, the tolerance of remote and ectopic organs or tissues is enhanced by repetitive occlusion of coronary, common carotid, and thoracic arteries to a greater extent than the tolerance increase observed in the heart, brain, and spinal cord, respectively. Repetitive ischemia of a limb on one side can result in increased tolerance to ischemia not only in the ipsilateral and contralateral limbs, but also in brain, heart, lung, kidney, intestine and other organs and tissues (Eddy et al., 1992; Giniset al., 1992; Kume et al., 1996; Oxman et al., 1997; Riepe et al., 1997; Song et al., 1998; Takaoka et al., 1999; Bruemmer-Smith et al., 2001; Harkin et al., 2002; Kharbanda et al., 2002; Konstantinov et al., 2004).
Similar to HPC, there are also various paradigms for IPC timing and repetition cycles. In a canine model, the duration of IPC was 10 min (Li et al., 1998). In a rabbit model of lung ischemia, hilar blood flow in the left lung was blocked for 10 min and then released for 15 min (Li et al., 1999). In a similar rabbit model, 15 min of IPC was more effective than 5 min (Gasparri et al., 1999). However, in another rabbit model, 5 min of IPC was better than 10 min in preventing subsequent ischemia-reperfusion injury (Friedrich et al., 2001). In a clinical study, the pulmonary hilum was clamped for 10 min with 10 min of reperfusion in patients undergoing major lung resection (Chen et al., 1999; Yang et al., 2002), or perfusion with chemotherapeutic agents (Zhang and Chen, 2001). Related studies have been conducted in a variety of preparations, including brain, brain slices in vitro, and cultured nerve cells (Schurr et al., 1986; Perez-Pinzon et al., 1996, 1997; Chen and Simon, 1997; Dawson and Dawson, 2000; Kirino, 2002; Schaller and Graf, 2002; Dirnaglet al., 2003; Pong, 2004).
4. From hypoxia to ischemia: mechanism of neuroprotection
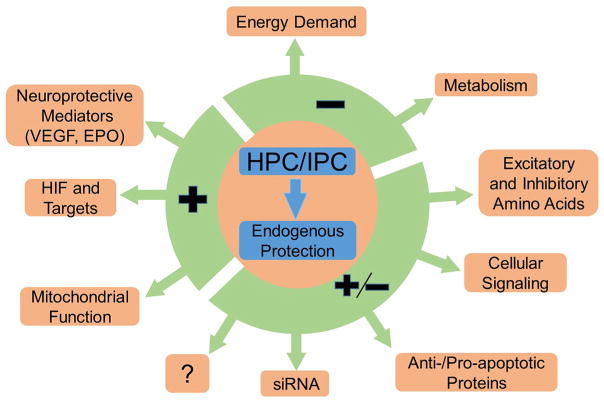
Although HPC and IPC target the same pathologic process (oxygen deprivation leading to cell damage or death), the terms are not interchangeable. Hypoxia is a state of relative oxygen deprivation; the extreme case is anoxia, or complete lack of oxygen. Hypoxia is typically considered in relation to a specific body region or tissue type. Ischemia refers to decreased blood supply to a body part or tissue, and unlike hypoxia, necessarily affects a particular vascular territory. Both HPC and IPC result in hypoxia, so it is likely that they share common mechanisms (Fig. 1).
Fig. 1.
Effect of hypoxic and ischemic preconditioning (HPC/IPC) on molecular mechanisms. HPC and IPC beneficially upregulate or downregulate different cellular processes that confer endogenous protection in various organ systems. HPC and IPC both depress cerebral metabolism and energy demand by, for example, decreasing ATPase activity and protein synthesis. On the other hand, HPC and IPC increase neuroprotective mediators, such as VEGF and EPO, HIF and its targets (e.g. hypoxic response elements), and promote mitochondrial function. HPC and IPC have a multitude of beneficial neuroprotective effects on intercellular signaling, excitatory and inhibitory neurotransmission, apoptosis pathways and siRNAs.
4.1. Depression of cerebral metabolism and activity following HPC and IPC
A commonality between the mechanisms that underlie the HPC and IPC is the metabolic depression of cerebral metabolism and activity to produce a hibernation-like state. Early studies showed that a decrease in metabolic rate, which is common in invertebrates and lower vertebrates, also frequently occurs in newborn and adult mammals during hypoxia. This seems to largely depend on thermoregulation (Mortola and Matsuoka, 1993). Thus, the ability to regulate body temperature and thus metabolic rate lies at the center of metabolic depression.
Previous studies have shown that decreasing body temperature and metabolic rate is the common response of many animals to hypoxia (Wood and Gonzales, 1996). Since the 1950s, this phenomenon has been well known to occur in a wide range of small mammals (Hill, 1959) and in newborn larger mammals, including humans (Cross et al., 1958). Hypoxia-induced reduction in body temperature and resulting metabolic inhibition plays an important protective role by decreasing oxygen demand, removing energy-expensive thermogenesis, improving oxygen affinity, and decreasing the cost of ventilation (Gautier et al., 1987, 1993; Wood and Malvin, 1991; Aboagye and Allen, 2014). Many studies support hypoxia reducing the hypothalamic thermoregulatory set-point (Mortolaet al., 1989; Clark and Fewell, 1996) and suggest that this must be a regulated process.
The metabolic suppression response is notable in the intact heart, where reduced oxygen delivery results in reduced contractile activity and oxygen consumption, in a phenomenon called “hibernating myocardium.” (Budinger et al., 1998). For example, one study showed that ischemic preconditioning lowers myocardial energy demand during post-IPC oxygen deprivation (Murry et al., 1990). This results in slower high energy phosphate usage and is accompanied by a reduced rate of anaerobic glycolysis. The study concluded that metabolic suppression is due to either preservation of ATP or reduction of the cellular load of catabolites. Other changes also occur during hibernation: blood flow and metabolism are reduced by 10% (Frerichs et al., 1994), body temperature and leukocyte counts decrease, and stress kinases and heat shock proteins are activated (Azzam et al., 2000). Furthermore, Azzam et al. observed a unique lipid and protein sequestration pattern in the neuronal endoplasmic reticulum. Although the mechanisms of the observed rapid increase in cerebral blood flow and severe oxidative stress during arousal of hibernating animals remains unknown, understanding these events could help illiminate the challenges faced by the human brain during post-ischemic reperfusion.
4.2. Metabolic mechanisms and signaling pathways at the cellular level
At the cellular level, hypoxia has profound influences on metabolism. These include improving the efficiency of energy-producing pathways via improved anaerobic glycolytic activity as well as reducing energy-consuming processes (Cerretelli, 1992). Regarding the latter, it is important to appreciate that ion-motive ATPases and protein synthesis hold the top position amongst the energy-consuming processes at standard metabolic rates, occupying more than 90% of ATP consumption in rat skeletal muscle and almost 66% in rat thymocytes (Cerretelli, 1976). Therefore, it is logical that these processes must be decreased during hypoxic conditions.
As the ATP supply is depleted, reallocation of energy lies at the crux of the ability to survive decreased oxygen levels. For example, studies of the liver have found that protein synthesis is largely reduced in response to hypoxia (Chandelet al., 2000). This reallocation is not arbitrary, but rather stems from the hierarchy of the ATP-consuming processes. A recent study concluded that proteins associated with ATP synthesis and the citric acid cycle were down regulated following HPC (Cui et al., 2015). RNA/DNA synthesis and protein synthesis are the first to be inhibited due to energy limitation. However, Na+/K+ pumping and Ca2+ cycling also takes high priority and is inhibited as hypoxia reaches a more severe stage. This phenomenon, called oxygen conformance, includes precise regulatory mechanisms such as the point of translation initiation (Buttgereit and Brand, 1995).
Extrapolating further, the varying sensitivity to hypoxia-induced cell death amongst various cell types appears to be a function of the cells’ dependence on electrical activity (i.e. the relative importance of the ATPase ion pumps versus other ATP-consuming processes). For example, ion pumping accounts for as much as 80% of ATP consumption in neurons but only 20% in skeletal muscle cells. Therefore, in severe oxygen deficit, the most excitable cells cannot continue to fulfill the energy demands of their ion transporting systems, resulting in cell death (Boutilier and St-Pierre, 2000). This relates back to the importance of reallocationg cellular energy to cell types that are vital, yet have high ATP consumption rates.
4.2.1. ERK
Extracellular-signal-regulated kinases, also known as mitogen-activated protein kinases (MAPK), play an important role in cell proliferation and activation (Boulton et al., 1991). This is most notably appreciated in neuron survival (Anderson and Tolkovsky, 1999). The initial investigations exploring the role of ERK in hypoxic states were contradictory. In one experiment, brains of mice who underwent HPC showed decreased expression and phosphorylation of ERK1/2, and increased phosphorylation of p38-MAPK (Zhang et al., 2007). More recently, Zhao et al. found that not only was HPC-induced neuroprotection associated with increased phosphorylation of p38-MAPK, but also that administration of a p38-MAPK inhibitor abolished the observed benefits of HPC (Zhao et al., 2013a, 2013b). In other studies, it was found that activation of ERK1/2 contributes to protection of adult and neonatal rat brains (Jones and Bergeron, 2004; Qin et al., 2007). ERK1/2 activity induced by cytokines, reactive oxidative species (ROS), and inflammatory mediators may worsen ischemic damage, whereas when activated by exogenous growth factors, estrogens, and preconditioning, ERK1/2 is neuroprotective (Sawe et al., 2008; Zhan et al., 2013).
4.2.2. Akt
Several investigations have shown that phosphatidylinositol 3-kinase (PI3-K) and its downstream mediator Akt (a serine/threonine kinase, protein kinase B), mediate growth factor-induced neuronal survival (Crowder and Freeman, 1998; Datta et al., 1997; Dudeket al., 1997; Zhan et al., 2010; Kim et al., 2014; Vazquez de la Torre et al., 2013). Earlier investigations showed that Akt-1 and PI3-K expression increased at 3–8 h in the penumbral region of an infarcted zone, and then declined at 24 h (Kitagawa et al., 1999). Ouyang et al. suggested that Akt may serve as one of the proteins responsible for delayed neuronal death in the first 24 h of global ischemia (Ouyang et al., 1999). HPC had no beneficial effect on the level of phosphorylated Akt or the Akt substrate glycogen synthase kinase 3β (Jones and Bergeron, 2004). However, under hypothermic conditions, Akt may have a minor role in neuroprotection of the brain (Tomimatsu et al., 2001). Conversely, Miyawaki et al. found that preconditioning involves PI3 K/Akt, which blocks the cell death cascade due to ischemia by inhibiting mitochondrial translocation of Bad, cleavage of Bcl-x(L), assembly of Bad with Bcl-x(L), activation of conductance channels in the outer membrane of the mitochondria, production of cytochrome c, and the subsequent caspase cascade that leads to cell death (Miyawaki et al., 2008). A recent study found that cerebrospinal fluid from hypoxia-preconditioned rats modulated apoptosis-related protein expression in neurons by upregulating Bcl-2 expression and downregulating Bax expression (Zhang et al., 2015; Kew and First, 1972). Thus, modulation of apoptotic protein expression is crucial in establishing hypoxic tolerance.
4.2.3. PKC
Protein kinase C and several of its isoforms have been shown to be involved in HPC (Niu et al., 2005). However, other studies suggests that nPKCσ is produced by focal brain ischemia via NMDA receptors, and that neuronal populations in the ischemic area are not affected by other PKC isoforms (Miettinenet al., 1996). mRNA levels of calcium-independent isoforms nPKCσ and aPKCζ are temporally induced by ischemic damage in the brain, suggesting that they play a crucial role in post-ischemic neuronal injury (Savithiry and Kumar, 1994). Curiously, cPKCβII and cPKCγ were involved in early phase tolerance, and cPKCγ was involved in the late phase (Niu et al., 2005). Jia et al. found that HPC (as well as oxygen-glucose deprivation) induced changes in nPKCε that were NMDA-receptor mediated (Jia et al., 2007). Lastly, Bu et al. found that inhibition of cPKCβII blocked the neuroprotective effects of HPC in a middle cerebral artery occlusion (MCAO) model (Bu et al., 2011; Zhan et al., 2011). These findings seem to be consistent with a recent study that examined rat hearts that underwent ischemic post-conditioning—a phenomenon in which the conditioning stimulus is applied after, rather than before, the pathologic event (e.g. during post-ischemic reperfusion). In this study, heat shock protein 90 was critical in cardioprotection because of its effect on PKCε, which targets the mitochondria to inhibit pro-apoptotic mediators (Zhong et al., 2014).
4.2.4. Glycolytic enzymes
Under ischemic conditions, cells use anaerobic processes such as glycolysis to produce energy. To produce energy at the quantity equivalent to oxidative phosphorylation, the cells would require a large amount of glucose, therefore the enzymes related to glucose transport and glycolysis may play critical roles in the hypoxic tolerance induced by HPC. Lack of glucose is a major cause of anoxia-related synaptic damage, and glycolysis can help prevent this damage in vitro given sufficient glucose (Tian and Baker, 2000). HPC can increase the expression of glucose transporters 1 and 3 (GLUT1 and GLUT3) in the brain (Jones and Bergeron, 2001; Yu et al., 2008). Furthermore, glycolytic enzymes include LDH, aldolase (ALD), phosphoenolpyruvate carboxylase, phosphogluco-kinase-1/L/C, pyruvate kinase, enolase, adrenomedullin and prolyl 4-hydroxylase, which are all regulated by hypoxia-inducible transcription factor 1 (HIF-1) (Sharp et al., 2004). The levels of mRNA encoding 13 different glucose transporters and glycolytic enzymes, including ALD A, enolase-1, GLUT1, and LDH A, were reduced in HIF-1α-deficient stem cells (Bergeron et al., 1999; Iyer et al., 1998; Wenger and Gassmann, 1997). HPC increases phosphofructokinase (PFK), ALD and LDH protein levels, with significant elevations noted 6 (LDH), 12 (GLUT1), and 24 (PFK and ALD) hours after hypoxia (Jones and Bergeron, 2001). Similar results were found in a proteomic analysis of mouse brain after HPC. The authors concluded that proteins associated with glycolysis and oxygen-binding were up-regulated following HPC (Cui et al., 2015).
4.3. Mitochondrial function (ROS, anti-, and pro-apoptotic molecules)
Apoptosis is an evolutionary adaption to prevent multi-cellular organisms from propagating cells that are abnormal, infected, or otherwise damaged. Regulation of apoptosis depends on upstream molecules which either prevent or induce cleavage of caspases. When activated, caspases play an integral role in apoptotic cell death.
There are two pathways by which apoptosis can be initiated: extrinsic (death-receptor pathway) and intrinsic. The intrinsic pathway heavily involves the mitochondria, which store apoptotic regulators as well as the signals that initiate caspase activation (Wang and Youle, 2009). Apoptosis is regulated by the Bcl-2 family of proteins including such members as Bcl-2, Bcl-x, BAD, and Bax. These proteins are candidates for the protective effects of HPC (Gustavsson et al., 2007). Furthermore, Bcl-2 and Bcl-x have been found to be regulated by both HIF-1 and GATA-binding protein 4 (Park et al., 2007). A study published in 2014 found similar results with respect to upstream regulation of Bcl-2 (Samoilov et al., 2014) involving enhanced expression of neuronal phosphorylated cAMP response element-binding (pCREB) and brain-derived neurotrophic factor (BDNF). Both are involved in pro-survival pathways after repetitive trials of hypoxic preconditioning. BDNF’s downstream effects include activation of adaptive proteins, such as Bcl-2, and antioxidants. pCREB’s downstream effects also target the cell-death inhibitors activating transcription factor 3 (ATF3) and activity-regulated inhibitor of death (AID).
HPC may also depend on the suppression of caspases, which was observed in wb-HPC and hyperbaric oxygen preconditioning (HBO-PC) models (Li et al., 2008; Zhang et al., 2008). Specifically, caspase-3 or -9 may be important targets for suppressing apoptosis in the preconditioned brain. Inhibition of caspase-3 by diazoxide provided a significant reduction in protein expression of cyto-chrome c and cleaved caspase-3, and a subsequent decrease in injury caused by cerebral ischemia and reperfusion (He et al., 2008). Mice deficient in p53, another protein involved in regulation of apoptosis, showed decreased infarct volumes after MCAO (Crumrine et al., 1994). Furthermore, HIF-1 signaling pathways activated in cortical neurons delayed death in a p53-dependent manner, thus demonstrating its importance in regulation (Halterman et al., 1999).
Several studies have shown ROS are involved in oxidative damage to proteins, lipids, and nucleic acids (Christophe and Nicolas, 2006). However, ROS also act as signaling molecules (Hensley et al., 2000). For example, ROS may be necessary to induce expression of endogenous antioxidant enzymes. Furuichi et al. demonstrated that ROS may play a key role in the development of brain preconditioning (Furuichi et al., 2005). Zhao et al. also concluded that HPC decreased infarct size by inhibiting free radical generation (Zhao et al., 2006). A recent study found that IPC reduces oxidative stress (Pinheiro et al., 2016). Enzymes like catalase, superoxide dismutase (SOD), lactoperoxidase, and glutathione peroxidase have been investigated to determine their roles in HPC (Duan et al., 1999; Garnier et al., 2001; Koca et al., 2010; Li et al., 2008; Stroev et al., 2005). In some studies, SOD was found to be significantly increased in HPC treatment groups. A recent study confirms these findings for ischemia-induced hippocampal damage: both ischemic pre- and post-conditioning attenuated oxidative stress and inflammatory, excitotoxic and apoptotic markers induced by ischemia (Saad et al., 2015).
4.4. Hypoxia-inducible transcription factor (HIF) and its target gene
HIF-1 is a key modulator of protein synthesis. HPC has been found to increase HIF-1 in the brain of neonatal and adult animals (Jones et al., 2006; Shao et al., 2005; Zhu et al., 2014). HIF-1, -2, and -3 are composed of alpha and beta subunits and are members of the basic helix-loop-helix-PAS superfamily. They all can be induced by hypoxia (Gu et al., 1998; Wiesener et al., 1998). HPC inhibits prolyl hydroxylase (PHD)-2, which regulates HIF-1α function by controlling its degradation (Jones et al., 2006). Under physiologic conditions, HIF-1α is hydroxylated by the PHD family (Bruick and McKnight, 2001; Epstein et al., 2001). It is further polyubiquitinylated, which targets it for proteasomal degradation. However, under hypoxic conditions, HIF-1α escapes this breakdown and instead translocates to the nucleus and dimerizes with HIF-1β and subsequently binds to hypoxia response elements on target genes, leading to transcriptional activation of several dozen genes including erythropoietin (EPO) and vascular endothelial growth factor (VEGF) (Sakanaka et al., 1998), both of which have been found to play a role in neuroprotection (Hayashi et al., 1998; Ruscher et al., 1998). VEGF plays a protective role by inducing vasculogenesis and angiogenesis in the brain, whereas EPO has been implicated in increased neural progenitor cell function (Noguchi et al., 2007). Similarly, intermittent hypoxia accelerates cognitive functional recovery after brain ischemia in mice through HIF-1 activation (Qiao et al., 2015).
4.4.1. EPO
Although EPO is produced extensively in the fetal liver and adult kidney, it is also synthesized in brain astrocytes and neurons (Bond and Rex, 2014; Marti, 2004). Studies in animal models of brain ischemia and trauma have demonstrated reduced injury with EPO administration. Intrinsic EPO production is impoprtant for in the brain protection because only low levels of EPO from the periphery can cross the blood-brain barrier, even when injected in high doses intravenously. EPO is released by the brain in a hypoxia-sensitive manner (Bernaudin et al., 2002) via hypoxia response elements (HRE) in the 3′ region of the EPO gene, with reporter genes exhibiting transactivation by dimeric HIF-1 (Chin et al., 2000; Yu et al., 2002). Although transcription of EPO receptor (EPO-R) gene is not believed to be regulated by HIF-1, hypoxic states do induce increased expression of EPO-R in neuronal cells, which enhances cells sensitivity to EPO (Chin et al., 2000). Mice that lack EPO or EPO-R exhibit enhanced neural apoptotic activity during development (Noguchi et al., 2007).
The significance of EPO production in HPC for cerebral infarction in mice was found by administration of soluble EPO-R (which act as decoys for EPO) into the cerebral ventricles; this reduced EPO’s protective effect by 40–88% (Malhotra et al., 2006; Prass et al., 2003). In an in vitro model of cerebral ischemia, Ruscher et al. showed that EPO mediated ischemic tolerance in primary cortical neurons, whereas soluble EPO-R, anti-EPO-R antibody and JAK2 inhibitors blocked neuroprotection (Ruscher et al., 2002). In a study exploring the effects of EPO on retinal degeneration models, elevated expression of EPO conferred protection against light-induced apoptosis of retinal cells, but not genetically determined retinal degeneration (Grimm et al., 2004).
4.4.2. VEGF
VEGF is an important signaling protein that is produced in response to hypoxia by the kidney, testis, lungs, liver, heart and brain. VEGF can be found in neurons, astrocytes, and microglia in the brain (Ogunshola et al., 2000). VEGF stimulates endothelial cells to improve the sprouting of blood vessels (Neufeld et al., 1999). As implied by these experiments, it appears that VEGF also acts directly on different types of neural cells, including neural stem cells (Storkebaum and Carmeliet, 2004). Furthermore, it has been shown that NO can control VEGF synthesis by increasing HIF-1 binding to the VEGF promoter’s hormone response element, leading to its transcription (Kimura et al., 2000). Three forms of human VEGF were found from the coding regions, resulting in chains of 189, 165, and 121 amino acids (Tischer et al., 1991). The biological effects of VEGF are mediated largely by two tyrosine kinase receptors, Flt1 (VEGFr-1) and KDR (VEGFr-2) (Compernolle et al., 2002; Ferrara and Gerber, 2001). Animal models have shown that with HPC, there is an increase in VEGF expression detected by RT-PCR, western blot, and mRNA and protein microarray (Bernaudin et al., 2002; Shao et al., 2005). In neonatal animals, the protective effects of VEGF was inhibited by anti-VEGFr1/2 monoclonal antibodies. Furthermore, mutant models using VEGF-A genes lacking the HRE found that HPC instead exacerbated brain lesions when compared to wild-type littermates (Laudenbach et al., 2007).
4.4.3. Nitric Oxide (NO)
It is believed that NO plays an important role in preconditioning due to its vasodilatory effect, as well as its effect on mitochondria (Murillo et al., 2011). Increased circulating nitrite from shear stress-related stimulation of endothelial nitric oxide synthase contributes to cardioprotection (Rassaf et al., 2014). Interestingly, inducible nitric oxide synthase (iNOS) is a target gene of HIF-1, and its expression is upregulated by HIF-1 under hypoxic conditions (Belaidi et al., 2008).
4.5. Excitatory and inhibitory amino acids and their receptors
The process by which neurons are damaged by excessive neurotransmitter activation is referred to as excitoxicity. Glutamate can bind to and activate N-methyl-D-aspartate (NMDA-), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA-), or kainic acid-preferring glutamatergic receptors on neuronal cell membranes, leading to excessive influx of calcium. This activates catabolic enzymes and leads to damage to structural cell components and DNA (Manev et al., 1989). Excitotoxicity is well known to occur during neuronal cell death, including that related to brain or spinal cord injury (Hulsebosch et al., 2009). Cell death releases additional glutamate and exacerbates injury, including ischemic brain injury. This can cause delayed death of a cell population that was not a target of the initial insult. If we can reduce the rate of cell death by preconditioning, it may also reduce secondary damage caused by dying cells.
Gong et al. found that cerebral ischemic preconditioning decreases glutamate excitotoxicity through the up-regulation of GLT-1 uptake activity (Gong et al., 2014). An alternative approach would be to reduce the degree of NMDA receptor stimulation. Tremblay et al. found that cultured neurons exposed to an NMDA receptor antagonist (MK801) developed long-term tolerance to excitotoxic stimulation (Tremblay et al., 2000). Slomka et al. applied the same protocol to cultured cerebellar cells and observed an increased tolerance as well (Slomka et al., 2014). There have been significant efforts to understand how HPC/IPC confers neuroprotection via its effects on glutamate (Liu et al., 2009) and other neurotransmitters, and new experimental models are testing chemical preconditioning of NMDA receptors to reduce subsequent excitotoxic cell death (Makarewicz et al., 2014; Stetler et al., 2014; Constantino et al., 2015). In a recent study of transient cerebral ischemia and neurons in the gerbil hippocampal CA1 region, kynurenic acid immunoreactivity was maintained or even increased. Kynurenic acid, a metabolite of kynurenine pathway for tryptophan degradation, plays a vital role in the CNS by selectively antagonizing NMDA receptors, which protects neurons from excitotoxicity. Kynurenic acid also has inflammatory, vascular, and antioxidant effects (Lee et al., 2015). Therefore, NMDA receptor modulation may be an important approach for conferring neuroprotection and, potentially, therapeutic intervention.
Besides NMDA receptors, the role of other key receptors in neuroprotection has been studied. Mefloquine, an inhibitor of the pannexin 1/P2 × 7 purinoceptor, abolished neuroprotection by IPC (Mahi et al., 2015). Further research will likely discover more receptors involved in conferring neuroprotection.
4.6. Small interfering RNAs (siRNA) in preconditioning
A relatively new direction in molecular biology is the study of small interfering RNAs (siRNA). These double-stranded stretches of short RNA are involved in RNA interference (RNAi), a pathway that seems to inhibit translation of specific mRNA transcripts. One class of siRNAs are microRNAs (miR), which are about 22 base-pairs in length and are key regulators in protein synthesis. Recent experiments have identified candidate miR targets of HPC. Peng et al. identified miR181b in their MCAO and oxygen-glucose deprivation model (showing a decrease in miR181b levels under conditions of ischemia), as well as two effector proteins: heat-shock protein A5 (HSPA5), and ubiquitin carboxy-terminal hydrolase isozyme L1 (UCHL1) (Peng et al., 2013). Antagonists of miR181b decreased cleavage of caspase-3 and thus reduced apoptotic activity. Furthermore, siRNA targeting HSPA5 and UCHL1 reduced the neuroprotective action of the miR181b antagonist. In a similar MCAO and oxygen-glucose deprivation study, Chi et al. (Chi et al., 2014) identified miR134, which is thought to inhibit HSPA12B translation. They found that MCAO or oxygen-glucose deprivation led to an upregulation in miR134, which was related to increased cell death. Furthermore, antagonism of miR134 resulted in a lower degree of cell death, and siRNA against HSPA12B reduced the neuroprotective effects of miR134 inhibition (Chi et al., 2014). Therefore, siRNAs play an important role in preconditioning and can be utilized in future studies as potential therapeutic agents.
5. Translational perspectives and prospective studies of hypoxic and ischemic preconditioning
5.1. Clinical applications
Although much of the reviewed experimental literature concerns hypoxic conditioning with regard to muscular, myocardial, or brain infarcts, there have been many investigations conducted to explore the role of HPC and IPC in other clinical conditions.
One small study (n = 8) found that HPC could enhance ventilatory function in patients with cervical or thoracic spinal cord injury (SCI) (Tester et al., 2014). Exposure to hypoxia, by breathing air with a low oxygen content, increased minute ventilation for 30 min compared to patients breathing room air. Furthermore, improvement persisted for the 10 days of HPC exposure, and in some patients for another 10 days.
Several studies have shown that HPC has anti-hypertensive properties. In one study, 37 men with stage 1 hypertension were treated in 20 sessions with 4–10 cycles of hypoxia (FiO2 = 10%), each lasting 3 min, whereas a control group received room air (Lyamina et al., 2011). Blood pressure was reduced in the treatment group, and in some patients the reduction persisted for up to 3 months.
IPC in the brain shares many features of transient ischemic attacks (TIA). Many studies have observed improved outcomes from stroke in patients with previous TIAs (Wegener et al., 2004; Schaller, 2005; Arboix et al., 2004). However, some studies have found no significant tolerance to stroke after TIAs (Morte et al., 2008). Overall, TIAs appear most likely to provide protection when they are recent, occur multiple times, and are of shorter duration. These are precisely the conditions under which experimental IPC optizes outcome.
Inducing IPC in the brain may come with unforeseen complications and risks as compared to limb or heart IPC, which is much more easily performed during surgery. Therefore, most clinical trials on brain function and tolerance have involved RIPC. One study showed improved outcomes after carotid endarterectomy when the patients underwent RIPC of the thigh with blood pressure cuffs (Walsh et al., 2010). Another study showed reduced cell damage and faster recovery time in patients undergoing decompression of the spinal cord with upper limb RIPC (Hu et al., 2010).
IPC can enhance the heart’s tolerance to ischemia, and recent studies using RIPC have achieved a similar effect. In a randomized controlled trial, 333 adult patients with suspected myocardialinfarction (MI) were assigned to one of two groups: upper-arm ischemia induced by a blood pressure cuff, or a control group (Ndrepepa et al., 2004). The authors found that those receiving RIPC had increased myocardial salvage, an indicator of therapeutic benefit. Similar studies have been performed on patients with ST-segment elevation MI, with concordant findings (White et al., 2015).
The feasibility, safety and efficacy of limb remote ischemic preconditioning (LRIPC) is indicated by two randomized clinical trials and another clinical study in patients with atherosclerotic disease, including intracranial atherosclerotic stenosis (ICAS) (Meng et al., 2012, 2015; Li et al., 2015). In 10 patients with unilateral MCA stenosis and 24 healthy volunteers, LRIPC was repeated for five cycles. These consisted of 5-min inflations of a blood pressure cuff to 200 mm Hg around an upper limb, followed by 5 min of reperfusion. LRIPC not affect heart rate, oxygenation index, or mean blood flow velocity (Li et al., 2015). Instead, healthy volunteers showed reduction in blood pressure 30 min following the final cycle. Another major study from the same group involved a randomized clinical trial of 68 Chinese patients with a recent history of stroke or transient ischemic attack (TIA) and intracranial arterial stenosis (Meng et al., 2012). This study evaluated the protective effects of brief repetitive bilateral arm ischemic preconditioning (BAIPC) on stroke recurrence in patients with symptomatic atherosclerotic ICAS. All patients received standard medical care. As in Li’s safety study (Li et al., 2015), 38 patients in the BAIPC group underwent 5 brief cycles consisting of bilateral upper limb ischemia (inflations of a blood pressure cuff to 200 mmHg) followed by reperfusion. BAIPC was performed twice daily over 300 days. Incidence of recurrent stroke in BAIPC-treated patients was reduced to 5% and 7.9% at 90 and 300 days, respectively, compared with 23.3% and 26.7% in the untreated control group (n = 30). In the BAIPC group, the average recovery rate, determined by the modified Rankin Scale, was also increased. Cerebral perfusion, measured by SPECT and transcranial Doppler sonography, was also markedly in the BAIPC-treated brain. This study concluded that BAIPC may be an effective way to improve cerebral perfusion and reduce recurrent strokes in patients with ICAS. In the most recent study from the same group (Meng et al., 2015), the effect of BAIPC on symptomatic intracranial arterial stenosis was evaluated in Chinese octo- and nonagenarians. Since endovascular treatment, such as stenting, may not be suitable for octogenarians with systemic diseases, less invasive methods are particularly needed. In this randomized, controlled prospective study, all 58 patients received standard medical management for 180 consecutive days. In the BAIPC group, patients underwent the same five cycles consisting of bilateral arm ischemia followed by reperfusion twice daily. Control patients underwent sham-BAIPC. BAIPC had no adverse effects on blood pressure, heart rate, local skin integrity, or plasma myoglobin. BAIPC appears a promising intervention in the elderly due to its limited profile of adverse effects and the absence of hemorrhagic complications.
Several clinical studies of IPC and RIPC have also shown benefit with regard to spinal cord function (Trumbower et al., 2012; Schega et al., 2013; Hayes et al., 2014).
5.2. Future direction and considerations
There are currently a plethora of novel and exciting applications of preconditioning being explored, which include both HPC and IPC. As our review has shown, evidence from controlled laboratory animal models favors the therapeutic benefits of preconditioning. However, routine clinical use is still lacking. Many human trials of HPC or IPC have small study populations, and do not reflect the heterogeneity of clinical disease. The optimal duration, frequency and magnitude of a preconditioning stimulus may vary across different diseases, necessitating extensive human trials. In addition, the efficacy of pharmacological and hypoxic preconditioning may be optimized by combination with other (e.g. cell-based) therapy.
One major limitation of HPC or IPC is that benefit is short-lived, usually lasting only a few days, whereas many target diseases are unpredictable in their time course. By the time the signs of a stroke or heart attack are apparent, for example, it is too late to begin preconditioning. HPC or IPC might be more readily used as pre-operative interventions. For example, Zarbock et al. explored the therapeutic benefits of RIPC on kidney function for patients undergoing cardiac surgery, and found a decrease in the occurrence of acute post-operative kidney injury (Zarbock et al., 2015).
The first experiments on preconditioning were very narrow in scope. Over time this simple concept has diverged to include many stimuli, techniques, and disease states to which it can be applied. This added complexity requires more thorough research to solidify our understanding of interventions and disease targets. Even with all of the limitations and considerations mentioned above, our group and many others continue to pursue this approach. There is something exciting about being able to prevent damage to cells by inducing the very process that causes it, albeit to a milder degree.
Acknowledgments
We thank Zhiqian Yan (Shanghai Jiao Tong University) for her help in our manuscript preparation. This project was supported by the China National Funds for Distinguished Youth Scientist (81325007), Distinguished Professor of Cheung Kong Scholars Program (T2014251) and The Capital Health Research and Development of Special (2016-4-1032), as well as American Heart Association Grant-in-Aid (14GRNT20460246) and Merit Review Award (I01RX-001964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service.
Abbreviations
- ALD
aldolase
- Akt
protein kinase B
- ATF3
activating transcription factor 3
- BAIPC
bilateral arm ischemic preconditioning
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- CPB
cardiopulmonary bypass
- ERK
extracellular signal-regulated kinase
- EPO
erythropoietin
- Glut-1
glutamate transporter-1
- Glut-3
glutamate transporter-3
- GSH-PX
glutathione peroxidase
- HBO-PC
hyperbaric oxygen preconditioning
- HIF-1
hypoxia-inducible transcription factor 1
- HPC
hypoxic preconditioning
- HSPA5
heat-shock protein A5
- ICAS
intracranial atherosclerotic stenosis
- IPC
ischemic preconditioning
- I/R
Ischmia/reperfusion
- LDH
lactate dehydrogenase
- LiHPC
local in situ hypoxic preconditioning
- LPO
lactoperoxidase
- LRIPC
limb remote ischemic preconditioning
- MAPK
mitogen activated protein kinase
- MCAO
middle cerebral artery occlusion
- NO
nitric oxide
- PC
preconditioning
- pCREB
phosphorylated cAMP response element-binding
- PFK
phosphofructokinase
- PHD
prolyl hydroxylase
- PI3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- ReIPC
remote ectopic ischemic preconditioning
- RIPC
remote ischemic preconditioning
- ROS
reactive oxygen species
- SIAS
symptomatic intracranial arterial stenosis
- siRNA
small interfering RNAs
- SOD
superoxide dismutase
- STEMI
ST-segment elevation MI
- TIA
transient ischemic attack
- UCHL1
ubiquitincarboxy-terminal hydrolase isozyme L1
- VEGF
vascular endothelial growth factor
- wb-HPC
whole body HPC
References
- Aboagye DL, Allen PJ. Metabolic and locomotor responses of juvenile paddlefish Polyodon spathula to hypoxia and temperature. Comp Biochem Physiol. 2014;169:739–757. doi: 10.1016/j.cbpa.2013.12.016. (51–59. l design 12) [DOI] [PubMed] [Google Scholar]
- Aksoyek S, Cinel I, Avlan D, Cinel L, Ozturk C, Gurbuz P, Nayci A, Oral U. Shock. Vol. 18. Augusta, Ga: 2002. Intestinal ischemic preconditioning protects the intestine and reduces bacterial translocation; pp. 476–480. [DOI] [PubMed] [Google Scholar]
- Anderson CN, Tolkovsky AM. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent: JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci. 1999;19:664–673. doi: 10.1523/JNEUROSCI.19-02-00664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboix A, Cabeza N, Garcia-Eroles L, Massons J, Oliveres M, Targa C, Balcells M. Relevance of transient ischemic attack to early neurological recovery after nonlacunar ischemic stroke. Cerebrovasc Dis. 2004;18:304–311. doi: 10.1159/000080356. [DOI] [PubMed] [Google Scholar]
- Azzam NA, Hallenbeck JM, Kachar B. Membrane changes during hibernation. Nature. 2000;407:317–318. doi: 10.1038/35030294. [DOI] [PubMed] [Google Scholar]
- Belaidi E, Beguin PC, Levy P, Ribuot C, Godin-Ribuot D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am J Physiol Heart Circ Physiol. 2008;294:H901–908. doi: 10.1152/ajpheart.00715.2007. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Bond WS, Rex TS. Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front Immunol. 2014;5:523. doi: 10.3389/fimmu.2014.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Boutilier RG, St-Pierre J. Surviving hypoxia without really dying. Comp Biochem Physiol. 2000;126:481–490. doi: 10.1016/s1095-6433(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Bruemmer-Smith S, Stuber F, Schroeder S. Protective functions of intracellular heat-shock protein (HSP) 70-expression in patients with severe sepsis. Intensive Care Med. 2001;27:1835–1841. doi: 10.1007/s00134-001-1131-3. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Bu X, Zhang N, Yang X, Liu Y, Du J, Liang J, Xu Q, Li J. Proteomic analysis of cPKCβII-interacting proteins involved in HPC-induced neuroprotection against cerebral ischemia of mice. J Neurochem. 2011;117:346–356. doi: 10.1111/j.1471-4159.2011.07209.x. [DOI] [PubMed] [Google Scholar]
- Budinger GR, Duranteau J, Chandel NS, Schumacker PT. Hibernation during hypoxia in cardiomyocytes. Role of mitochondria as the O2 sensor. J Biol Chem. 1998;273:3320–3326. doi: 10.1074/jbc.273.6.3320. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretelli P. Limiting factors to oxygen transport on Mount Everest. J Appl Physiol. 1976;40:658–667. doi: 10.1152/jappl.1976.40.5.658. [DOI] [PubMed] [Google Scholar]
- Cerretelli P. Muscle energetics and ultrastructure in chronic hypoxia. Respiration Int Rev Thorac Dis. 1992;59(Suppl 2):24–29. doi: 10.1159/000196117. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. doi: 10.1212/wnl.48.2.306. [DOI] [PubMed] [Google Scholar]
- Chen S, Li G, Long L. Clinical research of ischemic preconditioning on lung protection. Hunan yi ke da xue xue bao=Hunan yike daxue xuebao=Bull Hunan Med Univ. 1999;24:357–359. [PubMed] [Google Scholar]
- Chi W, Meng F, Li Y, Wang Q, Wang G, Han S, Wang P, Li J. Downregulation of miRNA-134 protects neural cells against ischemic injury in N2A cells and mouse brain with ischemic stroke by targeting HSPA12B. Neuroscience. 2014;26:111–122. doi: 10.1016/j.neuroscience.2014.06.062. [DOI] [PubMed] [Google Scholar]
- Chin K, Yu X, Beleslin-Cokic B, Liu C, Shen K, Mohrenweiser HW, Noguchi CT. Production and processing of erythropoietin receptor transcripts in brain. Brain Res Mol Brain Res. 2000;81:29–42. doi: 10.1016/s0169-328x(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Christophe M, Nicolas S. Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm. 2006;12:739–757. doi: 10.2174/138161206775474242. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Fewell JE. Decreased body-core temperature during acute hypoxemia in guinea pigs during postnatal maturation: a regulated thermoregulatory response. Can J Physiol Pharmacol. 1996;74:331–336. [PubMed] [Google Scholar]
- Clinton M, Jr, Thorn GW, Davenport VD. Studies on normal human subjects-Effect of repeated short expousures to reduced atmospheric pressure. Bull Johns’ Hopkins Hosp. 1946;79:70–89. [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation: whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Constantino LC, Vandresen-Filho S, Tasca CI. Neuroprotection induced by NMDA preconditioning as a strategy to understand brain tolerance mechanism. Neural Regener Res. 2015;10:542–543. doi: 10.4103/1673-5374.155415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross KW, Tizard JP, Trythall DA. The gaseous metabolism of the newborn infant breathing 15% oxygen. Acta Paediatr. 1958;47:217–237. doi: 10.1111/j.1651-2227.1958.tb07879.x. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. [DOI] [PubMed] [Google Scholar]
- Cui C, Zhou T, Li J, Wang H, Li X, Xiong J, Xu P, Xue M. Proteomic analysis of the mouse brain after repetitive exposure to hypoxia. Chem Biol Interact. 2015;236:57–66. doi: 10.1016/j.cbi.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dahl NA, Balfour WM. Prolonged anoxic survival due to anoxia pre-exposure: brain atp, lactate, and pyruvate. Am J Physiol. 1964;207:452–456. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Neuronal ischaemic preconditioning. Trends Pharmacol Sci. 2000;21:423–424. doi: 10.1016/s0165-6147(00)01560-1. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Duan C, Yan F, Song X, Lu GW. Changes of superoxide dismutase: glutathione perioxidase and lipid peroxides in the brain of mice preconditioned by hypoxia. Biol Signals Recept. 1999;8:256–260. doi: 10.1159/000014595. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Science. Vol. 275. New York, N. Y: 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt; pp. 661–665. [DOI] [PubMed] [Google Scholar]
- Eddy LJ, Goeddel DV, Wong GH. Tumor necrosis factor-alpha pretreatment is protective in a rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 1992;184:1056–1059. doi: 10.1016/0006-291x(92)90698-k. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fazekas JF, Alexaander FAD, Himwich HE. Tolerance of the newborn to anoxia. Am J Physiol. 1941;134:281–287. [Google Scholar]
- Featherstone RL, Chambers DJ, Kelly FJ. Ischemic preconditioning enhances recovery of isolated rat lungs after hypothermic preservation. Ann Thorac Surg. 2000;69:237–242. doi: 10.1016/s0003-4975(99)01134-0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Bhatt AJ. Corticosteroid responses following hypoxic preconditioning provide neuroprotection against subsequent hypoxic-ischemic brain injury in the newborn rats. Int J Dev Neurosci. 2015;44:6–13. doi: 10.1016/j.ijdevneu.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Heredia N, Grande L, Gomez G, Rimola A, Marco A, Gelpi E, Rosello-Catafau J, Peralta C. Hepatology. Vol. 36. Baltimore, Md: 2002. Preconditioning protects liver and lung damage in rat liver transplantation: role of xanthine/xanthine oxidase; pp. 562–572. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Friedrich I, Spillner J, Lu EX, Bartling B, Barnscheid M, Sablotzki A, Schade U, Reidemeister JC, Silber RE, Gunther A, Borgermann J. Ischemic preconditioning of 5 minutes but not of 10 minutes improves lung function after warm ischemia in a canine model. J Heart Lung Transplant. 2001;20:985–995. doi: 10.1016/s1053-2498(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Fuller TF, Freise CE, Feng S, Niemann CU. Ischemic preconditioning improves rat kidney graft function after severe ischemia/reperfusion injury. Transplant Proc. 2005;37:377–378. doi: 10.1016/j.transproceed.2004.12.274. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- Garnier P, Demougeot C, Bertrand N, Prigent-Tessier A, Marie C, Beley A. Stress response to hypoxia in gerbil brain: HO-1 and Mn SOD expression and glial activation. Brain Res. 2001;893:301–309. doi: 10.1016/s0006-8993(01)02009-1. [DOI] [PubMed] [Google Scholar]
- Gasparri RI, Jannis NC, Flameng WJ, Lerut TE, Van Raemdonck DE. Ischemic preconditioning enhances donor lung preservation in the rabbit. Eur J Cardiothorac Surg. 1999;16:639–646. doi: 10.1016/s1010-7940(99)00335-8. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M, Zaoui D. Influence of halothane on control of breathing in intact and decerebrated cats. J Appl Physiol. 1987;63:546–553. doi: 10.1152/jappl.1987.63.2.546. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M, Trinh HC. Ventilatory and metabolic responses to cold and CO2 in intact and carotid body-denervated awake rats. J Appl Physiol. 1993;75:2570–2579. doi: 10.1152/jappl.1993.75.6.2570. [DOI] [PubMed] [Google Scholar]
- Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebrovascular ischemic protection by pre- and post-conditioning. Brain Circ. 2015;1:97–103. [Google Scholar]
- Ginis I, Zaner K, Wang JS, Pavlotsky N, Tauber AI. Comparison of actin changes and calcium metabolism in plastic- and fibronectin-adherent human neutrophils. J Immunol. 1992;149:1388–1394. [PubMed] [Google Scholar]
- Gong J, Gong S, Zhang M, Zhang L, Hu Y, Liu Y, Li W. Cerebral ischemic preconditioning reduces glutamate excitotoxicity by up-regulating the uptake activity of GLT-1 in rats. Amino Acids. 2014;46:1537–1545. doi: 10.1007/s00726-014-1723-1. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M, Wilson MA, Mallard C, Rousset C, Johnston MV, Hagberg H. Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res. 2007;61:444–450. doi: 10.1203/pdr.0b013e3180332be4. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Dammann O, Mallard C, Leviton A. Preconditioning and the developing brain. Semin Perinatol. 2004;28:389–395. doi: 10.1053/j.semperi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hakim AM. Could transient ischemic attacks have a cerebroprotective role? Stroke J Cereb Circ. 1994;25:715–717. doi: 10.1161/01.str.25.3.715. [DOI] [PubMed] [Google Scholar]
- Haldane JB. Carbon monoxide as a tissue poison. Biochem J. 1927;21:1068–1075. doi: 10.1042/bj0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999;19:6818–6824. doi: 10.1523/JNEUROSCI.19-16-06818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin DW, Barros D’Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg. 2002;35:1264–1273. doi: 10.1067/mva.2002.121981. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8:619–629. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Mo X, Gu H, Chen F, Gu Q, Peng W, Qi J, Shen L, Sun J, Zhang R, Kj Y. Neuroprotective effect of diazoxide on brain injury induced by cerebral ischemia/reperfusion during deep hypothermia. J Neurol Sci. 2008;268:18–27. doi: 10.1016/j.jns.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015a;11:698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- Hess DC, Khan MB, Morgan JC, Hoda MN. Remote ischemic conditioning: a treatment for vascular cognitive impairment. Brain Circ. 2015b;1:133–139. doi: 10.4103/2394-8108.172885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JR. The oxygen consumption of new-born and adult mammals. Its dependence on the oxygen tension in the inspired air and on the environmental temperature. J Physiol. 1959;149:346–373. doi: 10.1113/jphysiol.1959.sp006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Rupert JL, Monge C. Adaptation and conservation of physiological systems in the evolution of human hypoxia tolerance. Comp Biochem Physiol. 1999;124:1–17. doi: 10.1016/s1095-6433(99)00079-3. [DOI] [PubMed] [Google Scholar]
- Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Botker HE, Ostergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke J Cereb Circ. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- Houston CS, Riley RL. Respiratory and circulatory changes during acclimatization to high altitude. Amer J Physiol. 1947;149:565–588. doi: 10.1152/ajplegacy.1947.149.3.565. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ, Yang LF, Xiong L. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010;22:46–52. doi: 10.1097/ANA.0b013e3181c572bd. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder A, Laue C, Schrezenmeir J. Metabolic aspects of neonatal rat islet hypoxia tolerance. Transpl Int. 2010;23:80–89. doi: 10.1111/j.1432-2277.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Alterations in lysosomes (Intracellular enzymes) during shock; effects of preconditioning (Tolerance) and protective drugs. Int Anesthesiol Clin. 1964;2:251–269. doi: 10.1097/00004311-196402000-00008. [DOI] [PubMed] [Google Scholar]
- Jia J, Wang X, Li H, Han S, Zu P, Li J. Activations of nPKC epsilon and ERK1/2 were involved in oxygen-glucose deprivation-induced neuroprotection via NMDA receptors in hippocampal slices of mice. J Neurosurg Anesthesiol. 2007;19:18–24. doi: 10.1097/01.ana.0000211020.88431.e2. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001;21:1105–1114. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J Neurochem. 2004;89:157–167. doi: 10.1111/j.1471-4159.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- Jones NM, Lee EM, Brown TG, Jarrott B, Beart PM. Hypoxic preconditioning produces differential expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and its regulatory enzyme HIF prolyl hydroxylase 2 in neonatal rat brain. Neurosci Lett. 2006;404:72–77. doi: 10.1016/j.neulet.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Kew MC, First RG. A trial of Ro 4–2137 in the treatment of hypertension. Curr Ther Res Clin Exp. 1972;14:343–348. [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Li J, Konstantinov IE, Cheung MM, White PA, Frndova H, Stokoe J, Cox P, Vogel M, Van Arsdell G, MacAllister R, Redington AN. Remote ischaemic preconditioning protects against cardiopulmonary bypass-induced tissue injury: a preclinical study. Heart (British Cardiac Society) 2006;92:1506–1511. doi: 10.1136/hrt.2004.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Shutov LP, Gnanasekaran A, Lin Z, Rysted JE, Ulrich JD, Usachev YM. Nerve growth factor (NGF) regulates activity of nuclear factor of activated T-cells (NFAT) in neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen synthase kinase 3beta (GSK3beta) pathway. J Biol Chem. 2014;289:31349–31360. doi: 10.1074/jbc.M114.587188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K. Immunoreactive Akt: PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett. 1999;274:45–48. doi: 10.1016/s0304-3940(99)00676-x. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Yellon D. Does ischemic preconditioning occur in patients? J Am Coll Cardiol. 1994;24:1133–1142. doi: 10.1016/0735-1097(94)90880-x. [DOI] [PubMed] [Google Scholar]
- Koca K, Yurttas Y, Bilgic S, Cayci T, Topal T, Durusu M, Kaldirim U, Akgul EO, Ozkan H, Yanmis I, Oguz E, Tunay S, Korkmaz A, Basbozkurt M. Effect of preconditioned hyperbaric oxygen and ozone on ischemia-reperfusion induced tourniquet in skeletal bone of rats. J Surg Res. 2010;164:e83–89. doi: 10.1016/j.jss.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genom. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T, Yamaoka Y. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251–258. doi: 10.1016/s0022-2143(96)90026-8. [DOI] [PubMed] [Google Scholar]
- Laskey WK, Beach D. Frequency and clinical significance of ischemic preconditioning during percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:998–1003. doi: 10.1016/s0735-1097(03)00909-4. [DOI] [PubMed] [Google Scholar]
- Laudenbach V, Fontaine RH, Medja F, Carmeliet P, Hicklin DJ, Gallego J, Leroux P, Marret S, Gressens P. Neonatal hypoxic preconditioning involves vascular endothelial growth factor. Neurobiol Dis. 2007;26:243–252. doi: 10.1016/j.nbd.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lee JC, Tae HJ, Cho GS, Kim IH, Ahn JH, Park JH, Chen BH, Cho JH, Shin BN, Cho JH, Bae EJ, Park J, Kim YM, Choi SY, Won MH. Ischemic preconditioning protects neurons from damage and maintains the immunoreactivity of kynurenic acid in the gerbil hippocampal CA1 region following transient cerebral ischemia. Int J Mol Med. 2015;35:1537–1544. doi: 10.3892/ijmm.2015.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesar MA, Stoddard MF, Xuan YT, Tang XL, Bolli R. Nonelectrocardiographic evidence that both ischemic preconditioning and adenosine preconditioning exist in humans. J Am Coll Cardiol. 2003;42:437–445. doi: 10.1016/s0735-1097(03)00658-2. [DOI] [PubMed] [Google Scholar]
- Li G, Chen S, Lou W, Lu E. Ischemic preconditioning enhances donor lung preservation in canine lung transplantation. Chin Med J (Engl) 1998;111:870–873. [PubMed] [Google Scholar]
- Li G, Chen S, Lu E, Hu T. Ischemic preconditioning reduces lung ischemia reperfusion injury in vivo rabbits. Hunan Yi Ke Da Xue Xue Bao. 1999;24:319–321. [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Li S, Ma C, Shao G, Esmail F, Hua Y, Jia L, Qin J, Ren C, Luo Y, Ding Y, Borlongan CV, Ji X. Safety and feasibility of remote limb ischemic preconditioning in patients with unilateral middle cerebral artery stenosis and healthy volunteers. Cell Transplant. 2015;24:1901–1911. doi: 10.3727/096368914X683520. [DOI] [PubMed] [Google Scholar]
- Lindhardt TB, Gadsboll N, Kelbaek H, Saunamaki K, Madsen JK, Clemmensen P, Hesse B, Haunso S. Pharmacological modulation of the ATP sensitive potassium channels during repeated coronary occlusions: no effect on myocardial ischaemia or function. Heart (British Cardiac Society) 2004;90:425–430. doi: 10.1136/hrt.2002.006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin. 2009;30:1071–1080. doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Chen C, Li XR, Ran YY, Xu T, Zhang Y, Geng XK, Zhang Y, Du HS, Leak RK, Ji XM, Hu XM. Remote ischemic preconditioning-Mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci Ther. 2016;22:43–52. doi: 10.1111/cns.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu GW, Cui XY, Zhao BM. Alteration of oxygen consumption and energy metabolism during repetitive exposure of mice to hypoxia. Neurochem Res. 1999;24:625–628. doi: 10.1023/a:1021092023253. [DOI] [PubMed] [Google Scholar]
- Lu GW, Yu S, Li RH, Cui XY, Gao CY. Hypoxic preconditioning: a novel intrinsic cytoprotective strategy. Mol Neurobiol. 2005;31:255–271. doi: 10.1385/MN:31:1-3:255. [DOI] [PubMed] [Google Scholar]
- Lu GW. Adaption to hypoxia via tissue and cell approches. Zhongguo ying yong sheng li xue za zhi=Zhongguo yingyong shenglixue zazhi= Chin J Appl Physiol. 1963:169–273. [Google Scholar]
- Luh SP, Yang PC. Organ preconditioning: the past current status, and related lung studies. J Zhejiang Univ Sci. 2006;7:331–341. doi: 10.1631/jzus.2006.B0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Zhang H, Zhang WW, Huang JT, Song EL, Chen SG, He F, Xu J, Wang HQ. Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci Lett. 2011;498:227–231. doi: 10.1016/j.neulet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Nilsson GE. Vertebrate brains at the pilot light. Respiratory Physiol Neurobiol. 2004;141:285–296. doi: 10.1016/j.resp.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Lutz PL. Mechanisms for anoxic survival in the vertebrate brain. Annu Rev Physiol. 1992;54:601–618. doi: 10.1146/annurev.ph.54.030192.003125. [DOI] [PubMed] [Google Scholar]
- Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens. 2011;29:2265–2272. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- Mahi N, Kumar A, Jaggi AS, Singh N, Dhawan R. Possible role of pannexin 1/P2x7 purinoceptor in neuroprotective mechanism of ischemic postconditioning in mice. J Surg Res. 2015;196:190–199. doi: 10.1016/j.jss.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Makarewicz D, Sulejczak D, Duszczyk M, Malek M, Slomka M, Lazarewicz JW. Delayed preconditioning with NMDA receptor antagonists in a rat model of perinatal asphyxia. Folia Neuropathol. 2014;52:270–284. doi: 10.5114/fn.2014.45568. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- McCormick PH, Chen G, Tlerney S, Kelly CJ, Bouchier-Hayes DJ. Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. J Surg Res. 2003;109:24–30. doi: 10.1016/s0022-4804(02)00035-5. [DOI] [PubMed] [Google Scholar]
- Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12:667–677. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen S, Roivainen R, Keinanen R, Hokfelt T, Koistinaho J. Specific induction of protein kinase C delta subspecies after transient middle cerebral artery occlusion in the rat brain: inhibition by MK-801. J Neurosci. 1996;16:6236–6245. doi: 10.1523/JNEUROSCI.16-19-06236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA. Ischemic preconditioning blocks BAD translocation Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Zipes DP. Protection against autonomic denervation following acute myocardial infarction by preconditioning ischemia. Circ Res. 1989;64:437–448. doi: 10.1161/01.res.64.3.437. [DOI] [PubMed] [Google Scholar]
- Morte DD, Abete P, Gallucci F, Scaglione A, D’Ambrosio D, Gargiulo G, Rosa GD, Dave KR, Lin H, Cacciatore F, Mazzella F, Uomo G, Rundek T, Perez-Pinzon MA, Rengo F. Transient ischemic attack before nonlacunar ischemic stroke in the elderly. J Stroke Cerebrovasc Dis. 2008;17:257–262. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Matsuoka T. Interaction between CO2 production and ventilation in the hypoxic kitten. J Appl Physiol. 1993;74:905–910. doi: 10.1152/jappl.1993.74.2.905. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Rezzonico R, Lanthier C. Ventilation and oxygen consumption during acute hypoxia in newborn mammals: a comparative analysis. Respir Physiol. 1989;78:31–43. doi: 10.1016/0034-5687(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respiratory Physiol Neurobiol. 2004;141:345–356. doi: 10.1016/j.resp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011;25:70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- Nayak GH, Prentice HM, Milton SL. Neuroprotective signaling pathways are modulated by adenosine in the anoxia tolerant turtle. J Cereb Blood Flow Metab. 2011;31:467–475. doi: 10.1038/jcbfm.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndrepepa G, Mehilli J, Schwaiger M, Schuhlen H, Nekolla S, Martinoff S, Schmitt C, Dirschinger J, Schomig A, Kastrati A. Prognostic value of myocardial salvage achieved by reperfusion therapy in patients with acute myocardial infarction. J Nucl Med. 2004;45:725–729. [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Niu C, Li J, Cui X, Han S, Zu P, Li H, Xu Q. Changes in cPKC isoform-specific membrane translocation and protein expression in the brain of hypoxic preconditioned mice. Neurosci Lett. 2005;384:1–6. doi: 10.1016/j.neulet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119:139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjö BK, Hu BR. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt: release of cytochrome C and Activation of caspase-like proteases. J Cereb Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Ovize M, Kloner R, Przyklenk K. Preconditioning and myocardial contractile function. In: Przyklenk K, Kloner R, Yellon D, editors. Ischemic Preconditioning: The Concept of Endogenous Cardioprotection. Springer; US: 1994. pp. 41–60. [Google Scholar]
- Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. American J Physiol. 1997;273:H1707–1712. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- Pan LN, Zhu W, Li Y, Xu XL, Guo LJ, Lu Q, Wang J. Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS One. 2014;9:e99526. doi: 10.1371/journal.pone.0099526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AM, Nagase H, Vinod Kumar S, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol Heart Circ Physiol. 2007;292:751–757. doi: 10.1152/ajpheart.01016.2006. [DOI] [PubMed] [Google Scholar]
- Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–1547. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- Peng Z, Li J, Li Y, Yang X, Feng S, Han S, Li J. Downregulation of miR-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1. J Neurosci Res. 2013;91:1349–1362. doi: 10.1002/jnr.23255. [DOI] [PubMed] [Google Scholar]
- Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, Gelpi E, Rosello-Catafau J. Hepatology. Vol. 33. Baltimore, Md: 2001. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat; pp. 100–113. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Lutz PL, Sick TJ, Rosenthal M. Adenosine a retaliatory metabolite, promotes anoxia tolerance in turtle brain. J Cereb Blood Flow Metab. 1993;13:728–732. doi: 10.1038/jcbfm.1993.93. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–694. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Pinheiro DF, Fontes B, Shimazaki JK, Heimbecker AM, de Jacysyn JF, Rasslan S, Montero EF, Utiyama EM. Ischemic preconditioning modifies mortality and inflammatory response. Acta cirurgica brasileira/Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2016;31:1–7. doi: 10.1590/S0102-865020160010000001. [DOI] [PubMed] [Google Scholar]
- Pong K. Ischaemic preconditioning: therapeutic implications for stroke? Expert Opin Ther Targets. 2004;8:125–139. doi: 10.1517/14728222.8.2.125. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Liu Z, Yan X, Luo C. Effect of intermittent hypoxia on neuro-functional recovery post brain ischemia in mice. J Mol Neurosci MN. 2015;55:923–930. doi: 10.1007/s12031-014-0447-8. [DOI] [PubMed] [Google Scholar]
- Qin Z, Song S, Xi G, Silbergleit R, Keep RF, Hoff JT, Hua Y. Preconditioning with hyperbaric oxygen attenuates brain edema after experimental intracerebral hemorrhage. Neurosurg Focus. 2007;22:E13. doi: 10.3171/foc.2007.22.5.14. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- Ren C, Liu K, Li N, Cui X, Gao J, Ji X, Ding Y. Neural transmission pathways are involved in the neuroprotection induced by post- but not per-ischemic limb remote conditioning. Brain Circ. 2015;1:159–166. [Google Scholar]
- Riepe MW, Esclaire F, Kasischke K, Schreiber S, Nakase H, Kempski O, Ludolph AC, Dirnagl U, Hugon J. Increased hypoxic tolerance by chemical inhibition of oxidative phosphorylation: chemical preconditioning. J Cereb Blood Flow Metab. 1997;17:257–264. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Isaev N, Trendelenburg G, Weih M, Iurato L, Meisel A, Dirnagl U. Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci Lett. 1998;254:117–120. doi: 10.1016/s0304-3940(98)00688-0. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MA, Abdelsalam RM, Kenawy SA, Attia AS. Ischemic preconditioning and postconditioning alleviates hippocampal tissue damage through abrogation of apoptosis modulated by oxidative stress and inflammation during transient global cerebral ischemia-reperfusion in rats. Chem Biol Interact. 2015;232:21–29. doi: 10.1016/j.cbi.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilov M, Churilova A, Gluschenko T, Rybnikova E. Neocortical pCREB and BDNF expression under different modes of hypobaric hypoxia: role in brain hypoxic tolerance in rats. Acta Histochem. 2014;116:949–957. doi: 10.1016/j.acthis.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Samoilov M, Churilova A, Gluschenko T, Vetrovoy O, Dyuzhikova N, Rybnikova E. Acetylation of histones in neocortex and hippocampus of rats exposed to different modes of hypobaric hypoxia: implications for brain hypoxic injury and tolerance. Acta Histochem. 2016;118:80–89. doi: 10.1016/j.acthis.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Savithiry S, Kumar K. mRNA levels of Ca(2+)-independent forms of protein kinase C in postischemic gerbil brain by northern blot analysis. Mol Chem Neuropathol Spons Int Soc Neurochem World Fed Neurol Res Groups Neurochem Cerebrospinal Fluid. 1994;21:1–11. doi: 10.1007/BF03160080. [DOI] [PubMed] [Google Scholar]
- Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R. Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 2002;249:1503–1511. doi: 10.1007/s00415-002-0933-8. [DOI] [PubMed] [Google Scholar]
- Schaller B. Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevance. Neurosci Lett. 2005;377:206–211. doi: 10.1016/j.neulet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Schega L, Peter B, Torpel A, Mutschler H, Isermann B, Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59:316–323. doi: 10.1159/000350927. [DOI] [PubMed] [Google Scholar]
- Schurr A, Reid KH, Tseng MT, West C, Rigor BM. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–248. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- Selle WA, Witten TA. Survival of the respiratory(gasping) mechaism in young animals subjected to anoxia. Proc Soc Exp Biol Med. 1941;47:495–497. [Google Scholar]
- Shao G, Gao CY, Lu GW. Alterations of hypoxia-inducible factor-1 alpha in the hippocampus of mice acutely and repeatedly exposed to hypoxia. Neurosignals. 2005;14:255–261. doi: 10.1159/000088641. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 2004;1:26–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol. 1999;123:221–234. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Singer D. Metabolic adaptation to hypoxia: cost and benefit of being small. Respiratory Physiol Neurobiol. 2004;141:215–228. doi: 10.1016/j.resp.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Slomka M, Kuszczyk M, Lazarewicz JW, Makarewicz D. NMDA receptor antagonists MK-801 and memantine induce tolerance to oxygen and glucose deprivation in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2014;74:396–404. doi: 10.55782/ane-2014-2002. [DOI] [PubMed] [Google Scholar]
- Soncul H, Oz E, Kalaycioglu S. Role of ischemic preconditioning on ischemia-reperfusion injury of the lung. Chest. 1999;115:1672–1677. doi: 10.1378/chest.115.6.1672. [DOI] [PubMed] [Google Scholar]
- Song D, O’Regan MH, Phillis JW. Mechanisms of amino acid release from the isolated anoxic/reperfused rat heart. Eur J Pharmacol. 1998;351:313–322. doi: 10.1016/s0014-2999(98)00318-5. [DOI] [PubMed] [Google Scholar]
- Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB. Anoxia tolerance in turtles: metabolic regulation and gene expression. Comp Biochem Physiol. 2007;147:263–276. doi: 10.1016/j.cbpa.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004;113:14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroev SA, Gluschenko TS, Tjulkova EI, Rybnikova EA, Samoilov MO, Pelto-Huikko M. The effect of preconditioning on the Cu: zn superoxide dismutase expression and enzyme activity in rat brain at the early period after severe hypobaric hypoxia. Neurosci Res. 2005;53:39–47. doi: 10.1016/j.neures.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Suryana E, Jones NM. The effects of hypoxic preconditioning on white matter damage following hypoxic-ischaemic injury in the neonatal rat brain. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2014;37:69–75. doi: 10.1016/j.ijdevneu.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, Kinoshita M. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of remote preconditioning. J Am Coll Cardiol. 1999;33:556–564. doi: 10.1016/s0735-1097(98)00559-2. [DOI] [PubMed] [Google Scholar]
- Teoh LK, Grant R, Hulf JA, Pugsley WB, Yellon DM. The effect of preconditioning (ischemic and pharmacological) on myocardial necrosis following coronary artery bypass graft surgery. Cardiovasc Res. 2002;53:175–180. doi: 10.1016/s0008-6363(01)00435-7. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med. 2014;189:57–65. doi: 10.1164/rccm.201305-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Dave KR, Young JI, Perez-Pinzon MA. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics. 2013;10:789–797. doi: 10.1007/s13311-013-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Baker AJ. Glycolysis prevents anoxia-induced synaptic transmission damage in rat hippocampal slices. J Neurophysiol. 2000;83:1830–1839. doi: 10.1152/jn.2000.83.4.1830. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Tomimatsu T, Fukuda H, Endo M, Watanabe N, Mu J, Kohzuki M, Fujii E, Kanzaki T, Murata Y. Effects of hypothermia on neonatal hypoxic-ischemic brain injury in the rat: phosphorylation of Akt, activation of caspase-3-like protease. Neurosci Lett. 2001;312:21–24. doi: 10.1016/s0304-3940(01)02178-4. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Chakravarthy B, Hewitt K, Tauskela J, Morley P, Atkinson T, Durkin JP. Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of death signals. J Neurosci. 2000;20:7183–7192. doi: 10.1523/JNEUROSCI.20-19-07183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Vande Loock K, Ciardelli R, Decordier I, Plas G, Haumont D, Kirsch-Volders M. Preterm newborns show slower repair of oxidative damage and paternal smoking associated DNA damage. Mutagenesis. 2012;27:573–580. doi: 10.1093/mutage/ges022. [DOI] [PubMed] [Google Scholar]
- Vazquez de la Torre A, Junyent F, Folch J, Pelegri C, Vilaplana J, Auladell C, Beas-Zarate C, Pallas M, Verdaguer E, Camins A. PI3k/akt inhibition induces apoptosis through p38 activation in neurons. Pharmacol Res. 2013;70:116–125. doi: 10.1016/j.phrs.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg. 2010;44:434–439. doi: 10.1177/1538574410369709. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JN, Ding P, Huang YZ, Luo LN, Guo LY, Kong X, Shao F. The protective effect of PEP-1-SOD1 preconditioning on hypoxia/reoxygenation injury in cultured human umbilical vein endothelial cells. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:750–756. [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain?: A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Wiegand F, Liao W, Busch C, Castell S, Knapp F, Lindauer U, Megow D, Meisel A, Redetzky A, Ruscher K, Trendelenburg G, Victorov I, Riepe M, Diener HC, Dirnagl U. Respiratory chain inhibition induces tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1229–1237. doi: 10.1097/00004647-199911000-00007. [DOI] [PubMed] [Google Scholar]
- Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- Wood SC, Gonzales R. Hypothermia in hypoxic animals: mechanisms mediators, and functional significance. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:37–43. doi: 10.1016/0305-0491(95)02045-4. [DOI] [PubMed] [Google Scholar]
- Wood SC, Malvin GM. Physiological significance of behavioral hypothermia in hypoxic toads (Bufo marinus) J Exp Biol. 1991;159:203–215. doi: 10.1242/jeb.159.1.203. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen SX, Zhang WX. Effect of ischemic preconditioning on human lung cell apoptosis in vivo and the expression of regulating gene bcl-2. Hunan yi ke da xue bao=Hunan yike daxue xuebao=Bull Hunan Med Univ. 2002;27:43–45. [PubMed] [Google Scholar]
- Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P, Fan M. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 2008;1211C:22–29. doi: 10.1016/j.brainres.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Yun J, Li J, Zuo Z. Transferred inter-cell ischemic preconditioning-induced neuroprotection may be mediated by adenosine A1 receptors. Brain Res Bull. 2014;103:66–71. doi: 10.1016/j.brainresbull.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- Zhan L, Wang T, Li W, Xu ZC, Sun W, Xu E. Activation of Akt/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hypoxic pre-conditioning in adult rats. J Neurochem. 2010;114:897–908. doi: 10.1111/j.1471-4159.2010.06816.x. [DOI] [PubMed] [Google Scholar]
- Zhan L, Peng W, Sun W, Xu E. Hypoxic preconditioning induces neuroprotection against transient global ischemia in adult rats via preserving the activity of Na(+)/K(+)-ATPase. Neurochem Int. 2011;59:65–72. doi: 10.1016/j.neuint.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Zhan L, Yan H, Zhou H, Sun W, Hou Q, Xu E. Hypoxic preconditioning attenuates neuronal cell death by preventing MEK/ERK signaling pathway activation after transient global cerebral ischemia in adult rats. Mol Neurobiol. 2013;48:109–119. doi: 10.1007/s12035-013-8436-4. [DOI] [PubMed] [Google Scholar]
- Zhang CF, Chen SX. Clinical study of ischemic preconditioning on isolated lung perfusion with chemotherapeutic agents in the treatment of unresectable lung cancer. Hunan yi ke da xue xue bao=Hunan yike daxue xuebao=Bull Hunan Med Univ. 2001;26:51–54. [PubMed] [Google Scholar]
- Zhang T, Zhang F, Zhou YA, Wang YJ, Li XF, Chen DF. Effect of ischemic preconditioning on cytokines during lung ischemia-reperfusion injury. Zhonghua wai ke za zhi [Chinese J Surg] 2003;41:545–547. [PubMed] [Google Scholar]
- Zhang N, Gao G, Bu X, Han S, Fang L, Li J. Neuron-specific phosphorylation of c-Jun N-terminal kinase increased in the brain of hypoxic preconditioned mice. Neurosci Lett. 2007;423:219–224. doi: 10.1016/j.neulet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Zhang YB, Lu GW, Yang MF, Niu JZ, Sun BL. Changes in Bcl-2 and Caspase-3 expressions in cortex of hypoxic preconditioning mice. Sheng Li Xue Bao. 2008;60:249–253. [PubMed] [Google Scholar]
- Zhang YB, Guo ZD, Li MY, Li SJ, Niu JZ, Yang MF, Ji XM, Lu GW. Cerebrospinal fluid from rats given hypoxic preconditioning protects neurons from oxygen-glucose deprivation-induced injury. Neural Regener Res. 2015;10:1471–1476. doi: 10.4103/1673-5374.165519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao L, Liu X, Liang J, Han S, Wang Y, Yin Y, Luo Y, Li J. Phosphorylation of p38 MAPK mediates hypoxic preconditioning-induced neuroprotection against cerebral ischemic injury via mitochondria translocation of Bcl-xL in mice. Brain Res. 2013a;150:378–388. doi: 10.1016/j.brainres.2013.01.051. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zhang Z, Cease A, Harrison J, Kang L. Efficient utilization of aerobic metabolism helps Tibetan locusts conquer hypoxia. BMC Genom. 2013b;14:631. doi: 10.1186/1471-2164-14-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong GQ, Tu RH, Zeng ZY, Li QJ, He Y, Li S, He Y, Xiao F. Novel functional role of heat shock protein 90 in protein kinase C-mediated ischemic postconditioning. J Surg Res. 2014;189:198–206. doi: 10.1016/j.jss.2014.01.038. [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhan L, Liang D, Hu J, Lu Z, Zhu X, Sun W, Liu L, Xu E. Hypoxia-inducible factor 1alpha mediates neuroprotection of hypoxic postconditioning against global cerebral ischemia. J Neuropathol Exp Neurol. 2014;73:975–986. doi: 10.1097/NEN.0000000000000118. [DOI] [PubMed] [Google Scholar]