Abstract
Auditory sensory gating, assessed in a paired-click paradigm, indicates the extent to which incoming stimuli are filtered, or “gated”, in auditory cortex. Gating is typically computed as the ratio of the peak amplitude of the event related potential (ERP) to a second click (S2) divided by the peak amplitude of the ERP to a first click (S1). Higher gating ratios are purportedly indicative of incomplete suppression of S2 and considered to represent sensory processing dysfunction. In schizophrenia, hallucination severity is positively correlated with gating ratios, and it was hypothesized that a failure of sensory control processes early in auditory sensation (gating) may represent a larger system failure within the auditory data stream; resulting in auditory verbal hallucinations (AVH).
EEG data were collected while patients (N = 12) with treatment-resistant AVH pressed a button to indicate the beginning (AVH-on) and end (AVH-off) of each AVH during a paired click protocol. For each participant, separate gating ratios were computed for the P50, N100, and P200 components for each of the AVH-off and AVH-on states. AVH trait severity was assessed using the Psychotic Symptoms Rating Scales AVH Total score (PSYRATS).
The results of a mixed model ANOVA revealed an overall effect for AVH state, such that gating ratios were significantly higher during the AVH-on state than during AVH-off for all three components. PSYRATS score was significantly and negatively correlated with N100 gating ratio only in the AVH-off state.
These findings link onset of AVH with a failure of an empirically-defined auditory inhibition system, auditory sensory gating, and pave the way for a sensory gating model of AVH.
Keywords: Auditory sensory gating, hallucinations, auditory verbal hallucinations, schizophrenia, EEG, ERP, auditory processing
1. Introduction
Auditory verbal hallucinations (AVH) involve the perception of speech in the absence of external auditory stimulation. AVH often contain derogatory and threatening content, thereby increasing patient anxiety and possibly encouraging social withdrawal (Delespaul et al. 2002). While often benign, AVH have also been reported in non-clinical populations; yet the consequences and content of AVH in patients with schizophrenia tend to be more negative and severe. Many authors have linked the etiology of schizophrenia AVH to auditory processing abnormalities. For example, the efference-copy model of AVH developed by Ford and colleagues (2001, 2007, 2012), suggests that AVH arise from an impaired ability to correctly label auditory verbal processing as either internally self-generated, or as externally generated. For example, Ford et al (2001) showed that a pattern of larger N100 response to externally-generated speech sounds relative to the N100 response to their own recorded speech sounds found in healthy controls was not observed in patients with schizophrenia. Similarly, other research has suggested that the auditory system of patients with schizophrenia may preferentially respond to emotionally salient or voice-like sounds, as indicated by increased reported detection of speech sounds in noise (Vercammen et al. 2008). Patients with more severe schizotypy have also been shown to report a stronger perception of emotionally salient voice sounds in noise (Galdos et al. 2011). In a parallel line of research, Woodruff et al (1997) showed that BOLD signal response in language-processing areas of the brain in patients reporting hallucinations were reduced in response to speech sounds; they hypothesized that this result was due to competition between hallucinations and the external stimuli for temporal cortical processing sites.
One means of directly measuring auditory perceptual abnormality is by measurement of auditory sensory gating. When assessed in terms of a paired-click paradigm, sensory gating is one means of measuring modulation of incoming auditory information as early as 50 milliseconds into cortical processing. The paired-click paradigm involves presentation of two clicks in rapid succession. In the auditory event-related potential (AERP), a large reduction of the response to the second stimulus (S2) relative to that of the first (S1) is interpreted as effective suppression of redundant stimulus information, and a reduction of 67% (S2/S1 = 1/3) or more is common in healthy neurotypical control subjects (Cromwell et al. 2008). Smaller reduction, or lack of reduced relative AERP amplitude to S2 is typical of patients with schizophrenia (Patterson et al. 2008). Two recent studies have directly tested the hypothesis that sensory gating, as a measure of schizophrenia abnormality of auditory perceptual processing, is related to AVH. Using the PANSS Item P3, a general measure of hallucination frequency and severity across sensory modalities, Faugère et al (2016) demonstrated higher AVH scores (item P3) in a group of schizophrenia patients with greater P50 sensory gating impairment relative to that of a group without P50 sensory gating impairment. Smith et al (2013) demonstrated a positive correlation between the extent of P50 sensory gating deficit and the severity of AVH, assessed with the psychotic symptom rating scales (PSYRATS).
Sensory gating ratios are stable across time and are extremely reliable in healthy neurotypical subjects (Rentzsch et al., 2008; Fuerst et al., 2007). In patients with schizophrenia, gating ratios can be far more variable (Smith et al., 1994), but the sources of that variability are unknown. The present study was designed to test whether auditory sensory gating is a state characteristic that varies as does AVH state (AVH-on versus AVH-off), or whether it may be better considered as a trait marker for AVH. Using a button-press protocol to have patients indicate when AVH-related voices started and stopped, distinct periods of AVH-on and AVH-off were identified. It was predicted that if sensory gating is sensitive to state characteristics within the auditory processing system, sensory gating ratios would evince greater impairment during AVH-on than during AVH-off.
2. Experimental/Materials and methods
2.1. Participants
Participants were recruited through referral by other researchers, from the University of New Mexico Health Sciences Center and other community clinics, and through ads posted on bulletin boards throughout the Albuquerque metropolitan area. All data were collected only after review and approval of the study by the University of New Mexico Health Sciences Center Human Subjects Protections Office (HRPO). All participants provided written informed consent and were informed that they could leave the study at any time without penalty. Inclusion criteria required age range was 18–60 years old, and diagnoses of schizophrenia or schizoaffective disorder, which was confirmed with SCID-CV. All participants had a history of frequent AVH documented in the medical record, and AVH frequency of at least two AVH per hour mixed with non-AVH periods, as determined by a hallucination “diary” which was filled out across the week preceding scanning. Exclusionary criteria were history of head injury with more than 5 minutes of unconsciousness, diagnosis of neurological disorder or disease, and current alcohol or other substance dependence. All participants underwent urine analysis and Breathalyzer to exclude acute drug or alcohol intoxication during data collection. All scans were acquired at the Mind Research Network (MRN) neuroimaging facility in Albuquerque, NM. Magnetoencephalography (MEG) data were simultaneously collected with EEG data for future analysis and structural MRI data were collected for signal localization. As these data were collected as part of a larger study, prior to enrollment, prospective participants’ head size was measured to assure they would fit within the MEG helmet and metal screening information was collected to assure that they were safe and comfortable within the MRI environment prior to study enrollment.
2.2. Data Collection Procedures
Twelve participants met all study criteria and completed the EEG scanning protocol. Of those, ten participants were administered the Positive and Negative Schizophrenia Syndromes Scale (PANSS; Kay et al., 1989) and the Psychotic Symptoms Rating Scales (PSYRATS; Haddock et al. 1999) on the day of scanning to assess the frequency and severity of their AVH. In the days prior to scanning, all participants were administered a structured button-press training course in which they were trained to reliably recognize the onset and off-set of AVH and to press appropriate buttons to indicate the beginning (AVH-on) and end (AVH-off) of each AVH. Practice on button-press procedures was administered again immediately prior to scanning for review. To assure adherence to AVH-reporting procedures, participants were continuously monitored by study personal during scanning to assure that the sequencing of button presses were appropriate to the AVH-on and AVH-off pattern. Additionally, during data analysis, epochs occurring during periods in which there were multiple consecutive button presses with the same hand were considered artifactual and those data were removed from further analysis.
Participants were seated in a comfortable reclining chair in which head motion was minimized by the use of pillows and foam cushions around the head, neck, and if necessary, the lower back and under the knees. Participants were equipped with gloves fitted with buttons for right and left index fingers, indicating AVH-on with a single right-hand button press (and immediate release) and AVH-off with a single left-hand button press. All EEG data were collected in a single session. EEG data were collected with eyes open, and participants were instructed to gaze at a continuous fixation point (small black cross) projected on the center of a white projection screen placed 36 inches from their face. EEG scans were conducted in a magnetically shielded and acoustically insulated room (Vacuumschmelze GmbH & Co. KG).
Click stimuli were created with Audacity® software, were 3 milliseconds (ms) in duration, square-wave pulses with spectral power across the 8–22,000 Hz range. Stimuli were delivered using Presentation® software into the participant’s ear canal using Etymotic earphones. Foam ear inserts were affixed within both ears and hearing thresholds for click stimuli were determined for each ear, for each subject. Click intensity was set to 30 dB above the participant’s hearing threshold within the Presentation software. The Presentation software was tested independently using a sound meter to ensure that software dB settings were calibrated. Click pairs were then presented binaurally to the participant with a 500 ms inter-stimulus interval and variable inter-trial-intervals that varied pseudo-randomly by 1s intervals between 8 and 12 seconds.
EEG data were collected using a 10–20 electrode array, but latency and amplitude measurements were computed on the basis of data derived from electrode Cz referenced to left earlobe. Maximum impedance of 10 kOhm was allowed for all electrodes. EEG data were digitized at 1200 Hz during data collection and down-sampled to 300 Hz offline prior to analysis. Eye motion was monitored within independent bipolar vertical electrooculogram (VEOG) and horizontal electrooculogram (HEOG). Epochs in which raw EEG or EOG amplitude greater than 100μV were discarded as artifacts and a minimum of 150 trials of artifact-free trials were collected for all participants. EEG highpass (0.1 Hz) and lowpass (330 Hz) filters were set to minimal system allowable levels during data collection.
After scanning, participants were administered a post-scan interview to review their experiences during scanning, including level of comfort during the scan, stress-level, and confirmation that button presses coincided with AVHs.
2.3. EEG Data Analysis Procedures
EEG waveforms were averaged off-line after initial data processing using Elekta Neuromag software and the MNE Suite (Gramfort et al., 2014), with trials averaged from 100 ms prior to stimulus onset to 450 ms post-stimulus onset. Epochs were first divided into bins for AVH-on and AVH-off conditions and within each bin data time-locked to S1 and S2 were averaged separately. Data were bandpass filtered at 1–30 Hz prior to assessment of ERP amplitude and latency using standard Elekta Neuromag filter software. Prestimulus baseline amplitude was computed in the averaged data from −100 to −10 ms prior to stimulus onset and was used for ERP normalization. The P50 component associated with S1 was defined as the most positive peak in electrode Cz occurring between 40 and 80 ms post stimulus. If two equal-amplitude peaks were present, the later peak was selected. Amplitude was measured relative to the immediately preceding negativity, though this trough could not have a latency less than 30 ms poststimulus (i.e. the trough search was stopped if a horizontal slope was not encountered by 30 ms poststimulus, and the 30 ms point was then used as the trough amplitude). The S1 amplitude was then computed as the difference between the P50 maxima and the minima associated with the preceding trough. The P50 component associated with S2 was defined as the most positive point at Cz following S2 onset within a latency range of ±10 ms around the latency of the selected S1 P50 peak. S2 amplitude was calculated relative to the immediately preceding trough applying the same 30 ms latency limitation used for S1. The N100 component associated with S1 was operationalized as the largest negative deflection between 80 and 150 ms post-stimulus. The N100 component associated with S2 was defined as the most negative trough following S2 onset within a latency range of ±15 ms around the latency of the selected S1 N100 peak. N100 peak amplitudes were computed as sum of the absolute value of the amplitude from the preceding P50 peak to the N100 minima (Hu et al., 2012). The P200 component associated with S1 was defined as the largest positive deflection between 150 and 250 ms post-stimulus. The P200 component associated with S2 was defined as the most negative trough following S2 onset within a latency range of ±20 ms around the latency of the selected S1 P200 peak. P200 peak amplitudes were computed as the sum of the absolute value of the preceding N100 minima and the P200 maxima.
2.4. AVH Assessment Procedures
AVH were assessed in two ways; as a state measure, participants pushing buttons to indicate AVH-on and AVH-off during scanning; and as a trait measure, based upon participants’ responses to items on the Psychotic Symptoms Rating Scales (PSYRATS; Haddock, 1999) AVH assessment. The PSYRATS is a multidimensional instrument, measuring AVH in terms of eleven scales scored using a Likert scale ranging from 0–4. A PSYRATS Total AVH score was computed as the sum of the PSYRATS scale scores for each participant.
3. Results
3.1. Participant Characteristics
Participants ranged in age from 29 to 55 years-old, with a mean age of 41. The group was comprised of eight males and four females. All participants PANSS Hallucinatory Behavior Scale scores were equal to four or greater. Aggregate AVH severity was assessed in terms of PSYRATS AVH Total score, which ranged from 22 to 37, with a mean of 29.67 and standard deviation of 5.41. PSYRATS scores and detailed clinical data were unavailable for two participants for whom only EEG and core diagnostic and demographic data were collected. Table 1 details the demographic and clinical information for each participant. In post-scan interviews, ten participants denied elevated stress levels during data collection and two reported mild elevations, one due to mild claustrophobia and one due to fatigue during scanning.
Table 1.
Participant Demographic and Diagnostic Information
| Subject | Primary Diagnosis | Age | Gender | Handedness | Duration of Illness (yrs) | Antipsychotic Medication* (dose**) |
|---|---|---|---|---|---|---|
| 1 | Schizophrenia | 28 | M | R | 11 | olanzapine (20) |
| 2 | Schizophrenia | 39 | M | R | 17 | clozapine/aripiprazole (30) |
| 3 | Schizophrenia | 50 | M | R | 12 | haloperidol (6.65) |
| 4 | Schizoaffective | 20 | F | R | 30 | none |
| 5 | Schizoaffective | 53 | F | R | 32 | risperidone/clozapine (46.64) |
| 6 | Schizophrenia | 55 | F | R | 17 | perphenazine (¥) |
| 7 | Schizophrenia | 39 | M | R | 18 | Risperidone/clozapine (13.32) |
| 8 | Schizophrenia | 46 | M | R | 5 | aripiprazole (6.7) |
| 9 | Schizophrenia | 50 | M | R | 26 | clozapine/aripiprazole (22.56) |
| 10 | Schizophrenia | 29 | M | L | 41 | clozapine (20) |
| 11 | Schizophrenia | 41 | M | du | du | du |
| 12 | Schizophrenia | 37 | F | du | du | du |
du= data unavailable
primary/secondary antipsychotic medication
dosages reported in total olanzapine mg/day equivalent (Gardner et al., 2010)
dosage information not available
3.2. EEG Data Processing
Due to the nature of the symptom capture protocol, the number of epochs within each bin for AVH state varied across conditions. Mean number of epochs within the AVH-on state was 56.75 (standard deviation = 22.84) and epochs for the AVH-off state was 49.50 (standard deviation = 24.09), which was not a significant difference (p = .64). For one participant, the number of epochs in the AVH-on state was 9 (AVH-off epochs = 79) and for a second, the number of epochs in the AVH-off state was 12 (AVH-on epochs = 42) and these data were removed from the analysis.
3.3. Auditory Sensory Gating
Distributions of gating ratios (S2/S1) all met Shapiro-Wilk criteria for normality. As such, gating ratios were analyzed using a mixed model ANOVA with component (P50, N100, P200) and AVH State (AVH-on, AVH-off) as independent variables. The main effect of Component was significant (F(2,10) = 6.48, p = .02, partial eta-squared = .62), indicating that gating ratios were highest for N100, across AVH state (see Table 2). The main effect of AVH state was also significant (F(2,10) = 8.58, p = .02, partial eta-squared = .49), such that gating ratios were significantly greater during the AVH-off condition than in the AVH-on condition. The two-way Component*AVH state interaction did not approach the level of significance (p = .86; partial eta-squared = .04), suggesting that the effect of AVH state on gating ratio was uniform across components.
Table 2.
Descriptive statistics for gating ratio by component and condition (N = 12)
| Component | Gating ratio (mean(sd)) | Range | ||
|---|---|---|---|---|
|
| ||||
| AVH-off | AVH-on | AVH-off | AVH-on | |
| P50 | .46(.35) | .69(.35) | .03–1.21 | .23–1.33 |
| N100 | .54(.31) | .86(.39) | .08–1.06 | .24–1.44 |
| P200 | .49(.25) | .70(.25) | .17–.90 | .30–1.24 |
3.4. Analysis of ERP Amplitudes and Latencies
Figure 2 depicts grand-averaged waveforms for S1 and S2 in the AVH-on and AVH-off states.
Figure 2.
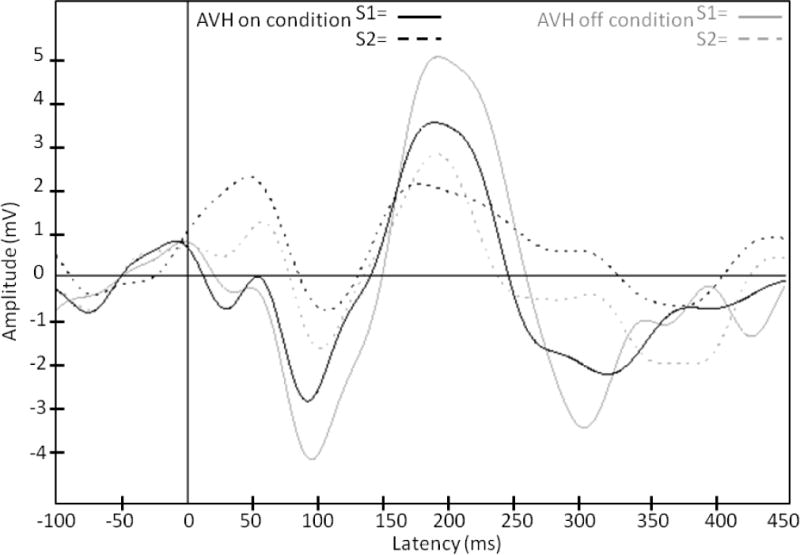
Group mean ERP waveforms depicting S1 and S2 during AVH-on (solid lines) and AVH-off (dotted lines).
Since gating ratios can vary based on either S1 or S2 amplitude in (μV), and because not all distributions of S1 and S2 amplitudes met criteria for normality, the effect of AVH state on AERP amplitudes was analyzed using Wilcoxon signed rank tests. There was a trend toward an effect of AVH state on N100 S1 amplitude (z(10) = −1.78, p = .07), such that N100 amplitude was greater during AVH-off relative to AVH-on. No other comparisons approached the level of significance.
Since S2 latency is by definition, linked to S1 latency for each component, only S1 latencies were investigated. Using Wilcoxon signed rank tests, there was no evidence of an effect for AVH state on AERP latency across components.
3.5. Analysis of PSYRATS Total AVH score
Pearson correlation coefficients were computed to test the hypothesis that higher gating ratio would predict more severe AVH, assessed in terms of PSYRATS Total AVH score. The only association between component gating ratio and AVH PSYRATS was a significant negative correlation between N100 gating ratio during AVH-off (r = −.94, p < .001).
To further investigate this effect, analysis of N100 S1 and S2 amplitudes in the AVH-off state showed that PSYRATS Total score was significantly and negatively correlated with S2 amplitude (r = −.65, p = .04) and positively, but non-significantly correlated with S1 (r = .44, p = .19). Of the PSYRATS subscale scores, N100 sensory gating ratio significantly predicted the Amount of Distress (r = −.76, p = .02), Intensity of Distress (r = .80, p = .009), and Disruption (r = −.94, p <.001) subscale scores.
To investigate whether overall gating ratios were predictive of PSYRATS Total score, mean gating ratios were computed for the P50, N100, and P200 components across AVH states ((AHV-on+AVH-off)/2). Correlations between PSYRATS total score and the P50 (r = −.18, p = .64), N100 (r = −.34, p = .36), and P200 (r = −.44, p = .24) mean gating ratios did not approach the level of significance.
3.8. Analysis of Demographic and Clinical Variables
Potential confounding variables including age, gender, handedness, and duration of illness were considered as covariates across all sensory gating analyses. Medication was assessed in terms of dummy-coded nominal variables for each medication type, in terms of novel (N = 8) and conventional (N = 2) antipsychotic, and in terms of an olanzapine-equivalent, continuous variable. No measures of medication had a significant effect on reported results.
4. Discussion
Gating ratios during AVH-off were in the range that is typical for schizophrenia (Hu et al., 2012). During AVH-on, however, gating ratios were significantly greater, suggesting significantly greater gating impairment, which was consistent with the hypothesis that auditory sensory gating ratio is sensitive to AVH-state. These results were true for gating assessed across the P50, N100, and P200 AERP components, suggesting that there is an overall disconnect or failure between sensory systems and gating modulation systems. The present results complement the AVH neurophysiology literature in that they link failure of an empirically defined inhibitory mechanism to the occurrence of AVH. As auditory sensory gating is considered to be one of the core inhibitory systems operating to control auditory input (Boutros et al., 2017), the present findings showing failure of sensory gating during AVH-on is further confirmation of the notion that AVH reflect a failure of inhibitory processes within auditory and linguistic systems (Hoffman et al., 2002, Kompas et al., 2011). Failure of the system used to inhibit incoming auditory input during self-generated internal speech is purported to underlie an individual’s ability to appropriately label speech as being generated internally or externally during AVH (Ford et al., 2012).
All three components assessed in the present study, P50, N100, and P200, are known to be subject to the gating effect (Buchsbaum, 1977; Boutros and Belger, 1999), and bottom-up models of sensory gating suggest that gating deficits early in perception may lead to information overload in downstream processing systems and ultimately result in the positive symptoms of schizophrenia (McGhie and Chapman, 1961). A more recent model of the control of gating suggests that the earliest AERP components are most subject to modulation based upon stimulus characteristics, but that the control network gets more elaborate and increasingly under top down control by prefrontal and parietal regions as component latency progresses (Boutros et al., 2013). In the present analyses, stimulus characteristics were identical between the AVH-off and AVH-on states, minimizing the effect of stimulus characteristics, and there was no interaction between AVH state and component. This would suggest that there has been a collapse of the top-down modulatory networks controlling gating more generally. As such, the present neurophysiological analysis is consistent with that of our recent fMRI network analysis (Thoma et al., 2016), the results of which suggested that as one moves into the AVH-on state, pre-frontally mediated networks, presumably associated with executive control, go off-line, and a predominant temporal lobe AVH network emerges supporting the experience of AVH.
It is likely that there are multiple mechanisms working in parallel in support of sensory gating (Boutros et al., 2013) and the reduction of N100 S1 amplitude during the AVH-on state is perhaps evidence of a second independent process at work. The effect, consistent with the findings of Hubl et al (2007), was not found in the P200 S1, and the present analyses showing that it also is not characteristic of P50, suggests that this effect is truly specific to the N100. Hubl and colleagues (2007) interpreted this finding as evidence that the N100 amplitude reduction is due to competition for limited neuronal resources by AVH, but added that it may also be an indication that there is simply an inability to focus attention on auditory inputs. The present results serve to further emphasize the importance of N100 changes with regard to AVH, in that of the three components measured, only N100 gating was found to be predictive of PSYRATS-assessed AVH severity at the trait level. Although this correlation was primarily driven by changes in S2, there was also a non-significant positive correlation of moderate effect size between S1 amplitude and AVH severity suggesting perhaps a third possibility; that the acute reduction in N100 S1 amplitude during AVH-on is a corrective one representing an attempt to “turn down the volume” on perceived voices.
At the outset of this study it was expected that greater severity of a core positive symptom, AVH assessed at the trait level, would also be reflected as greater impairment in sensory gating, based on the positive correlation between PSYRATS score and P50 gating reported by Faugère et al (2016). Not only was the AVH severity and gating relationship specific to gating of the N100 component, the valence of that correlation was opposite to that predicted. An important difference between the current results and the previous report was that we divided the epochs into AVH-on and AVH-off conditions and the unexpected result only occurred during the AVH-off condition for the N100 component. This seemingly paradoxical relationship might be best explained if considered in terms of relative changes in S1 and S2. The suppression of S2 is thought to be the result of an active sensory filtering mechanism (Boutros et al., 2013) and one possibility is that during AVH, greater S2 suppression occurs as a second corrective mechanism. Hence, a more severe AVH experience results in a greater attempt to reduce the processing of invasive voices perceived to be incoming from the environment. A second possibility is that greater suppression of S2 is one consequence of AVH network formation (Thoma et al., 2016), resulting in reduced processing of stimuli from the external environment and increased processing of internally generated data, ultimately resulting in more severe AVH. Of course, it is possible that both mechanisms are operating simultaneously resulting in a vicious circle in which greater S2 suppression leads to increasing AVH severity and greater suppression of S2.
To assure a comprehensive analysis, medications were evaluated based on individual medication, classification (novel or conventional) and olanzapine equivalents. It was perhaps not surprising that no effect of medication was found on AVH or gating, as the participants in this study were selected for experiencing AVH despite ongoing pharmacologic treatment. Additionally, due to the small sample size, it is not possible to conclude that a lack of findings for medication effects may be interpreted as a true lack of a medication effect.
The conclusions made in the present study are hampered by small sample size although this is offset to some degree based on the within-subjects design, and this research would benefit from replication in a larger sample. Questions regarding the extent to which patients are able to successfully indicate the onset or offset of AVH may bear on the present conclusions, but data presented elsewhere demonstrated a high test-retest reliability of our button press protocol in an overlapping sample of schizophrenia patients (Thoma et al., 2016). Lastly, these findings emphasize the importance of using the temporal sensitivity of neurophysiological measures to better understand the state-related changes in neural functioning underlying schizophrenia symptoms.
In conclusion, during AVH-on state, auditory sensory gating becomes further impaired, indicating that sensory gating is sensitive to AVH-state. This result better defines the link between sensory gating and positive symptoms, and suggests that perhaps gating must also be considered as a marker of state, in addition to a trait characteristic of schizophrenia. The change in correlations between AVH severity and gating ratios depending upon AVH state serves to demonstrate the extent of change in auditory processing with AVH-onset (or offset), and suggests that perhaps a sensory gating model of AVH may help to advance our knowledge of the neurophysiology of schizophrenia hallucinations.
Figure1.
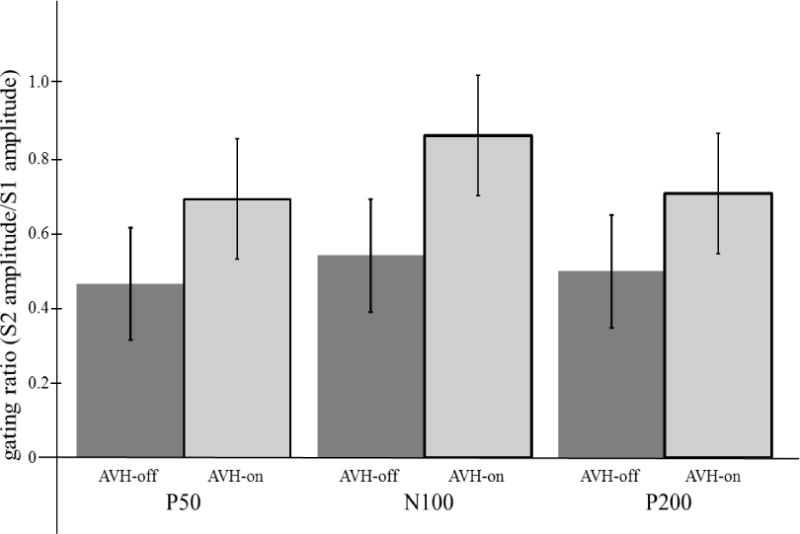
Group means and standard deviations for P50, N100, and P200 sensory gating ratios (S2/S1) for the AVH-on and AVH-off states.
Figure 3.
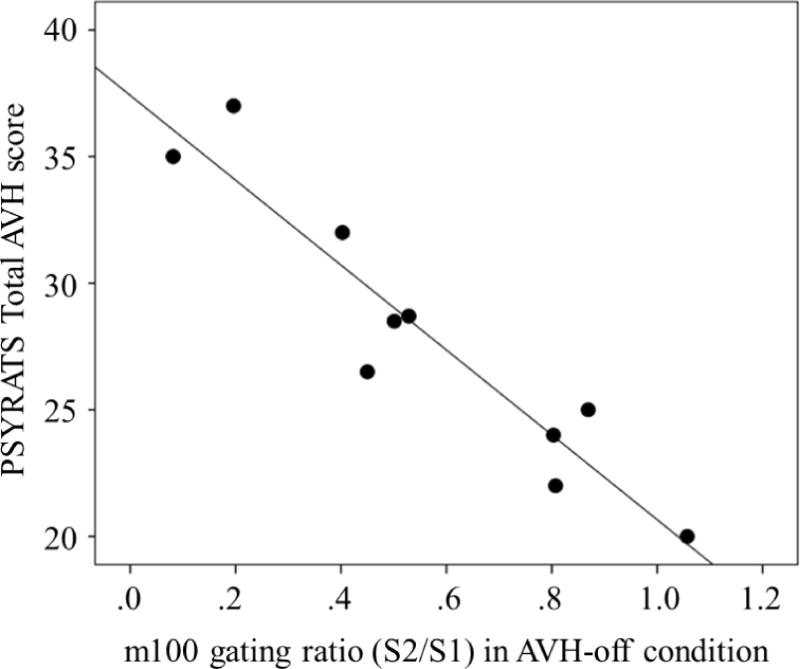
Scatterplot depicting the relationship between N100 sensory gating ratio and a trait measure of AVH severity (PSYRATS Total AVH score).
Figure 4.
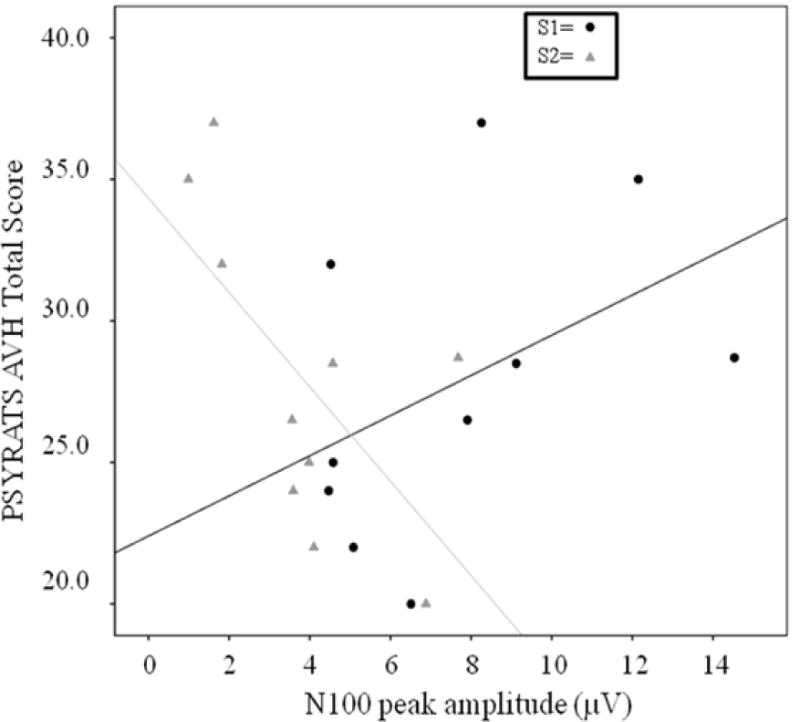
Scatterplot depicting the relationships between S1 and S2 amplitude and trait AVH severity (PSYRATS Total AVH score).
Table 3.
Descriptive statistics for peak amplitude (uV) and latency (ms) by component and condition (N = 12)
| Component | S1 AVH-off | S1 AVH-on | S2 AVH-off | S2 AVH-on | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| amplitude | latency | amplitude | latency | amplitude | latency | amplitude | latency | |
| P50 | 3.57(2.76) | 57.7(12.5) | 3.76(3.95) | 56.4(14.1) | 2.05(2.16) | 58.3(13.0) | 2.45(2.50) | 55.0(13.6) |
| N100 | 7.46(3.39) | 97.5(18.7) | 5.32(3.8) | 97.9(17.7) | 3.62(2.21) | 94.1(21.3) | 3.99(2.09) | 94.7(15.4) |
| P200 | 9.77(5.61) | 183.6(17.3) | 6.44(3.01) | 188.9(22.4) | 4.28(2.77) | 179.4(22.4) | 4.32(2.11) | 164.3(30.2) |
Acknowledgments
We thank Juan Bustillo, MD, Jessica Turner, Ph.D., and Cheryl Aine, Ph.D. for their support and guidance as this project progressed to its fruition.
Funding body agreements and policies
The first author (Thoma) was supported by an NIGMS COBRE (Phase I: P20GM103472) Early Investigator Award. The second author (Meier) by the UNM Postbaccalaureate Research and Education Program, funded by R25 GM075149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
Contributors
Dr. Thoma was directly involved in all aspects of the research. Dr. Calhoun worked with the first author on conceptualization and design of the research. Andrew Meier and Dr. Houck were responsible for data collection, database management, EEG preprocessing, and analysis. Dr. Lewine, Clark, and Stephen assisted with statistical analysis of the data and helped to guide the manuscript to completion. All authors participated in writing and editing the manuscript for final submission.
References
- Boutros NN, Belger A. Midlatency evoked potentials attenuation and augmentation reflect different aspects of sensory gating. Biol Psychiat. 1999;45(7):917–922. doi: 10.1016/s0006-3223(98)00253-4. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gjini K, Eickhoff SB, Urbach H, Pflieger M. Mapping repetition suppression of the P50 evoked response to the human cerebral cortex. Clin Neurophysiol. 2013;124(4):675–685. doi: 10.1016/j.clinph.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS. The middle evoked response components and schizophrenia. Schiz Bull. 1977;3:93–104. doi: 10.1093/schbul/3.1.93. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. P50 Suppression among schizophrenia and normal comparison subjects: A methodological analysis. Biol Psychiat. 1997;41(10):1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Mears RP, Wan L, Boutros NN. Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci. 2008;39(2):69–72. doi: 10.1177/155005940803900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97–104. doi: 10.1007/s001270200000. [DOI] [PubMed] [Google Scholar]
- Faugère M, Micoulaud-Franchi JA, Boyer L, Cermolacce M, Richieri R, Faget C, Vion-Dury J, Lançon C. Does sensory gating have a protective effect against hallucinatory behavior in schizophrenia? Clin Neurophysiol. 2016;127(2):1746–1748. doi: 10.1016/j.clinph.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. American J Psychiatry. 2007;164(3):458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- Ford JM, Dierks T, Fisher DJ, Herrmann CS, Hubl D, Kindler J, Koenig T, Mathalon DH, Spencer KM, Strik W, van Lutterveld R. Neurophysiological studies of auditory verbal hallucinations. Schiz Bull. 2012;38(4):715–723. doi: 10.1093/schbul/sbs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst DR, Gallinat J, Boutros NN. Range of sensory gating values and test–retest reliability in normal subjects. Psychophysiology. 2007;44(4):620–626. doi: 10.1111/j.1469-8986.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Galdos M, Simons C, Fernandex-Rivas A, Wichers M, Peralta C, Lataster T, Amer G, Myin-Germeys I, Allardyce J, Gonzalez-Torres MA, van Os J. Affectively salient meaning in random noise: A task sensitive to psychosis liability. Schiz Bull. 2011;37(6):1179–1186. doi: 10.1093/schbul/sbq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessu M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Parkkonen L, Hamalainen MS. MNE software for processing MEG and EEG data. Neuroimage. 2014;86:446–460. doi: 10.1016/j.neuroimage.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptoms rating scales (PSYRATS) Psychol Med. 1999;29(4):879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry. 2002;159(7):1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- Hu L, Boutros NN, Jansen BH. Sensory gating-out and gating-in in normal and schizophrenic participants. Clin EEG Neurosci. 2012;43(1):23–31. doi: 10.1177/1550059411429524. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. Brit J Psychiat. 2007;190:57–62. doi: 10.1192/bjp.bp.106.022954. [DOI] [PubMed] [Google Scholar]
- Kompus KR, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer J. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. Brit J Psychiat. 1989;155(7):59–65. [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Potkin S, Bunney WE. P50 sensory gating ratios in schizophrenics and controls: A review and data analysis. Psychiat Res. 2008;158(2):226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Jockers-Scherubl MC, Boutros NN, Gallinat J. Test–retest reliability of P50, N100 and P200 auditory sensory gating in healthy subjects. Int J Psychophysiol. 2008;67(2):81–90. doi: 10.1016/j.ijpsycho.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Sklar AL, Nixon SJ. Disruption of sensory gating by moderate alcohol doses. Psychopharmacology. 2014;231(22):4393–4402. doi: 10.1007/s00213-014-3591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliabilit of P50 auditory event-related potential indices of sensory gating. Psychophysiology. 1994;31(5):495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Smith DM, Grant B, Fisher DJ, Borracci G, Labelle A, Knott VJ. Auditory verbal hallucinations in schizophrenia correlate with P50 gating. Clin Neurophysiol. 2013;124(7):1329–1335. doi: 10.1016/j.clinph.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Chaze C, Lewine JD, Calhoun VD, Clark VP, Bustillo J, Houck J, Ford J, Bigelow R, Wilhelmi C, Stephen JM, Turner JA. Functional MRI evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Frontiers in Psychiatry. 2016;7(39):1–10. doi: 10.3389/fpsyt.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM. Auditory Hallucinations and the temporal cortical response to speech in schizophrenia: A functional magnetic resonance imaging study. Am J Psychiat. 1997;154(12):1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- Vercammen A, de Haan EH, Aleman A. Hearing a voice in the noise: auditory hallucinations and speech perception. Psychol Med. 2008;38(8):1177–1184. doi: 10.1017/S0033291707002437. [DOI] [PubMed] [Google Scholar]


