Abstract
Background and aims
The aim of this study was to measure the microhardness values of irradiated computer-aided design/computer-aided manufacturing (CAD/CAM) ceramics surfaces before and after thermal treatment.
Materials and Methods
Sixty CAD/CAM ceramic discs were prepared and grouped by material, i.e. lithium disilicate ceramic (Emax CAD) and zirconia ceramic (Emax ZirCAD). Laser irradiation at the material surface was performed with a carbon dioxide laser at 5 Watt (W) or 10 W power in continuous mode (CW mode), or with a neodymium:yttrium aluminum perovskite (Nd:YAP) laser at 10 W on graphite and non-graphite surfaces. Vickers hardness was tested at 0.3 kgf for lithium disilicate and 1 kgf for zirconia.
Results
Emax CAD irradiated with CO2 at 5 W increased microhardness by 6.32 GPa whereas Emax ZirCAD irradiated with Nd:YAP decreased microhardness by 17.46 GPa.
Conclusion
CO2 laser effectively increases the microhardness of lithium disilicate ceramics (Emax CAD).
Keywords: CAD/CAM ceramic, Microhardness, CO2 laser, Nd:YAP laser
Introduction
CAD/CAM ceramic blocks were first introduced to the dental market in the early 1980s 1). CAD-CAM ceramic restoration has since gained popularity in routine clinical practice as restorations including all-ceramic crowns, inlays, onlays, and veneers mimic natural teeth. Ceramic core materials are categorized into 3 different types: glass, alumina-based, and zirconia 2).
CAD/CAM ceramic materials have properties of great interest for various structural applications. The chemical stability of dental ceramics lends them good mechanical and optical properties and excellent biocompatibility 3). However, these ceramic materials are also notoriously brittle and susceptible to fracture, and this significant fragility has proven a major disadvantage limiting further applications.
To overcome some of the problems seen in earlier ceramic materials, different surface treatment methods have been proposed for CAD/CAM ceramics to provide roughness and promote micromechanical retention 4). Various authors do not recommend air-abrasion prior to cementation as it may affect the ceramic surface by creating microcracks and reducing the fracture strength of the ceramic 5). Other studies suggest that machining produces a rough surface, like the air-abraded surface in some machinable all-ceramic systems 6–8).
Prompted by advances in laser technology, some studies have suggested applying lasing media such as Er:YAG (erbium-doped yttrium aluminum garnet) and CO2 (carbon dioxide) lasers on zirconia ceramics to improve bonding to tooth structures 9–12). However, a number of studies have yielded contradictory results 13, 14).
Rocca et al. showed that presence of cracks on ceramic surfaces is related to the thermal effect of various laser intensities 15). However, there is limited literature on the effect of laser irradiation on the surface hardness of CAD/CAM ceramics.
The current study tested the working hypothesis that laser irradiation would influence mechanical properties, with a strong negative correlation between laser irradiation and surface hardness.
Aim of the study
The purpose of this in vitro study was to evaluate (1) the microhardness values of ceramic specimens (Emax CAD and Emax Zircad) irradiated with CO2 (10,600 nm) or Nd:YAP (1340 nm) laser and (2) the effect of thermal treatment by ceramic furnace before and after laser irradiation on the microhardness values of both lithium disilicate (Emax CAD) and zirconia (Emax ZirCAD) ceramic materials.
Materials and Methods
A). Lasers used
1). CO2 laser
A 10,600 nm-wavelength CO2 laser (Dream Pulse Lasers, Daeshin Enterprise Corp., Korea) was used with two different settings (5W and 10W) delivering two different power densities (6.37e4 W/cm2 and 1.25e5 W/cm2). Irradiation was conducted cross-pattern in continuous mode for 60 seconds (working distance: 2 mm, spot size of the aiming beam: 0.1 mm). The laser handpiece was held perpendicular to the irradiated surfaces (Fig. 1). CO2 laser parameters were selected based on results of previous studies for micromechanical retention due to its affinity to ceramics 15, 16).
Fig. 1:

CO2 laser.
2). Nd:YAP laser
Industry has proposed Nd :YAP laser as a surface modification technique to form a glazed surface layer on ceramics. A previous study 15) used an Nd:YAP laser (Nd:YAP Lokki, Lobel Medical, France, wavelength 1340 nm) with a 320 µm fiber at 10 W, corresponding to 31,831 W/cm2 of power density (Fig. 2). The ceramic surfaces were painted black with a soft pencil to enhance absorption at this wavelength. The irradiation parameters are summarized in Table 1.
Fig. 2:

Nd:YAP laser.
Table 1: Irradiation parameters.
| Laser/Parameter | CO2 | Nd:YAP | |
|---|---|---|---|
| Settings | 5 W | 10 W | 10 W |
| Operating mode | C.W | C.W | Pulsed |
| Surface area | 0.0314 cm2 | 0.0314 cm2 | 0.0008 cm2 |
| Density | 6.37e4 W/cm2 | 1.25e5 W/cm2 | 31,831W/cm2 |
B). Ceramic materials
1). Lithium disilicate (Emax CAD)
This material is composed of lithium silicate with micron-size lithium disilicate crystals in between, which are sub-micron lithium orthophosphate crystals creating a densely-packaed glass matrix 17–21).
2). Zirconia (Emax ZirCAD)
This material is partially stabilized by the addition of small amounts of other metal oxides 22).
The physical and chemical properties of tested ceramics are detailed in Table 2.
Table 2: Physical and chemical properties of the ceramics.
| Lithium | Disilicate | Zirconia | ||
|---|---|---|---|---|
| Composition (wt %) | SiO2 | 57.0–80.0 | ZrO2 | 87.0–95.0 |
| Li2O | 11.0–19.0 | Y2O3 | 4.0–6.0 | |
| ZrO2 | 0.0–8.0 | HfO2 | 1.0–5.0 | |
| MgO | 0.0–5.0 | Al2O3 | 0–1 | |
| K2O | 0.0–13.0 | |||
| P2O5 | 0.0–11.0 | |||
| ZnO | 0.0–8.0 | |||
| Al2O3 | 0.0–5.0 | |||
| Coloring oxides | ||||
| 0.0–8.0 | ||||
| Physical properties | Flexural strength (biaxial) 360 ± 60 MPa |
Flexural strength (biaxial) 900 × 50 MPa |
||
| Chemical solubility 40 ± 10 µg/cm2 |
Chemical solubility < 10 µg/cm2 |
|||
| Coefficient of thermal expansion (100–400°C) 10.15 ± 0.4×10−6 K 1 |
Coefficient of thermal expansion (100–400°C) 10.75 ± 0.25×10−6 K1 |
|||
A total of 30 discs of lithium disilicate (IPS e.max CADs, Lot: S24489, Ivoclar Vivadent, Liechtenstein) and 30 discs of zirconia (IPS e.max ZirCAD, Lot: R83002, Ivoclar Vivadent, Liechtenstein) were sliced to 3 mm thickness with a low-speed saw (ISOMET™, Buehler, ITW Company, USA) using an Arbor size 0.5/12.7 mm diamond blade (Buehler, ITW Company, USA) in wet conditions. The ceramic samples were then roughed by diamond dental bur (Grain size 100 µm), (Lot: 373384 Komet Dental, Germany). One bur passage was done on the ceramic surface, using a special holder to maintain the distance between ceramic surface and handpiece. Samples were immersed in demineralized water and cleaned with an ultrasonic cleaner (Fischer Scientific FB15047®) for three minutes. Note that all surfaces were cleaned with ethanol 100%. These ceramic samples were then randomly distributed into three main groups according to surface treatment:
Control group: Contains two control subgroups, i.e. Control 1 (Co1): Pure ceramic, Control 2 (Co2): Ceramic treated thermally with a ceramic furnace to the manufacturer's instructions.
LT group: ceramics laser-irradiated then treated thermally in a ceramic furnace to manufacturer's instructions; TL group: ceramics treated thermally in a ceramic furnace then laser-irradiated, including four subgroups, i.e. C5: surface irradiated with CO2 laser at 5W, C10: surface irradiated with CO2 laser at 10 W, NdG: graphite surface irradiated with Nd:YAP laser, Nd: non graphite surface irradiated with Nd:YAP laser. Table 3. Recaps the groups tested.
Table 3: Tested groups.
| Groups/Ceramics | Emax CAD | Emax ZirCAD | |
|---|---|---|---|
| Control group | Co1 | 3 | 3 |
| Co2 | 3 | 3 | |
| LT Group (Laser + thermal treatment) | C5 | 3 | 3 |
| C10 | 3 | 3 | |
| NdG | 3 | 3 | |
| Nd | 3 | 3 | |
| TL Group (Thermal treatment + laser) | C5 | 3 | 3 |
| C10 | 3 | 3 | |
| NdG | 3 | 3 | |
| Nd | 3 | 3 | |
C). Microhardness indentation (Vickers hardness, VH)
The Vickers indentation test method is widely used to evaluate the toughness of brittle materials 23–25). Vickers indentation testing requires a polished and perfectly flat surface and an indenter loading system.
The principle of the method is to apply the indenter under a known force (F) to the pyramidal contact area (A) of the indentation. Hardness was calculated with the formula H=1.854 F/d2, where (F) is the applied test load and (d) is the diagonal length left by the indenter 26).
For lithium disilicate ceramics, 5 VH measurements (GPa) per ceramic sample were done on the surfaces using a hardness indentation device (Buehler/Wilson VH3100, USA) at a force of 0.3 kg for 20 seconds. For zirconia ceramics, 5 VH measurements per ceramic sample were done at a force of 1 kg for 20 seconds. The five simultaneous measurements were made in two lines (three horizontally and two vertically) (Fig. 3).
Fig. 3:

VH measurements on ceramic surfaces.
D). Statistical analyses
Microhardness indentation (GPa) results were compared with a Mann-Whitney U-test where Vickers hardness was dependent variable and surface treatment was influencing factor. Statistical significance was set at p < 0.01.
Results
A). Lithium disilicate (Emax CAD)
In the control group, average hardness values (in GPa) were Co1: 6.44 ± 0.06 and Co2: 6.34 ± 0.24. In the LT group, average hardness values were C5: 6.32 ± 0.09, C10: 6.34 ± 0.17, NdG: 6.33 ± 0.04 and Nd: 6.35 ± 0.05. In the TL group, average hardness values were C5: 6.64 ± 0.12, C10: 6.44 ± 0.01, NdG: 5.52 ± 0.18 and Nd: 6.43 ± 0.07.
The VH test results on lithium disilicate ceramics (Emax CAD) are summarized in Fig. 4.
Fig. 4:
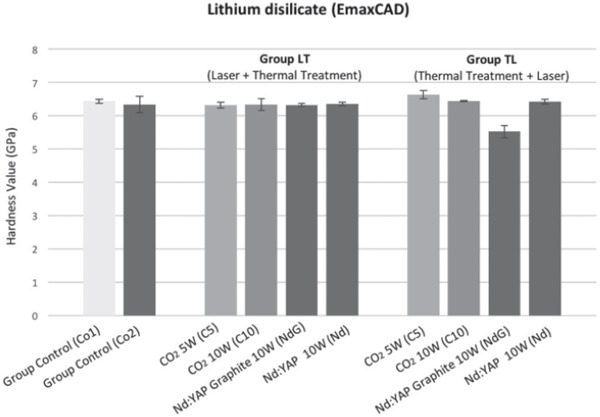
Hardness of lithium disilicate (Emax CAD).
B). Zirconia (Emax ZirCAD)
In the control group, average hardness values were Co1: 15.32 ± 0.89 and Co2: 23.03 ± 0.56. In the LT group, average hardness values were C5: 22.9 ± 1.74, C10: 20.83 ± 0.76, NdG: 21.70 ± 1.02 and Nd: 22.18 ± 1.70. In the TL group, average hardness values were C5: 21.78 ± 0.66, C10: 18.59 ± 0.22, NdG: 17.45 ± 0.29, and Nd: 22.14 ± 0.58.
The VH test results on zirconia ceramics (Emax ZirCAD) are summarized in Fig. 5. Statistical analysis (Wilcoxon–Mann-Whitney) is reported in Table 4.
Fig. 5:
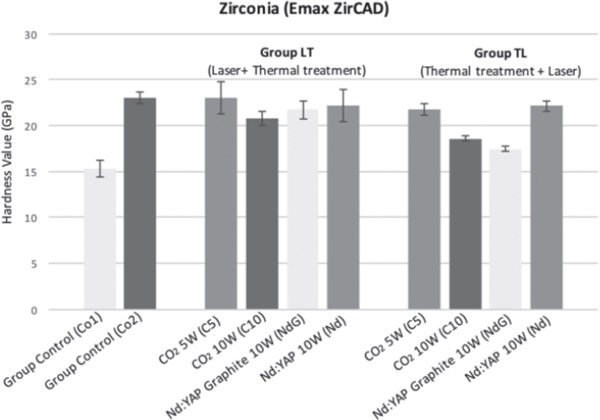
Hardness of zirconia (Emax ZirCAD).
Table 4: Statistical analyses.
| Ceramic/Group | Control | CO2 5 W (C5) | CO2 10 W (C10) | Nd:YAP Graphite (NdG) |
| Emax CAD | p-value: < 0.0003 S | p-value: < 0.0003 S | ||
| Emax ZirCAD | p-value: < 0.0003 S | p-value: < 0.0003 S | p-value: < 0.0003 S | |
Discussion
Dental ceramics are required to combine long-term durability with excellent physical properties in the oral cavity, but they also exhibit inherent flaws or surface defects, and all the systems proposed for ceramic surface conditioning have demonstrated several limits 27–28). One of these defects is surface hardness, which is a relative measure of resistance to an external indentation force. Indentation hardness is considered a predictor of the wear resistance of a material 29).
The current study sets out to investigate the effect of laser irradiation on the microhardness values of CAD/CAM ceramics and evaluate the influence of temperature on surface hardness.
The thickness of ceramics used in this study was designed to be as close as possible to clinical practice. An increase in Vickers hardness thus indicates an increase in resistance to abrasion of ceramic surfaces 30).
CO2 laser is well suited to ceramic surface treatment as its emission wavelength is almost totally absorbed by ceramics 15, 16). Laser irradiation with CO2 and Nd:YAP lasers can form rough ceramic surfaces. Moreover, after treatment with the CO2 10W & Nd:YAP lasers, ceramic surfaces showed micro-cracks caused by the severe physical stress created in the re-hardening ceramic surface by the sharp increase in temperatures 11) .
Statistical analysis showed significant differences in microhardness among the CAD/CAM ceramics tested.
On the basis of the results reported here, the microhardness values of Emax CAD and Emax ZirCAD ceramics were affected by laser irradiation and temperature. In Emax CAD ceramics, temperature had no effect on the microhardness values of the ceramics tested, and the relationship between increased temperature regime and laser irradiation was not clearly observed in our experimental conditions. This was further confirmed when comparing control groups, i.e. pure and heated samples (p = 0.3681).
Comparison between LT and TL groups showed that microhardness values increased when these ceramics were lasered by CO2 at 5W (p < 0.0003) but remained unchanged when lasered by CO2 at 10W and Nd:YAP without graphite, and decreased significantly in Emax CAD when lasered by Nd:YAP with graphite (p < 0.0003).
In Emax ZirCAD, comparison of microhardness values between heated control specimens (Co2 group) and the manufacturer's specimen (Co1) showed that temperature had a significant effect on microhardness values, which increased in the heated ceramics (p < 0.0003). Comparison of the heated group (Co2) against LT and TL group ceramics showed no effect of CO2 at 5 W or Nd:YAP lasering on microhardness values whereas CO2 at 10W (p < 0.0001) and Nd:YAP with graphite (p < 0.0003) both significantly decreased microhardness values.
An explanation for the increased hardness value in lithium disilicate irradiated with CO2 at 5W is that a high surface roughness may mean that hardness measurements on rough surfaces might not correlate with the actual hardness of the ceramic material but instead characterize the shape of the surface 30). Moreover, the presence of micro-cracks on the irradiated ceramic surfaces largely contributed to decreasing hardness values.
Conclusion
Within the limits of the present study, the most significant finding of this experimental study is that CO2 laser irradiation at 5W increased the microhardness of lithium disilicate ceramics (Emax CAD) whereas CO2 at 10W or Nd:YAP laser irradiation decreased the microhardness of zirconia ceramic (Emax ZirCAD).
Further studies should be performed to study other physical properties, i.e. surface roughness and surface wettability. Moreover, mechanical properties have to be studied in terms of resistance to fracture, behaviour with bonding agents or cementation, adhesive bond strength and the potential for micro-leakage.
Conflict of interest
The authors of this article certify that they have no proprietary, financial, or other personal interest of any nature or kind in any product, service and/or company that is presented in this article.
References
- 1: Mörmann WH. (2006) The evolution of the CEREC system. J Am Dent Assoc 137:7S-3S [DOI] [PubMed] [Google Scholar]
- 2: Conrad HJ, Seong WJ, Pesun IJ. (2007) Current ceramic materials and systems with clinical recommendations: a systematic review. J Prosthet Dent 98(5):389-404 [DOI] [PubMed] [Google Scholar]
- 3: Rekow ED, Silva N, Coelho P.G, Zhang Y, Guess P. (2011) Performance of dental ceramics: challenges for improvements. J Dent Res 8:937-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4: Saracoglu ACC, Cotert HS. (2004) Effect of various surface treatment methods on the bond strength of the heat-pressed ceramic samples. J Oral Rehabil 31:790-797 [DOI] [PubMed] [Google Scholar]
- 5: Zhang Y, Lawn BR, Rekow ED, Thompson VP. (2004) Effect of sandblasting on the long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater 71:381-386 [DOI] [PubMed] [Google Scholar]
- 6: Soderholm KJ, Mondragon E, Garcea I. (2003) Use of zinc phosphate cement as a luting agent for Denzir trade mark copings: an in vitro study. BMC Oral Health 3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7: Derand T, Molin M, Kleven E, Haag P, Karlsson S. (2008) Bond strength of luting materials to ceramic crowns after different surface treatments. Eur J Prosthodont Restor Dent 16:35-38 [PubMed] [Google Scholar]
- 8: Derand P, Derand T. (2000) Bond strength of luting cements to zirconium oxide ceramics. Int J Prosthodont 13:131-135 [PubMed] [Google Scholar]
- 9: Ural C, Kulunk T, Kulunk S, Kurt M. (2010) The effect of laser treatment on bonding between zirconia ceramic surface and resin cement. Acta Odontol Scand 68(6):354-359 [DOI] [PubMed] [Google Scholar]
- 10: Casucci A, Mazzitelli C, Monticelli F, Toledano M, Osorio R, Osorio E, et al. (2010) Morphological analysis of three zirconium oxide ceramics: Effect of surface treatments. Dent Mater 26(8):751-760 [DOI] [PubMed] [Google Scholar]
- 11: Ersu B, Yuzugullu B, Ruya YA, Canay S. (2009) Surface roughness and bond strengths of glass-infiltrated aluminaceramics prepared using various surface treatments. J Dent 37(11):848-856 [DOI] [PubMed] [Google Scholar]
- 12: Akyil MS, Uzun IH, Bayindir F. (2010) Bond strength of resin cement to yttrium-stabilized tetragonal zirconia ceramic treated with air abrasion, silica coating, and laser irradiation. Photomed Laser Surg 28(6):801-808 [DOI] [PubMed] [Google Scholar]
- 13: Akin H, Ozkurt Z, Kirmali O, Kazazoglu E, Ozdemir AK. (2011) Shear bond strength of resin cement to zirconia ceramic after aluminum oxide sandblasting and various laser treatments. Photomed Laser Surg 29(12):797-802 [DOI] [PubMed] [Google Scholar]
- 14: Paranhos MP, Burnett LH, Jr, Magne P. (2011) Effect of Nd:YAG laser and CO2 laser treatment on the resin bond strength to zirconia ceramic. Quintessence Int 42(1):79-89 [PubMed] [Google Scholar]
- 15: Rocca JP, Fornaini C, Brulat N, Seif B, Darque Ceretti E. (2014) CO2 and Nd:YAP laser interaction with lithium disilicate and zirconia dental ceramics: A preliminary study. Opt Laser Technol 57:216-223 [Google Scholar]
- 16: El Gamal A, Rocca JP, Fornaini C, Muhammad O, Medioni E, Cucinotta A, Brulat-Bouchard N. (2016) The effect of CO2 and Nd:YAP lasers on CAD/CAM Ceramics: SEM, EDS and thermal studies. Laser Ther 25(1):27-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17: Albakry M, Guazzato M, Swain MV. (2003) Fracture toughness and hardness evaluation of three pressable all-ceramic dental materials. J Dent 31:181-188 [DOI] [PubMed] [Google Scholar]
- 18: Albakry M, Guazzato M, Swain MV. (2003) Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic materials. Prosthet Dent 89:374-380 [DOI] [PubMed] [Google Scholar]
- 19: Guazzato M, Albakry M, Ringer SP, Swain MV. (2004) Strength, fracture toughness and microstructure of a selection of all-ceramic materials. I. Pressable and alumina glass-infiltrated ceramics. Dent Mater 20:441-448 [DOI] [PubMed] [Google Scholar]
- 20: Höland W, Schweiger M, Frank M, Rheinberger V. (2000) A comparison of the microstructure and properties of the IPS empress 2 and the IPS empress glass ceramics. J Biomed Mater Res 53:297-303 [DOI] [PubMed] [Google Scholar]
- 21: DellaBona A, Mecholsky JJ, Anusavice KJ. (2004) Fracture behavior of lithia disilicate- and leucite-based ceramics. Dent Mater 20:956-962 [DOI] [PubMed] [Google Scholar]
- 22: Piwowarczyk A, Ottl P, Lauer HC, Kuretzky T. (2005) A clinical report and overview of scientific studies and clinical procedures conducted on the 3M ESPE Lava All-Ceramic System. Prosthodont 14:39-45 [DOI] [PubMed] [Google Scholar]
- 23: Shetty DK, Wright IG, Mincer PN, Clauer AH. (1985) Indentation fracture of WC–Co cermets. J Mater Sci 20(5):1873-1882 [Google Scholar]
- 24: Chinn R E. (2002) Ceramography preparation and analysis of ceramic microstructures. ASM International, USA [Google Scholar]
- 25: Wachtman JB. (1996) Mechanical properties of ceramics, John Wiley & Sons, Inc. [Google Scholar]
- 26: Coldea A, Swain MV, Thiel N. (2013) Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent Mater 29(4):419-426 [DOI] [PubMed] [Google Scholar]
- 27: Kasraei S, Atefat M, Beheshti M, Safavi N, Mojtahedi M, Rezaei-Soufi L. (2014) Effect of surface treatment with carbon dioxide (CO2) laser on bond strength between cement resin and zirconia. J Lasers Med Sci 5(3):115-120 [PMC free article] [PubMed] [Google Scholar]
- 28: Ural C, Kalyoncuo lu E, Balkaya V. (2012) The effect of different power outputs of carbon dioxide laser on bonding between zirconia ceramic surface and resin cement. Acta Odontol Scand 70(6):541-546 [DOI] [PubMed] [Google Scholar]
- 29: Lauvahutanon S, Takahashi H, Shiozawa M, Iwasaki N, Asakawa Y, Oki M, Finger WJ, Arksornnukit M. (2014) Mechanical properties of composite resin block for CAD/CAM. Dent Mater 33(5):705-710 [DOI] [PubMed] [Google Scholar]
- 30: Flury S, Peutzfeldt A, Lussi A. (2012) Influence of surface roughness on mechanical properties of two computer-aided design/computer-aided manufacturing (CAD/CAM) ceramic materials. Oper Dent 37(6):617-624 [DOI] [PubMed] [Google Scholar]


