Abstract
Achatina fulica (Lissachatina fulica) is one of the most invasive species found across the globe causing a significant damage to crops, vegetables, and horticultural plants. This terrestrial snail is native to east Africa and spread to different parts of the world by introductions. India, a hot spot for biodiversity of several endemic gastropods, has witnessed an outburst of this snail population in several parts of the country posing a serious threat to crop loss and also to human health. With an objective to evaluate the genetic diversity of this snail, we have sampled this snail from different parts of India and analyzed its haplotype diversity by means of 16S rDNA sequence information. Apart from this, we have studied the phylogenetic relationships of the isolates sequenced in the present study in relation with other global populations by Bayesian and Maximum-likelihood approaches. Of the isolates sequenced, haplotype ‘C’ is the predominant one. A new haplotype ‘S’ from the state of Odisha was observed. The isolates sequenced in the present study clustered with its conspecifics from the Indian sub-continent. Haplotype network analyses were also carried out for studying the evolution of different haplotypes. It was observed that haplotype ‘S’ was associated with a Mauritius haplotype ‘H’, indicating the possibility of multiple introductions of A. fulica to India.
Keywords: Achatina fulica, Haplotype, Indian sub-continent, 16S rDNA, Network analysis, Phylogeny
Introduction
Achatina fulica (Bowdich, 1882) is a pulmonate land snail found in different parts of the world. It has its origin from East Africa and has spread to different parts of the world by introductions (Albuquerque et al. 2008). It is distributed to all the continents except Antarctic. Madagascar is the first place of introduction outside the African continent from Kenya prior to the year 1800. From Madagascar, it was introduced to Mauritius. From Mauritius, it has spread to different parts of the world (Raut and Baker 2002). It is a pestiferous snail and is recorded as one among the 100 most invasive species found in the world by IUCN (Lowe et al. 2000). It is a vector for rat lung worm Angiostrongylus cantonensis that causes eosinophilic meningitis in humans, Angiostrongylus costaricensis that causes abdominal angiostrongyliasis, and another worm Angiostrongylus abstrusus, whose effects are yet to be known (Silvana et al. 2007; Jayashankar et al. 2014; Joseph 1966; Fernanda et al. 2010). Apart from causing damage to the human health, A. fulica is also a carrier of several pathogens that infests different crops (Rudra et al. 2009), thus damaging both the human health as well as agricultural crops.
Indian sub-continent harbours rich biodiversity of several endemic gastropods (Ramakrishna et al. 2010). In the recent past, the country has witnessed an outbreak of A. fulica in different parts of India causing a significant damage to agro-horticultural plants. As per the historical records, this snail was first introduced into the country at Kolkata (erst while Calcutta) in April 1847 by a famous malacologist, Sir. Henry Benson. He had collected two individuals of A. fulica from Mauritius in February, 1847. These were handed over to his friend, who had released them into his garden at Chowringhee, Kolkata (Naggs 1997). Later, it was spread to different parts of the country mostly by introductions. From West Bengal, it was introduced to Odisha and Bihar (Sheela 1999). From Odisha, these were introduced at Araku Valley in the Visakhapatnam district in the state of Andhra Pradesh (Rekha et al. 2015). This snail was introduced to North Bihar from Kolkata by a black smith (Sheela 1999). In 1959, a few snails were introduced to Nagaland from West Bengal by R.S. Bedi. He had brought these snails out of curiosity and released them into his garden at Dimapur. Later, it was spread to in and around areas of Dimapur and caused significant damages to the crop plants. It was introduced for the first time at Chennai (erst while Madras) in the south Indian state of Tamil Nadu at ‘My lady Garden’ during British period. From there, it was spread to different parts of Tamil Nadu (Raut and Ghose 1984). It was introduced to Andaman and Nicobar Islands around 1940s by Japanese soldiers during World War II. This has resulted in its present day dispersal in this region (Paras Nath 2007). This snail was also introduced at Mussoorie, a hill station in India by Captain Hutton. However, all the introduced specimens were dead due to severe winter (Mead 1961). Deliberate transport of this snail from one region to another region is one of the main reasons for its wide spread in India and to different parts of the world (Mead 1961; Budha and Naggs 2008). A. fulica has spread to different states in India, viz., Karnataka, Andhra Pradesh, Uttar Pradesh, Bihar, Tamil Nadu, Maharashtra, Kerala, Gujarat, Nagaland, Assam, Jharkhand, Daman and Diu, Tripura, Mizoram, Goa, Andaman and Nicobar Islands, and Lakshadweep Islands.
In India, Agriculture sustains the livelihood of 70% of its population and is a significant contributor to the nation’s economy. Agricultural yield in the country is dependent on several factors like onset of monsoon, availability of water resources, etc (Khan et al. 2009). Any change in the aforementioned parameters adversely affects the agricultural yield and thus affects the livelihood of farmers. Apart from these abiotic factors, yield is also significantly affected by several diseases and infestations by different pests. In such a scenario, the prevalence of A. fulica has become a great menace causing severe damage to crops, vegetables, and horticultural plants, thereby adversely affecting the economies of the families dependent on agriculture and agro-based industries.
The outburst of this snail was first reported in the country in Odisha as an epidemic form in 1928 (Nath 2007). It is a voracious feeder with diverse food habits and feeds on different horticultural, ornamental, and crop plants viz., banana (Mathai 2014), Vanilla (Vanitha et al. 2011), cucumber, brinjal, lady’s finger, cabbage, cauliflower, turmeric (Sheela 1999), mulberry, ground nut, French beans, marigold (Jadhav et al. 2016), etc. This has made its survival in the newly introduced areas a success and a great loss to the crop plants. It is overwhelming to see the list of wide varieties of crops, vegetables, horticultural, and ornamental plants that it feeds on (Albuquerque et al. 2008; Jadhav et al. 2016).
In the recent past, there are several unpublished reports that are growingly available with respect to the severity of its infestations in new areas and its damage to the agro-horticultural plants. In a recently published study carried out by Jadhav et al. (2016) in the Kolhapur district from the state of Maharashtra, this snail was found to cause severe damage to the foliage of Mulberry plants. Furthermore, the leaves were found unsuitable for feeding the silkworm because of the presence of mucus and excreta of this snail. As a result, the local sericulture industry was severely damaged and several farmers were choosing to opt out sericulture.
Introduced out of its native range, its outburst in different geographical regions is attributed mainly due to its high reproductive capacity, thus out competing the endemic snail populations which lead to their extinction, thereby posing a serious threat to the local biodiversity (Silvana et al. 2007). There are significant costs involved in managing this pestiferous snail, which has become a burden to the states. This includes the cost towards the purchase of various chemicals, expenses related to manpower, etc (Civeyrel and Simberloff 1996). For example, it costed an amount of 700,000 dollars to the United States for the complete eradication of A. fulica from Florida (Poucher 1975). Brazil has incurred agricultural losses up to US 42.6 billion dollars (Silvana et al. 2007). Most importantly, these expenses do not cover the losses to human health. Apart from this, its presence is a nuisance on daily basis in the backyards of homes, offices, universities (Mathai 2014), and roads (Rekha et al. 2015).
Given the severity of damage caused to the agricultural crops, an immediate step is required to prevent its further spread. Hence, there are several approaches which includes the intervention at human level by clearing the areas where these snails resides (Budha and Naggs 2008; Albuquerque et al. 2008); chemical control by spraying different molluscicides; and also by biological control mechanisms (Mathai 2014).
Apart from its negative impact on the agriculture and human health, it possesses medicinal properties and is also edible in different parts of the world. These are one of the main reasons for its introduction to Madagascar from its native range (Raut and Baker 2002; Fontanilla et al. 2014). However, people have deliberately introduced from one place to another place out of wonder and curiosity after they encountered this snail (Budha and Naggs 2008).
Achatina fulica has long historical association to India. However, genetic diversity of this snail is not explored in entirety. The present study is carried out with an objective to look for the presence of any new haplotypes in the Indian sub-continent by increasing the extent of sampling than that carried out by Fontanilla et al. (2014). The first step towards studying the haplotype diversity of this snail by 16S rDNA was carried out by Fontanilla et al. (2014). They have sampled this snail from different parts of the world. With respect to India, they have confined their sampling only to four localities from the state of Maharashtra. Hence, in the present study, we have sampled this snail from new areas. Because of the spread of this snail over wide geographical regions, and due to logistic constraints and limited man power, A. fulica from all the regions of India was not sampled. Especially, we were unable to sample this snail from Kolkata, which is considered to be the first place of introduction to India.
In the present study, we have chosen 16S rDNA for evaluating the haplotype diversity and phylogenetic relationships among the isolates of A. fulica sequenced in this study in relation with other global populations.
Materials and methods
In the present study, a total of eight individuals of A. fulica were collected from September 2014 to December 2015 from different geographical regions of India covering four different states, viz., Andhra Pradesh, Karnataka, Odisha, and Bihar (Table 1; Fig. 1) for studying their phylogenetic relationships.
Table 1.
List of NCBI accession numbers, places of collection, and haplotypes of the isolates of Achatina fulica sequenced in the present study
| Sr. no. | Organism (isolates) | Locality | Collector | NCBI GenBank™ accession number | No. of isolates sequenced | Haplotype |
|---|---|---|---|---|---|---|
| 1 | Achatina fulica (Af-Ban_1 and Af-Ban_2) | Bangalore, Karnataka | Vijaya Sai A | KP317640 | 2 | C |
| Vijaya Sai A | KX060743 | C | ||||
| 2 | Achatina fulica (Af-Od_1 and Af-Od_2) | Bharatpur, Odisha | Jena | KP317641 | 2 | S |
| Jena | KP119753 | S | ||||
| 3 | Achatina fulica (SKN-Af-Bhr_1, SKN-Af-Bhr_2 and SKN- Af-Bhr_3) | Bihar | Kishore Kunal | KX514436 | 3 | C |
| Kishore Kunal | KX514437 | C | ||||
| Kishore Kunal | KX514438 | C | ||||
| 4 | Achatina fulica (Af-SSSPN-PTP_1) | Puttaparthi, Andhra Pradesh | Vijaya Sai A | KX514435 | 1 | C |
Fig. 1.
India map depicting the states from where Achatina fulica was sampled for the present study. A.P Andhra Pradesh state
Isolation of genomic DNA
Interference of mucopolysaccharides and proteins from the gastropod tissues makes genomic DNA unsuitable for PCR amplification (Wade and Mordan 2000; Ayyagari et al. 2017) and (personal observation). To overcome this, we have standardized different isolation procedures for the isolation of good quality genomic DNA from the foot muscle tissue of A. fulica suitable for the amplification of 16S rDNA. They include the protocol of Ayyagari et al. (2017) and slightly modified procedure of Sokolov (2000) for the isolation of genomic DNA from the foot muscle tissue of A. fulica. For few isolates, small piece of foot muscle tissue was cut noninvasively. For these, genomic DNA isolation was carried out by employing NucleoSpin® Tissue kit (Macherey–Nagel) following the manufacturer’s instructions.
Amplification and sequencing of 16S rDNA
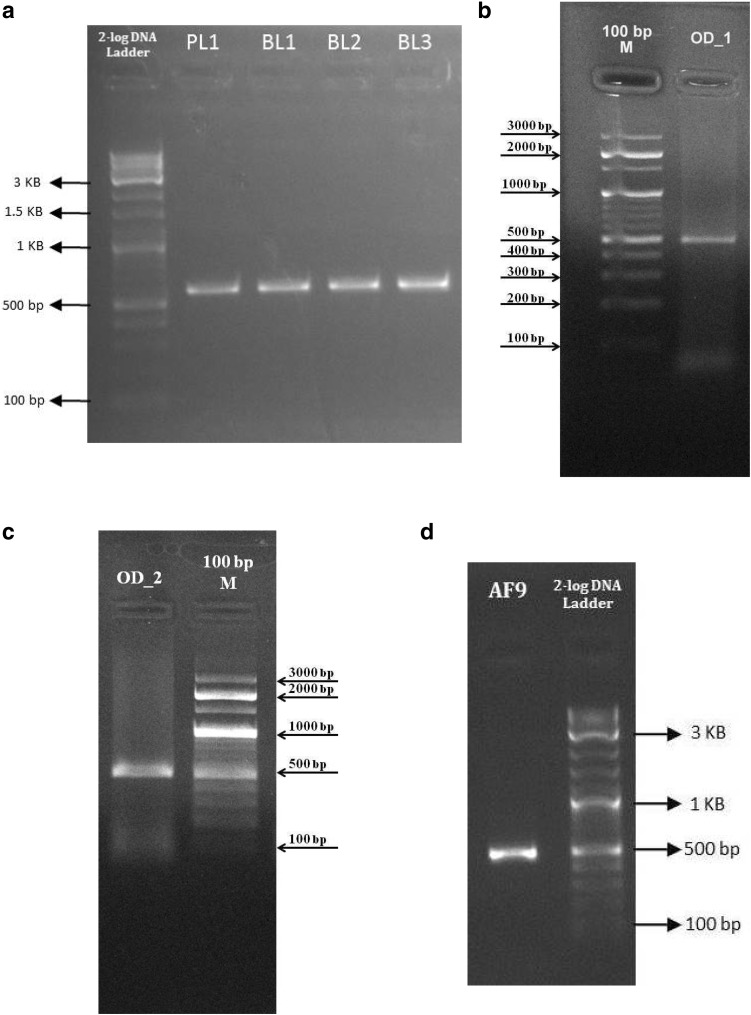
Genomic DNA was amplified for 16S rDNA using the primers of Palumbi et al. (2002). PCR conditions were as that of Ayyagari et al. (2017). Alternatively, PCR was performed in a 20 µl reaction volume which contained 1× Phire PCR buffer (contains 1.5 mM MgCl2), 0.2 mM dNTPs, 1 µl DNA, 0.2 µl Phire Hot start II DNA polymerase enzyme, 5 µM of forward and reverse primers, 0.1 mg/ml BSA, 3% DMSO, and 0.5 M betaine. The cycling conditions consisted of denaturation at 98 °C for 30 s followed by 40 cycles of denaturation at 98 °C for 5 s, annealing at 48 °C for 10 s, extension at 72 °C for 15 s, and a final extension at 72 °C for 1 min (Fig. 2a–d). PCR amplification was carried out in thermal cycler (GeneAmp PCR System 9700, Applied Biosystems).
Fig. 2.
a PCR amplification of 16S rDNA from the isolates of Achatina fulica. 2-Log DNA ladder is molecular weight marker. PL1, BL1, BL2, and BL3 are the different isolates of Achatina fulica. b PCR amplification of 16S rDNA from the isolate of Achatina fulica. 100 bp M is molecular weight marker. OD_1 is an isolate of Achatina fulica. c PCR amplification of 16S rDNA from the isolate of Achatina fulica. 100 bp M is molecular weight marker. OD_2 is an isolate of Achatina fulica. d PCR amplification of 16S rDNA from the isolate of Achatina fulica. 2-Log DNA ladder is molecular weight marker. AF9 is an isolate of Achatina fulica
PCR products were cleaned using ExoSAP-IT™ (GE Healthcare) following the manufacturer’s instructions. Purified amplicons were sequenced in both the directions with the same primers used for PCR at Regional facility for DNA fingerprinting, India. The chromatograms were visualized and edited for obtaining consensus sequences in DNA Baser v4.20.0 (www.DnaBaser.com). Resulting consensus sequences were submitted to NCBI GenBank™ and were assigned accession numbers KP317640–KP317641, KX514435–KX514438, KX060743, and KP119753 (Table 1).
Sequence analysis
We first report the longest 16S rDNA sequence of A. fulica so far submitted to the NCBI GenBank™/DDBJ/EMBL. The primers used in our study were different from the primers used by Fontanilla et al. (2014). The primers of Fontanilla et al. (2014) bind to two conserved regions that lies within the regions of 16S rDNA amplified by the primers used in our present study. To include only the common regions amplified by both the primers in our analyses, we have removed few nucleotide sequences obtained in our study lying outside the primer sequences of Fontanilla et al. (2014). Therefore, while performing multiple sequence alignment, only the common regions were included.
Apart from the nucleotide sequences obtained from the present study, we have retrieved 16S rDNA sequences of A. fulica from NCBI GenBank™ for studying the phylogenetic relationships of the sequenced specimens with those from the retrieved (Table 2).
Table 2.
List of nucleotide sequences retrieved from NCBI GenBank™
(Fontanilla et al. 2014)
| S. no. | Locality | Haplotype(s) | NCBI accession no. | Reference |
|---|---|---|---|---|
| 1 | East Africa: Kampala, Uganda | O | JQ436767 | Fontanilla et al. (2014) |
| 2 | East Africa: Dar Es Salaam, Tanzania | I, J, K, L, M, N |
JQ436761–JQ436766 KC682495 |
Fontanilla et al. (2014) |
| 3 | Indian Ocean Islands, Mayotte | A, B | JQ436753–JQ436754 | Fontanilla et al. (2014) |
| 4 |
Indian Ocean Islands Mayotte, Mauritius, Seychelles and Madagascar, Nepal, Sri Lanka India Pune, Talegaon South East Asia Burma, Thailand, Philippines, Singapore, Malaysia, Vietnam Pacific Islands Hahasima, Ogasawara, Polynesia, Hawaii Caribbean Martinique North America Florida, USA South America Ecuador, Bolivia, Brazil |
C | JQ436755 | Fontanilla et al. (2014) |
| 12 |
Indian Ocean Islands Mayotte and Mauritius, South America
Guayas, Ecuador, La Mana, Cotopaxi, Ecuador and Puerto Suarez, Bolivia |
D | JQ436756 | Fontanilla et al. (2014) |
| 13 | Mayotte | G | JQ436759 | Fontanilla et al. (2014) |
| 14 | Mayotte and Mauritius | H | JQ436760 | Fontanilla et al. (2014) |
| 15 |
Indian Sub-continent
Nagpur and Nashik |
P | JQ436751 | Fontanilla et al. (2014) |
| 16 |
South East Asia
Philippines |
E | JQ436757 | Fontanilla et al. (2014) |
| 17 |
Pacific Islands
New Caledonia Caribbean Barbados |
F | JQ436758 | Fontanilla et al. (2014) |
| 18 |
South America
Ecuador |
Q | JQ436752 | Fontanilla et al. (2014) |
Multiple sequence alignment was performed in MEGA v6.06 (Tamura et al. 2013) using the inbuilt muscle (Edgar 2004) programme by default parameters. The sequence alignment data were exported to fasta format using MEGA (Tamura et al. 2013), which was then converted to Nexus format suitable for usage in MrBayes v3.03 (Huelsenbeck and Ronquist 2001) by MESQUITE v3.2.5 (Maddison and Maddison 2015). The suitable substitution model for the data set implemented in MrBayes was set to HKY, which was determined by importing the Nexus file created by Mesquite to jMODELTEST v2.1.3 (Darriba et al. 2012) by choosing the Bayesian inference criterion (BIC).
Phylogenetic analyses
Bayesian inference (BI) of phylogenetic relationships of the haplotypes was carried out in MrBayes for 10,000,000 generations with two independent runs. Each run was carried out with four chains (3 heated and 1 cold) with default heating parameters. The chains were sampled at the end of every 1000th generation. Of the 10,000 trees generated, initial 2500 trees were discarded as burnin. The average standard deviation of the split frequencies was <0.05 and the potential scale reduction factor (PSRF) was 1.000. The phylogram was visualized using the ‘sumt’ command. The consensus tree generated was visualized in FigTree v1.4.2 (Rambaut 2012) by importing the ‘.con’ file. The convergence of markov chains towards the target distribution was assessed by importing the ‘P’ files into TRACER v1.6 (Rambaut et al. 2014) for estimating the effective sample size. The sample size was found to be >500.
Apart from Bayesian inference (BI), maximum-likelihood (ML) analysis was carried out based on HKY model in MEGA with 1000 bootstrap replicates.
Both rooted and unrooted trees were constructed by ML and BI methods. Isolates sequenced in the present study were searched against the NCBI GenBank database using Blast algorithm (Basic Local Alignment Search Tool; http://www.blast.ncbi.nlm.nih.gov) for choosing an appropriate outgroup. On the basis of percentage similarity, Achatina reticulata was chosen to root the phylogenetic trees.
Haplotype analyses
After multiple sequence alignment, haplotypes were identified by carefully observing the nucleotide differences between the sequences. Each haplotype was designated by an alphabet code. The nomenclature of haplotypes used was the same as that was used by Fontanilla et al. (2014), except for the newly observed haplotype ‘S’ (Table 3).
Table 3.
Nucleotide sequence variations in the 19 haplotypes (A–S) of Achatina fulica
| Haplotype | Nucleotide position | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 42 | 71 | 102 | 103 | 106 | 145 | 151 | 153 | 155 | 156 | 158 | 197 | 205 | 210 | 217 | 258 | 277 | 278 | 282 | 283 | |
| A | G | A | T | C | C | C | C | A | A | T | A | A | T | T | C | A | A | T | T | T | T |
| B | A | G | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| D | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | - | . | . | . |
| E | A | C | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| F | A | . | . | . | . | . | . | . | . | C | . | G | . | . | . | . | . | - | . | . | . |
| G | A | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | - | . | . | . |
| H | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . |
| I | A | . | . | T | . | . | . | G | . | . | T | . | C | . | . | . | . | . | . | . | . |
| J | A | . | . | T | . | . | . | G | . | . | T | . | . | . | . | . | . | - | . | . | A |
| K | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | - | - | . | . |
| L | A | . | . | G | . | . | . | G | . | . | T | . | C | C | . | . | . | - | - | . | . |
| M | A | . | . | T | . | . | . | G | . | . | T | . | c | . | . | . | . | - | . | . | . |
| N | A | . | . | T | . | . | . | G | . | . | T | . | . | . | . | G | . | - | . | . | . |
| 0 | A | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | - | . | A | . |
| P | A | . | . | . | T | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Q | A | . | C | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| R | A | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | - | - | . | . |
| Odisha_S | A | . | . | . | . | . | . | . | G | . | . | G | . | . | . | . | . | . | . | . | . |
| Odisha_S | A | . | . | . | . | . | . | . | G | . | . | G | . | . | . | . | . | . | . | . | . |
| Bangalore_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Bangalore_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Bihar_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Bihar_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Bihar_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
| Puttaparthi_C | A | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | - | . | . | . |
In Odisha_S, Bangalore_S, Bihar_C, and Puttaparthi_C, the prefix indicates the geographical location of the isolate sequenced in the present study and suffix indicates the haplotype of the isolate
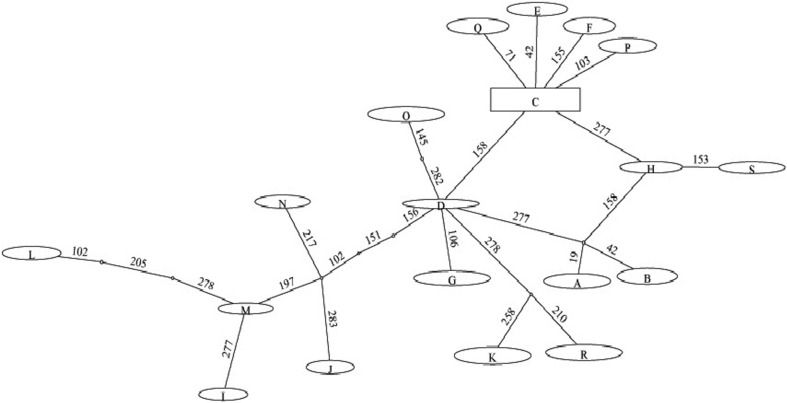
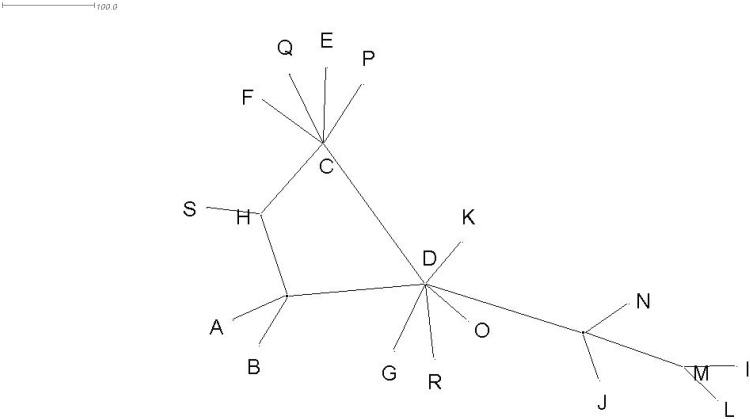
Haplotype network of the isolates of A. fulica was drawn using TCS v1.21 (Clement et al. 2000; Fig. 3) by importing the alignment file in Nexus format into this software. Apart from this, median-joining network of haplotypes was constructed using SplitsTree v4.14.3 (Huson and Bryant 2006; Fig. 4).
Fig. 3.
TCS network of the haplotypes of Achatina fulica. Network tree depicted was obtained by selecting the ‘spring tree’ option in the TCS software. Indicated on the lines connecting different haplotypes is the position of the nucleotide sequence variation. The size of the oval or square box do not exactly correspond to the haplotype frequency. The frequency of each haplotype was rounded off to one, except for the isolates sequenced in the present study
Fig. 4.
Median-joining network of haplotypes of Achatina fulica drawn using SplitsTree
Results
Till date, there is a little systematic evaluation of the genetic diversity of this snail collected from different parts of India. This study aims in evaluating the haplotype diversity of A. fulica collected from different parts of India and also to study the phylogenetic relationships of the isolates sequenced in this study in relationship with the isolates of A. fulica from different parts of the world.
Various parameters relating to the nucleotide sequences were estimated in MEGA (Tamura et al. 2013). Pairwise distance between the sequences was analyzed using the maximum composite likelihood (MCL) model in MEGA v6 (Table 4). The nucleotide frequencies for A, T, C, and G were 28.50, 35.79, 15.44, and 20.27%, respectively. The transition/transversion rate ratios were k 1 = 8.019 (purines) and k 2 = 6.022 (pyrimidines), and the overall transition/transversion bias was R = 3.186. The rates of substitution of one nucleotide to another were estimated using the MCL method. The rates of transitional substitutions computed for A → G = 18.07, T → C = 10.33, C → T = 23.96 and G → A = 25.4 and transversional substitutions computed for A → T = 3.98, A → C = 1.72, T → A = 3.17, T → G = 2.25, C → A = 3.17, C → G = 2.25, G → T = 3.98, and G → C = 1.72 (Table 4).
Table 4.
Maximum composite likelihood estimate of the pattern of nucleotide substitution
| A | T | C | G | |
|---|---|---|---|---|
| A | – | 3.98 | 1.72 | 18.07 |
| T | 3.17 | – | 10.33 | 2.25 |
| C | 3.17 | 23.96 | – | 2.25 |
| G | 25.4 | 3.98 | 1.72 | – |
The rates of transitional substitutions were highlighted in bold
The pairwise distance between the haplotypes varied from a maximum of 0.025 to a minimum of 0–0.003. With respect to the isolates sequenced in the present study, haplotype S was highly distant (0.025) from haplotype L and least distant (0.003) from haplotype C (Table 5).
Table 5.
Estimates of evolutionary divergence between sequences (the number of base substitutions per site from between sequences is shown)
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | s* | s* | C* | c* | c* | c* | c* | c* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | ||||||||||||||||||||||||||
| B | 0.007 | |||||||||||||||||||||||||
| C | 0.007 | 0.007 | ||||||||||||||||||||||||
| D | 0.003 | 0.003 | 0.003 | |||||||||||||||||||||||
| E | 0.010 | 0.007 | 0.003 | 0.007 | ||||||||||||||||||||||
| F | 0.010 | 0.010 | 0.003 | 0.007 | 0.007 | |||||||||||||||||||||
| G | 0.007 | 0.007 | 0.007 | 0.003 | 0.010 | 0.010 | ||||||||||||||||||||
| H | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | |||||||||||||||||||
| I | 0.017 | 0.017 | 0.017 | 0.014 | 0.021 | 0.021 | 0.017 | 0.017 | ||||||||||||||||||
| J | 0.017 | 0.017 | 0.017 | 0.014 | 0.021 | 0.021 | 0.017 | 0.017 | 0.007 | |||||||||||||||||
| K | 0.007 | 0.007 | 0.007 | 0.003 | 0.010 | 0.010 | 0.007 | 0.007 | 0.017 | 0.017 | ||||||||||||||||
| L | 0.021 | 0.021 | 0.021 | 0.017 | 0.025 | 0.025 | 0.021 | 0.021 | 0.007 | 0.014 | 0.021 | |||||||||||||||
| M | 0.017 | 0.017 | 0.017 | 0.014 | 0.021 | 0.021 | 0.017 | 0.017 | 0.000 | 0.007 | 0.017 | 0.007 | ||||||||||||||
| N | 0.017 | 0.017 | 0.017 | 0.014 | 0.021 | 0.021 | 0.017 | 0.017 | 0.007 | 0.007 | 0.017 | 0.014 | 0.007 | |||||||||||||
| 0 | 0.010 | 0.010 | 0.010 | 0.007 | 0.014 | 0.014 | 0.010 | 0.010 | 0.021 | 0.021 | 0.010 | 0.025 | 0.021 | 0.021 | ||||||||||||
| P | 0.010 | 0.010 | 0.003 | 0.007 | 0.007 | 0.007 | 0.010 | 0.003 | 0.021 | 0.021 | 0.010 | 0.025 | 0.021 | 0.021 | 0.014 | |||||||||||
| Q | 0.010 | 0.010 | 0.003 | 0.007 | 0.007 | 0.007 | 0.010 | 0.003 | 0.021 | 0.021 | 0.010 | 0.025 | 0.021 | 0.021 | 0.014 | 0.007 | ||||||||||
| R | 0.007 | 0.007 | 0.007 | 0.003 | 0.010 | 0.010 | 0.007 | 0.007 | 0.017 | 0.018 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.010 | 0.010 | |||||||||
| Odisha_S | 0.010 | 0.010 | 0.003 | 0.007 | 0.007 | 0.007 | 0.010 | 0.003 | 0.021 | 0.021 | 0.010 | 0.025 | 0.021 | 0.021 | 0.014 | 0.007 | 0.007 | 0.010 | ||||||||
| Odisha_S | 0.010 | 0.010 | 0.003 | 0.007 | 0.007 | 0.007 | 0.010 | 0.003 | 0.021 | 0.021 | 0.010 | 0.025 | 0.021 | 0.021 | 0.014 | 0.007 | 0.007 | 0.010 | 0.000 | |||||||
| Bangalore_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | ||||||
| Bangalore_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | 0.000 | |||||
| Bihar_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | 0.000 | 0.000 | ||||
| Bihar_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | 0.000 | 0.000 | 0.000 | |||
| Bihar_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Puttaparthi_C | 0.007 | 0.007 | 0.000 | 0.003 | 0.003 | 0.003 | 0.007 | 0.000 | 0.017 | 0.017 | 0.007 | 0.021 | 0.017 | 0.017 | 0.010 | 0.003 | 0.003 | 0.007 | 0.003 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
* Haplotypes obtained in the present study
There were a total of 293 nucleotide positions after multiple sequence alignment. Of these, 274 were conserved and 19 were variable. Of the 19 variable sites, six sites were parsimony informative.
Phylogenetic analyses
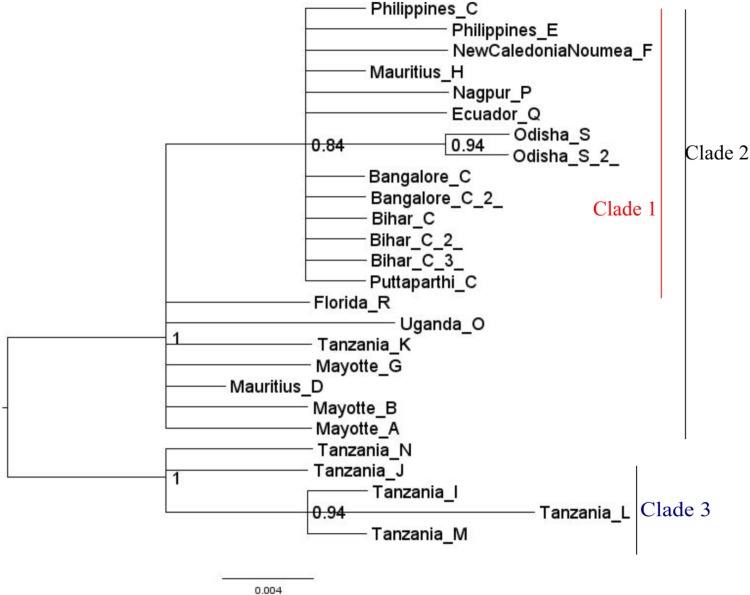
Bayesian Inference of phylogeny was carried out in MrBayes (Fig. 5). Bayesian tree consisted of three distinct clades. Clade 1 consisted of all the individuals of A. fulica that were sequenced in the present study. Apart from the isolates sequenced in our present study, it also harboured all the previously sequenced individuals of A. fulica by Fontanilla et al. (2014) that were originated from the Indian sub-continent that harboured haplotypes ‘C’ and ‘P’. Nested within Clade I were the haplotypes C, H, F, E, Q, and P that were evolved outside East Africa.
Fig. 5.
Midpoint—rooted Bayesian tree depicting the phylogenetic relationships of the isolates of Achatina fulica based on 16S rDNA. Indicated on the nodes are the Bayesian probability values. Scale bar represents 0.004 substitutions per site
Of the eight isolates of A. fulica sequenced in the present study, six haplotypes were of a previously reported haplotype ‘C’ and the remaining two isolates from Odisha were of a new haplotype ‘S’. These two isolates formed a distinct clade within Clade 1.
Outside Clade 1, within Clade 2 were haplotypes A, B, D, G, K, O, and R that showed polytomy. It is interesting to note that haplotypes K, O, and R were from Tanzania. Instead of clustering with the remaining Tanzanians in Clade 3, they were emerged with nonAfrican haplotypes in Clade 2. This is similar to the observation made by Fontanilla et al. (2014), when they performed median-joining network analysis for studying the evolution of different haplotypes; they noticed that the Tanzanian haplotypes K, O, and R were emerged outside the remaining haplotypes from Africa. The same was observed in the network analysis carried out in the present study. All the clades in the Bayesian tree were strongly supported (posterior probability >0.9).
Clade 3 emerged out from clade 2 and consisted of five different Tanzanian haplotypes. Within this clade, Tanzanian haplotypes L, I, and M formed a separate clade.
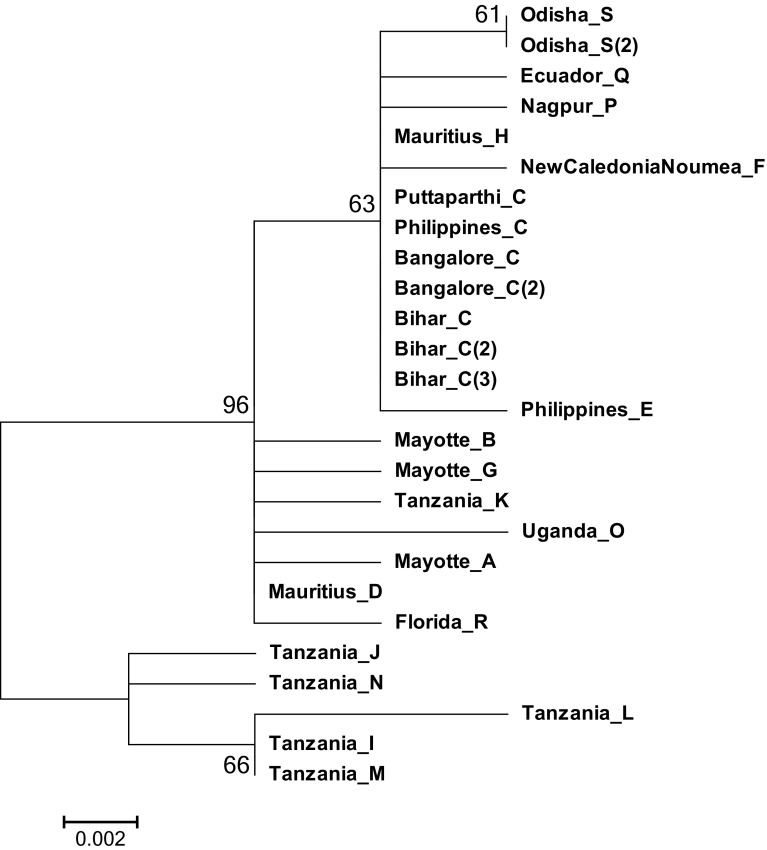
In addition to the Bayesian inference, phylogenetic relationships between the haplotypes were also studied using ML method (Fig. 6). Both of them have yielded similar topologies but differed in the node support values (Figs. 5, 6).
Fig. 6.
Phylogenetic relationships of the haplotypes of Achatina fulica constructed by maximum likelihood (midpoint rooted) in MEGA. Indicated on the nodes are the bootstrap values. Scale bar represents 0.002 nucleotide substitutions per site
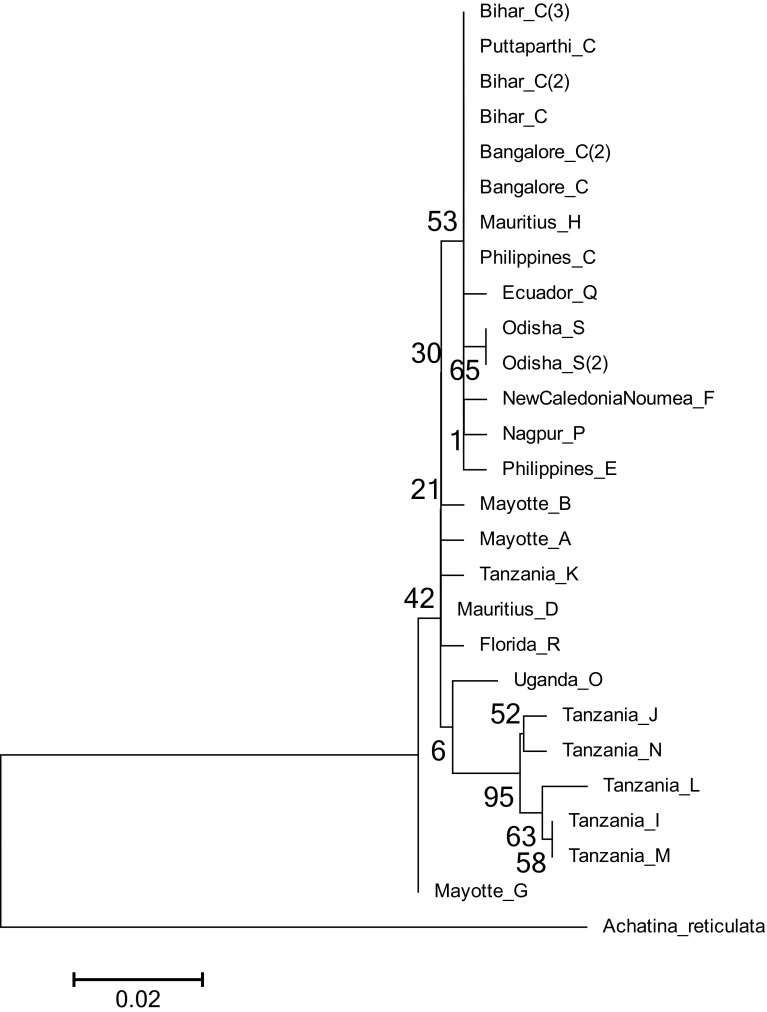
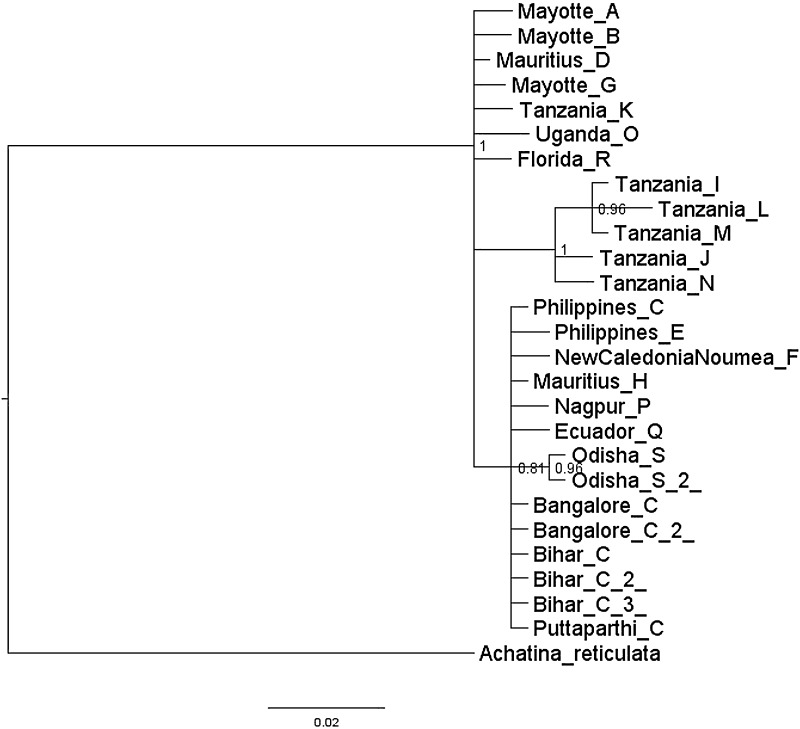
Apart from this, ML and Bayesian analyses were also carried out using Achatina reticulata as outgroup. In ML analysis (Fig. 7), it was observed that inner nodes are not clearly resolved when compared to the one constructed without outgroup (Fig. 6). However, it recovered two of the major clades consisting of Tanzanian haplotypes J, N, L, I, and M as a distinct clade and haplotypes C, H, Q, S, F, P, and E as another clade. In contrast, Bayesian tree constructed with an outgroup (Fig. 8) inferred the same relationships with the one constructed without outgroup (Fig. 5).
Fig. 7.
Phylogenetic relationships of the haplotypes of Achatina fulica constructed by maximum likelihood in MEGA. The phylogenetic tree is rooted by Achatina reticulata. Indicated on the nodes are the bootstrap values. Scale bar represents 2 substitutions per 100 nucleotides
Fig. 8.
Bayesian inference of phylogenetic relationships of the haplotypes of Achatina fulica. The phylogenetic tree is rooted by Achatina reticulata. Indicated on the nodes are the posterior probability values. Scale bar represents 2 substitutions per 100 nucleotides
Network analyses
Haplotype network drawn using TCS v1.21 (Fig. 3) showed that haplotype C gave rise to haplotypes E, F, P, and Q each with a single-nucleotide difference from haplotype C. Apart from these, haplotypes H and D were linked to Haplotype C each with a single mutation step. Haplotype S from the Indian sub-continent emerged from Mauritius haplotype ‘H’ by a single mutation step. Haplotypes H and D were linked by a hypothetical haplotype which was distant from these two haplotypes by a single-nucleotide difference. This hypothetical haplotype gave rise to two haplotypes A and B which were distant from it by a single mutation step. Emerged from haplotype D were the Tanzanian haplotypes I, J, L, M, and N which were linked to haplotype D by three mutation steps. Apart from these haplotypes, Tanzanian haplotypes R and K were linked to haplotype D each with two mutation steps. Furthermore, Tanzanian haplotype O and a Mayotte haplotype G linked to haplotype D with two and one mutation steps, respectively.
Apart from this, median-joining network analysis of haplotypes was carried out using SplitsTree (Fig. 4). The topologies of haplotype networks drawn using SplitsTree and TCS were similar except that haplotypes K and R emerged directly from haplotype D in the former instead of emerging from a hypothetical haplotype as seen in the latter.
Discussion
The comprehensive observations regarding the haplotype diversity of A. fulica in India were that two haplotypes C and P were observed from the studies of Fontanilla et al. (2014). In the present study, a new haplotype S was observed along with the haplotype C, which is the predominant one in the Indian Ocean islands (Fontanilla et al. 2014). Thus, in total, three haplotypes of A. fulica were observed in India to the extent it was sampled.
It is to be noted that there is limited genetic variation outside the East Africa as evident from the studies carried out by Fontanilla et al. (2014). However, it is also to be noted that the extent of sampling plays an important role in determining the haplotype diversity and in uncovering new haplotypes. High extent of sampling sites with increased sampling may possibly give a clear picture on the prevalence of new haplotypes.
Achatina fulica is native to East Africa and was spread to the entire world by introductions. The time scale or the time in which introductions started is very less compared to the evolution of A. fulica itself (Raut and Baker 2002). Hence, the prevalence of diverse numbers of haplotypes in the native regions of A. fulica is of no wonder. However, such a high prevalence of different haplotypes in the introduced regions of Mayotte and Madagascar (Fontanilla et al. 2014) raises several important questions as to how these haplotypes emerged in a short period of time in these countries when compared to the other regions of introduction. A possible explanation to this is that there could have been multiple introductions from Africa to Madagascar and Mauritius in the past which might have given rise to the existing haplotype diversity along with the emergence of new haplotypes during the course of evolution.
Lack of fossil evidence in Madagascar suggests East Africa to be the place of origin of this snail (Raut and Baker 2002). As per Raut and Baker (2002), A. fulica was introduced to Madagascar prior to 1800 from Kenya. However, Fontanilla et al. (2014) did not sample this snail from Kenya. Sampling from Kenya may give a clear picture about the prevalence of different haplotypes in that region. Further increased sampling from Madagascar also gives more insights regarding the prevalence of haplotypes in relationship to Kenya. Especially, it may be known whether haplotype C is prevalent in Kenya or not.
In the Indian populations of A. fulica, haplotype C is the predominant one. However, it cannot be established definitely that the two individuals of A. fulica introduced into the country a century ago were of C haplotype. On one hand, it may seem likely because haplotype C is the predominant one amongst the haplotypes sampled from Mauritius by Fontanilla et al. (2014) from where these were introduced into the country a century ago.On the other hand, it may be unlikely because there might had an ancestral haplotype which might have given rise to the present day ‘C’ haplotype in India which might have gone extinct or was not sampled. Another possibility which cannot be ruled out is that the two individuals of A. fulica introduced in India a century ago might have consisted of different haplotypes.
Haplotype network analyses showed that haplotype ‘S’ is linked to a Mauritius haplotype ‘H’ by a single mutation. Mauritius is considered to be the place from where A. fulica was introduced into the country (Raut and Baker 2002). In contrast, haplotype ‘P’ from India emerged from haplotype ‘C’. Further understanding is required with regard to the association of haplotype S observed from the Indian state of Odisha with Mauritius haplotype ‘H’. Is this because of unseen multiple introductions to India from Indian Ocean islands? As opined by Fontanilla et al. (2014), the possibility of unseen introductions into the country also cannot be ruled out. It is yet to be known whether A. fulica from Kolkata harbours haplotype(s) C, P, S or a new haplotype. This shall be known only after this snail could be sampled from Kolkata in future.
As this snail was introduced into the country deliberately by human introductions, strict quarantine checks prevent its further dispersal from one country to another country. Apart from this, public awareness needs to be created about the potential damage that it causes to the crops plants as well as to human beings. This limits the dispersal of this snail from one region to another region within a country. With the changing climatic patterns, there are chances that new areas may be prone for further invasions (Rekha et al. 2015). In this scenario, increased surveillance with respect to controlling its further spread in the country needs to be carried out. In conclusion, this study gives insights regarding the genetic diversity of this snail population in India despite several logistical constraints in sampling snails from different regions. In future, further sampling from different geographical regions gives more insights into the genetic diversity of this snail population in India.
Acknowledgements
This work has been funded by the Science and Engineering Research Board, Department of Science and Technology, Government of India (Sanction order: SB/SO/AS-138/2012 dated 30-10-2013). Authors gratefully acknowledge the experts from Malacological section, Zoological Survey of India, Kolkata for identifying the A. fulica specimen.
Compliance of ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Albuquerque FS, Peso-Aguiar MC, Assunção-Albuquerque MJT. Distribution, feeding behaviour and control strategies of the exotic land snail Achatina fulica (Gastropoda: Pulmonata) in the northeast of Brazil. Braz J Biol. 2008;68(4):837–842. doi: 10.1590/S1519-69842008000400020. [DOI] [PubMed] [Google Scholar]
- Ayyagari VS, Naravula J, Sreerama K. Optimization of the isolation procedure of genomic DNA from a mucus laden pulmonate gastropod, Achatina fulica. Natl Acad Sci Lett. 2017;40:109–112. doi: 10.1007/s40009-016-0535-0. [DOI] [Google Scholar]
- Budha PB, Naggs F. The giant African land snail Lissachatina fulica (Bowdich) in Nepal. Malacologist. 2008;50:19–21. [Google Scholar]
- Civeyrel L, Simberloff D. A tale of two snails: is the cure worse than the disease? Biodivers Conserv. 1996;5:1231–1252. doi: 10.1007/BF00051574. [DOI] [Google Scholar]
- Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9(10):1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernanda PO, Guimarães MCDA, Fernanda YT, Juliana ME. Current distribution of Achatina fulica, in the state of São Paulo including records of Aelurostrongylus abstrusus (Nematoda) larvae infestation. Rev Inst Med Trop Sao Paulo. 2010;52(4):211–214. doi: 10.1590/S0036-46652010000400009. [DOI] [PubMed] [Google Scholar]
- Fontanilla IKC, Maria IMPS, Garcia JRM, Ghate H, Naggs F, et al. Restricted genetic variation in populations of Achatina (Lissachatina) fulica outside of East Africa and the Indian Ocean islands points to the Indian Ocean islands as the earliest known common source. PLoS One. 2014;9(9):e105151. doi: 10.1371/journal.pone.0105151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist FR. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23(2):254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jadhav AD, Dubal RS, Bagade RP, Sanadi RA, Kamble PL, Belgumpe S, Sathe TV. Giant African snail, Achatina fulica Bowdich a destructive pest of V1 mulberry (Morus alba L.) by—a new report and control strategies from Kolhapur, Maharashtra, India. Biolife. 2016;4(1):184–188. [Google Scholar]
- Jayashankar M, Murthy GSS, Krishnappa DK, Reddy MS. Incidence of life stages of strongylid nematodes in the giant African snail, Achatina Fulica (Bowdich) in Bangalore region. Glob J Res Anal. 2014;3(4):193–194. doi: 10.15373/22778160/APR2014/67. [DOI] [Google Scholar]
- Joseph EA. The presence of Angiostrongylus Cantonensis in islands of the Indian ocean and probable role of the giant African snail, Achatina fulica in dispersal of the parasite to the Pacific islands. Can J Zool. 1966;44(6):1041–1049. doi: 10.1139/z66-111. [DOI] [PubMed] [Google Scholar]
- Khan SA, Kumar S, Hussain MZ, Kalra N. Climate change, climate variability and Indian agriculture: impacts vulnerability and adaptation strategies. In: Singh SN, editor. Climate change and crops, environmental science and engineering. Berlin: Springer; 2009. pp. 19–38. [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the World’s worst invasive alien species: a selection from the global invasive species database. Published by the Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12 pp. First published as special lift-out in Aliens 12, December 2000. Updated and reprinted version: November 2004. Auckland, New Zealand
- Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis. Version 3.03. http://mesquiteproject.org
- Mathai RS. The snail spurt—an issue of concern. Int Res J Environ Sci. 2014;3(6):88–91. [Google Scholar]
- Mead AR. The giant African snail: a problem in economic malacology. USA: The University of Chicago Press; 1961. [Google Scholar]
- Naggs Fred. William Benson and the early study of land snails in British India and Ceylon. Arch Nat Hist. 1997;24(1):37–38. doi: 10.3366/anh.1997.24.1.37. [DOI] [Google Scholar]
- Nath Paras. Emerging pest problems in India and critical issues in their management. In: Jain PC, Bhargava MC, editors. Entomology—novel approaches. New Delhi: New India Publishing Agency; 2007. pp. 43–96. [Google Scholar]
- Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Gra-bowski G (2002) The simple fool’s guide to PCR. Version 2.0, University of Hawaii Press, Honolulu
- Poucher C. Eradication of the giant African snail in Florida. Annu Meet Fla State Hort Soc. 1975;88:523–524. [Google Scholar]
- Ramakrishna Mitra SC, Dey A (2010) Annotated checklist of Indian land molluscs. Rec. Zool. Surv. India, Occasional paper no. 306, pp 1–359 (Published by the Director, Zoological Survey of India, Kolkata)
- Rambaut A (2012) FigTree v1.4.2: tree figure drawing tool. Available from http://tree.bio.ed.ac.uk/software/figtree/
- Rambaut A, Suchard MA, Xie W, Drummond AJ (2014) Tracer v1. 6. Available from http://beast.bio.ed.ac.uk/Tracer
- Raut SK, Baker GM. Achatina fulica Bowdich and other Achatinidae as pests in tropical agriculture. In: Baker GM, editor. Molluscs as crop pests. Wallingford: CAB International Publishing; 2002. pp. 55–114. [Google Scholar]
- Raut SK, Ghose KC. Pestiferous land snails of India. Tech Monogr Zool Surv India. 1984;11:1–151. [Google Scholar]
- Rekha SR, Munsi M, Aravind NA. Effect of climate change on invasion risk of giant African snail (Achatina fulica Ferussac, 1821: Achatinidae) in India. PLoS One. 2015;10(11):e0143724. doi: 10.1371/journal.pone.0143724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra NB, Robin G, Birinchi KB. Snail: from present perspective to the history of Assam. Asian Agri-Hist. 2009;13(3):227–234. [Google Scholar]
- Sheela T (1999) Management of giant African snail Achatina fulica Bowdich (Mollusca: Gastropoda) in North Bihar. Final project report submitted to Indian Council of Agricultural Research, New Delhi, India
- Silvana CT, Faraco FA, Norma CS, Robert HC, Monica AF. Rapid spread of an invasive snail in South America: the giant African snail, Achatina fulica, in Brazil. Biol Invasions. 2007;9:693–702. doi: 10.1007/s10530-006-9069-6. [DOI] [Google Scholar]
- Sokolov EP. An improved method for DNA isolation from mucopolysaccharide-rich molluscan tissues. J Moll Stud. 2000;66:573–575. doi: 10.1093/mollus/66.4.573. [DOI] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitha K, Karuppuchamy P, Sivasubramanian P. Record of gastropod pests on vanilla and their population dynamics in relation to weather parameters. Pest Manag Horticult Ecosyst. 2011;17(1):56–59. [Google Scholar]
- Wade CM, Mordan PB. Evolution within the gastropod molluscs: using the ribosomal RNA gene-cluster as an indicator of phylogenetic relationships. J Moll Stud. 2000;66:565–570. doi: 10.1093/mollus/66.4.565. [DOI] [Google Scholar]