Abstract
Thoracic endovascular aortic repair (TEVAR) in the current era has been chosen as a dominant and minimally invasive treatment for complicated aorta dissection. This study aimed to assess safety and feasibility of TEVAR in acute and chronic type B aortic dissection.
Between January 2011 and December 2013, 85 patients with complicated type B aortic dissection undergoing TEVAR were divided into acute aortic dissection (AAD) (n = 60) group and chronic aortic dissection (CAD) group (n = 25). Computed tomography was used to evaluate postoperative changes in maximal aortic diameter and true and false lumen diameters at 3 levels during a mean follow-up period of 26.4 ± 15.6 months.
The technical success rate was 100%. In-hospital and 30-day rates of death were 3.3% in acute group and 0 in chronic group. Postdischarge rates of type I leak, type II leak, and retrograde type A dissection were 6.7%, 5.2%, and 3.4% (acute) and 0%, 4.0%, and 4.0% (chronic), respectively. The maximal aorta diameter remained stable in all the 3 levels in both acute and chronic group. The cumulative freedom from all-cause mortality at 3 years was similar in acute and chronic groups (89.5% vs 95.5%, P = .308). The cumulative freedom from aortic-related mortality was also not significantly different in the acute and chronic groups (92.8% vs 95.2%, P = .531). In the thoracic aorta, TEVAR treatment resulted in a significant increase in true lumen (TL) diameter and decrease in false lumen (FL). However, in the abdominal aorta, TEVAR did not lead to significant change in TL and FL diameters. The rates of complete thrombosis thoracic false lumens were better than that in the abdominal false lumen.
TEVAR was a safe and effect therapy for complicated acute and chronic type B dissection with low early and mid-term mortality and morbidity.
Keywords: aortic remodeling, endoleak, retrograde type A dissection, thoracic endovascular aortic repair, type B aortic dissection
1. Introduction
Aortic dissection is the most common life-threatening disorder of the aorta, and the incidence has been reported approximately 3 cases per 100,000 people per year, with higher rates in men.[1,2] According to the International Registry of Acute Aortic Dissection (IRAD), about one third of all the acute aortic dissections (AADs) are of type B.[1] Among all the acute type B aortic dissection, about 25% of patients are complicated with malperfusion syndrome or hemodynamic instability at admission and untreated complicated dissection will result in irreversible end organ damage or death.[3] Similarly, recurrence of symptoms, aneurysmal dilation (55 mm), or an aortic yearly increase of 4 mm was indicative of complicated chronic type B aortic dissection, and these factors are indicative of higher risk.[3] Untreated complicated dissection will result in irreversible end organ damage or death. Therefore, efficient and timely measures should be adopted to save patients’ lives.[4]
Although great advances have been made over the years, surgical mortality was reported to exceed more than 30%[5,6] and considerable morbidity including spinal cord ischemia, cerebrovascular accident, and renal failure.[7] Thoracic endovascular aortic repair (TEVAR) in the current era has become the dominant and minimally invasive treatment for type B complicated aorta dissection.[8,9] The first report of TEVAR repair was firstly introduced by Dake et al 1994[10,11] and the first commercial device gained US Food and Drug Administration approval for this indication in 2005.[12] The aim of the treatment for the complicated aortic dissection is to deliver the blood flow through true lumen by sealing the proximal entry tear, abrogation of frank or impending aortic rupture, and relief of dynamic malperfusion. This process is termed as aortic remodeling, during which the false lumen is gradually thrombosed and true lumen is enlarged but without enlargement of the total aortic diameter.[13] Furthermore, aortic remodeling after TEVAR has been reported to be a significant prognostic factor for better outcome.[14] However, there are few studies on aortic remodeling for patients undergoing TEVAR in China, and early and midterm clinical efficiency of TEVAR in complicated type B aortic dissection required further investigations. Therefore, we performed this retrospective clinical study to evaluate the clinical outcomes and aortic remodeling of acute and chronic aortic dissection (CAD) in a Chinese population.
2. Materials and methods
2.1. Patient population
The study was performed according to the principles of the Declaration of Helsinki and approved by the ethics committee of our institution. Written informed consent was provided by all patients who participated in the study. Between January 2011 and December 2013, 85 consecutive patients with type B aortic dissection undergoing TEVAR in the Department of Vascular Surgery of the General Hospital of the People's Liberation Army were recruited in this study. Indications for complicated AAD included one or more followed clinical symptoms or anatomical characteristics: free or contained rupture; clinical or radiographic evidence of malperfusion; and refractory pain/impending rupture. Indications for complicated CAD included maximum aortic diameter >55 mm, an aortic increase of >5 mm within 3 months, detection of organ ischemia, and recurrence of other symptoms (pleural effusion, refractory pain, and resistant hypertension). Refractory pain was defined as ongoing symptoms of back and/or chest pain requiring narcotic medications in case of excellent blood pressure control. Patients with connective tissue disease (eg, Marfan syndrome), atypical aortic dissection (including intramural hematoma and penetrating atherosclerotic ulcer), residual type A aortic dissection, and trauma patients were excluded. Aortic dissection was defined as acute within 14 days from dissection onset and chronic 14 days after onset of acute symptoms, respectively. The whole 85 patients were divided into AAD group (n = 25) and CAD group (n = 60).
2.2. Surgical technique
All the procedures were performed under general anesthesia in the operation room. Patients should be evaluated by a team of cardiothoracic and vascular surgeons, interventional radiologists, and anesthesiologists before operation and only the qualified patients could receive TEVAR procedure. Detailed descriptions of procedural and technical information have been described previously.[15,16] In brief, arterial access was established and the stent grafts were deployed over a stiff wire. A proximal sealing zone of at least 15 mm was required. Oversizing by 10% to 20% was achieved according to the operator. Stent graft was deployed to prevent displacement of the stent graft with systolic pressure titrated to 80 to 90 mm Hg with sodium nitroprusside. Since the first description of TEVAR, it has been the 1st-line therapeutic option for acute complicated type B aortic dissection and increasingly used in the treatment of chronic complicated type B aortic dissection due to it provides relative low morbidity and mortality and satisfactory clinical outcome compared to open surgery. Therefore, the standard clinical practice should be performed.[17] Angiography was applied to evaluate the reestablishment of distal perfusion after the stent-graft was deployed in the hybrid operation room. Computed tomography angiography (CTA) was performed again to assess the reestablishment of distal perfusion during the follow-ups. Subsequent deployment of the bare metal stent component was recommended if branch vessel obstruction or false lumen perfusion persisted, and was performed at the discretion of each implanting physician.
2.3. Postprocedural imaging and follow-up
All patients underwent preoperative CTA of the entire aorta, including the bilateral carotid and pelvic arteries. Delayed scans were used for the detection of endoleaks. The Digital Imaging and Communications in Medicine data were transferred to 3mensio v6.1 (Pie Medical Imaging B.V. Inc, Maastricht, The Netherlands) for measurement and calculation of maximal aorta diameter (MD), true diameter (TD), and maximal false diameter (FD) using multiple plane reconstruction technique at 3 different levels.[14] The MD, TD, and FD were measured directly at the levels of the bronchial bifurcation (L1), the lower edge of left atrium (L2), and the celiac trunk (L3) (Fig. 1). Status of the false lumen was qualitatively assessed using CTA as patent (evidence of contrast without evidence of thrombus), partially thrombosed (evidence of both contrast and thrombus), or completely thrombosed (evidence of thrombus without evidence of contrast) in the descending thoracic and abdominal aorta.[18] The typical images in the same patient before and after TEVAR were shown in Fig. 2.
Figure 1.
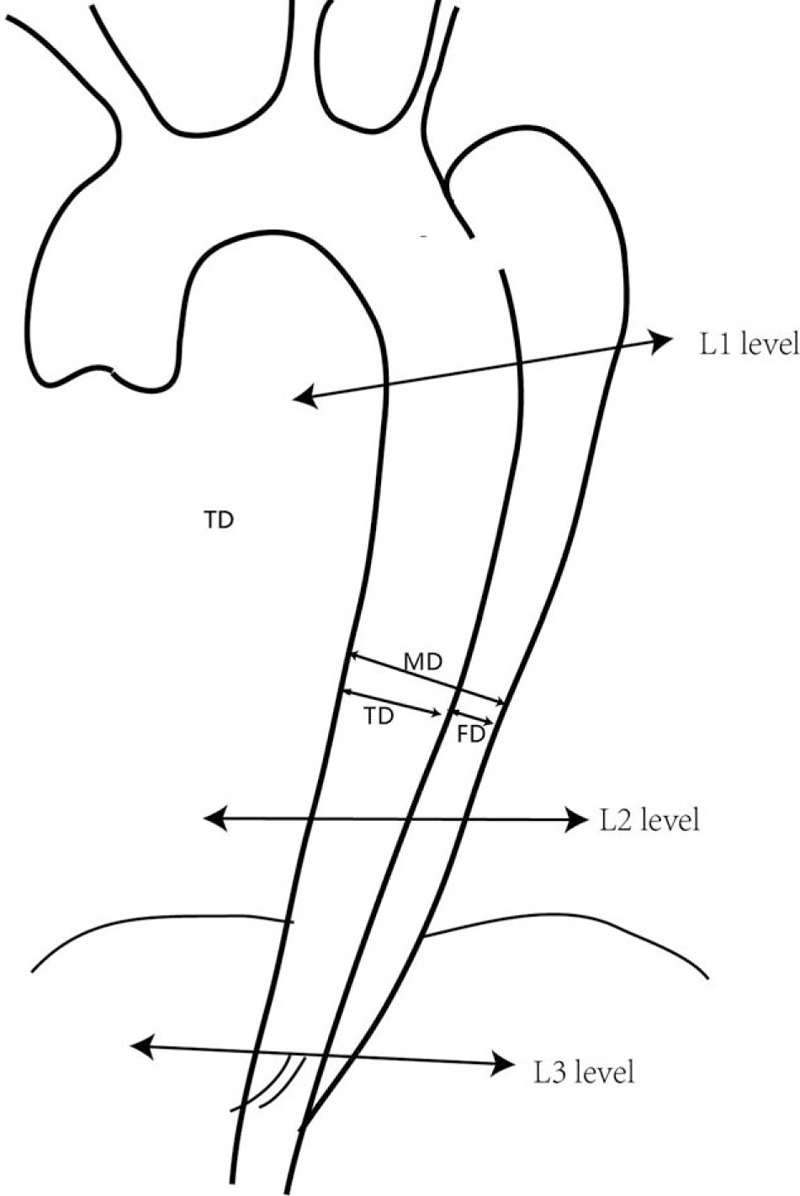
Levels on which the maximal aorta diameter, true diameter, and maximal false diameter were measured; the S1, S2, and S3 segments were indicated.
Figure 2.
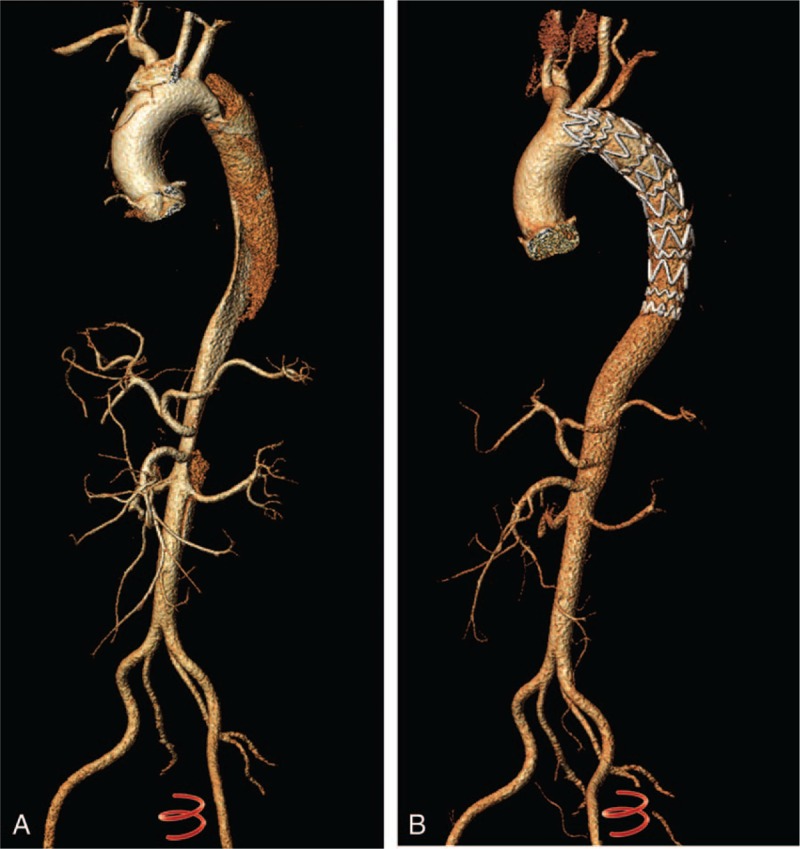
Three-dimensional CTA reconstruction demonstrated an acute type B aortic dissection (De Bakey III) (A) and the same patient 1 year after TEVAR procedure. No sign of dissection was seen. CTA = computed tomography angiography, TEVAR = thoracic endovascular aortic repair.
Postoperative CT was routinely performed at 3, 6, and 12 months, and yearly thereafter to measure the MD, TD, and FD. The entire descending aorta was divided into 3 segments distally from the stent graft to the celiac trunk: S1, stent covered aorta; S2, from the stent to the start of the celiac trunk; and S3, from the start of the celiac trunk to iliac branch arteries. The patency and remodeling of the false lumen were assessed by postoperative CTA. The diameter of the S1 segment was measured using curved planar reconstruction and the presence of distal perfusion in false lumen at the S1 segment, secondary entry tear of the end of the stent, and re-entries distal to the stent were recorded.
2.4. Statistical analysis
All data were analyzed using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range), according to whether they exhibited a normal distribution, and were compared by t test or a Wilcoxon rank-sum test. Categorical variables were expressed as percentages, and analyzed by chi-square and Fisher exact tests. Survival analyses were performed using the Kaplan–Meier method, and between-group comparisons were assessed by log-rank tests. Longitudinal data of total lumen, true lumen, and false lumen in acute and chronic group were compared with preoperative assessments. P < .05 was considered statistically significant.
3. Results
3.1. Patient demographic data and aortic dissection characteristics
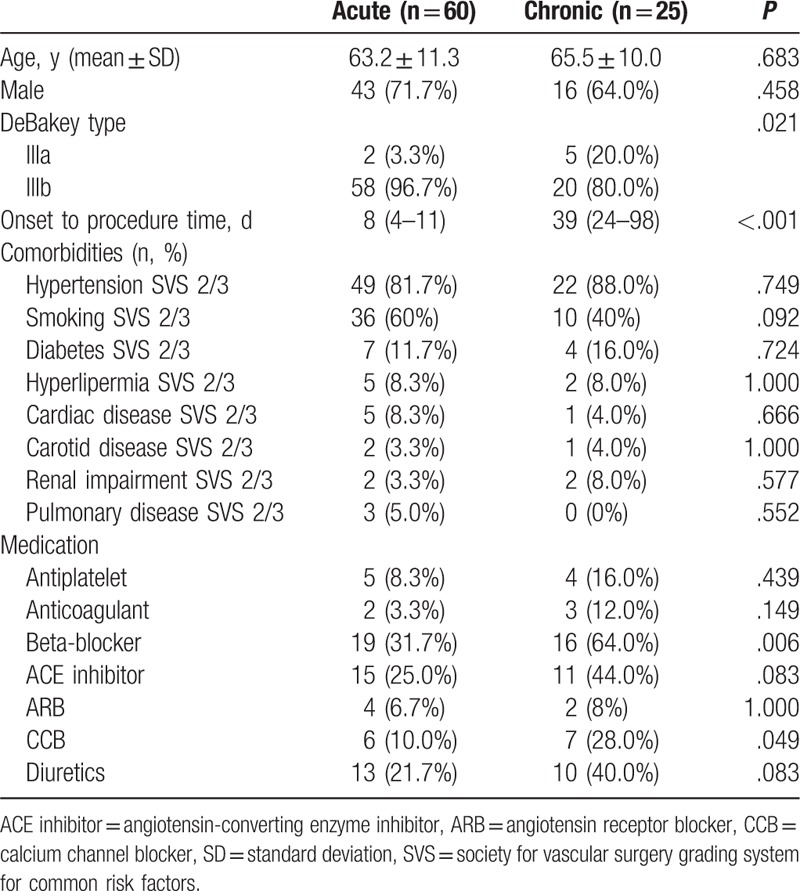
The demographics of the patient cohort are illustrated in Table 1. There were 43 and 16 male patients in the acute and chronic group, respectively. There was no significant difference between the 2 groups. More DeBakey type IIIb patients in the acute group compared to that in chronic group (96.7% vs 80.0%, P = .021). The mean onset to procedure time was 8 and 39 days in the acute and chronic group. Other detailed demographic information was also shown in Table 1. The FL was patent without evidence of thrombus formation in all patients. Beta-blocker and calcium channel blocker (CCB) were more prevalent in chronic group, and the other parameters were similar in the 2 groups.
Table 1.
Demographic information for patients with complicated acute and chronic aortic dissection.
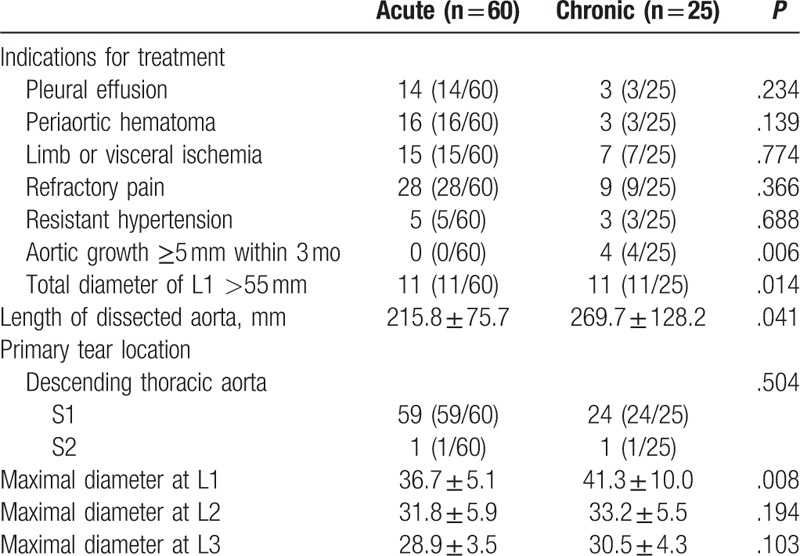
The indications for all patients receiving TEVAR were summarized in Table 2. A subset of patients in both acute and chronic group has 2 or more indications for TEVAR. The mean length of aorta covered in chronic group was significantly longer than that in the acute group (215.8 vs 269.7 mm, P = .041).
Table 2.
Dissection characteristics.
3.2. Clinical complications and secondary intervention
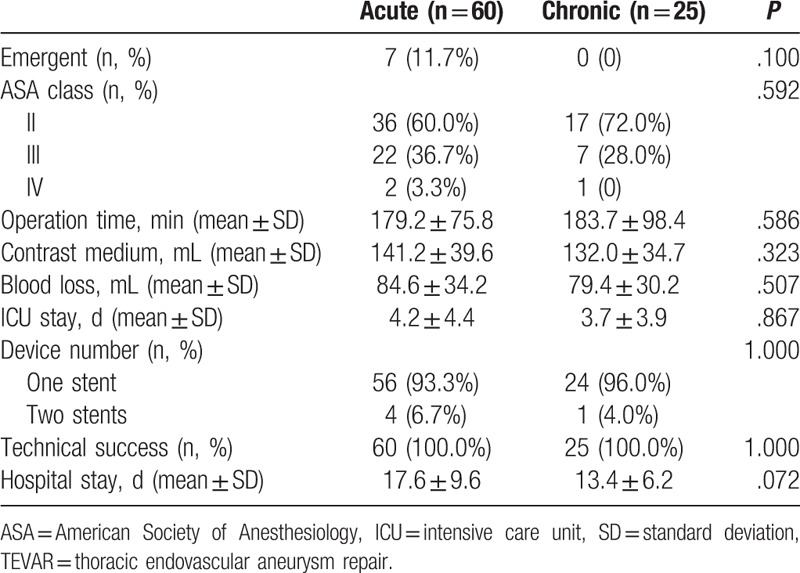
The technical success of TEVAR was achieved in all patients with correct deployment of the stent grafts and complete exclusion of the primary entry tear. All proximal landing zones were >2 cm. There were no conversions to open surgery and no type I endoleaks. Four patients (6.7%) in the acute group and 1 patient (4.0%) in the chronic group were implanted with 2 stents (Table 3). In addition, 3 (3/60, 5%) patients were implanted with a bare stent at the superior mesenteric artery, right renal artery, and right common iliac artery to provide a blood supply. The average postoperative intensive care unit (ICU) stay was 4.2 and 3.7 days in acute and chronic group, and no significant difference was found (P = .867). Hospital stay was potentially longer in the acute group, although the statistical significance was not strong (P = .072).
Table 3.
Operation data related with TEVAR for acute and chronic type B aortic dissection.
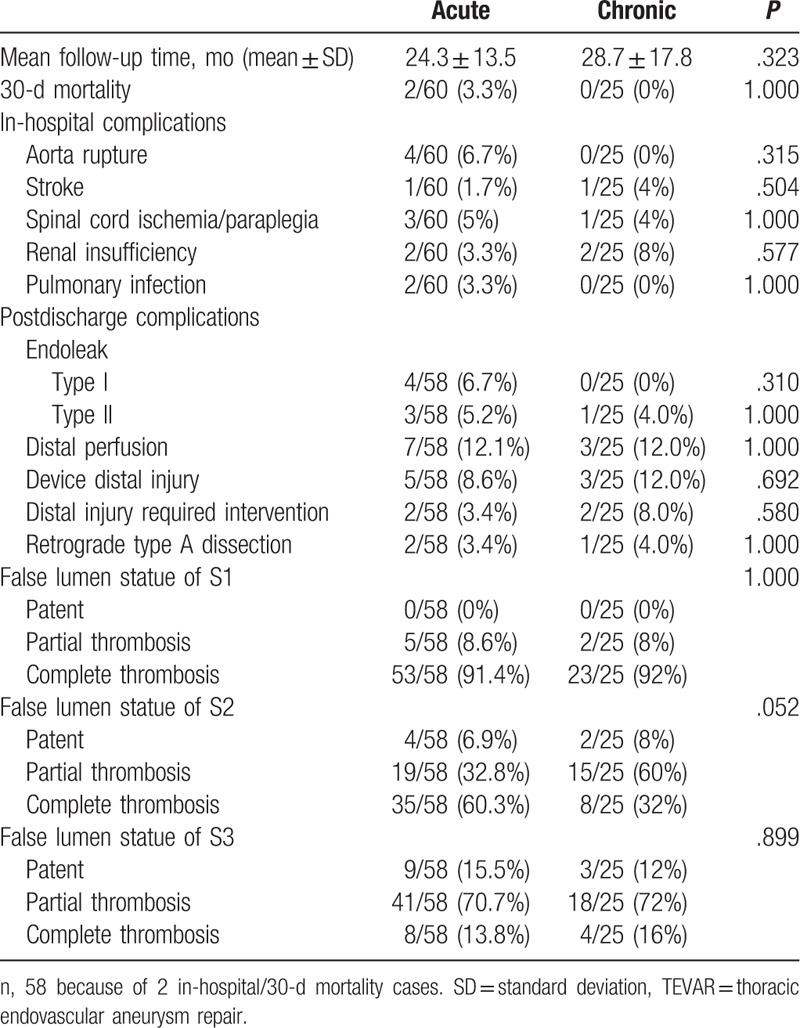
Type I endoleak only occurred in 4 acute dissections. Of them, 3 cases were successfully repaired by TEVAR, and the other case with small distal endoleaks experienced spontaneous resolution. Type II endoleak was found in 3 acute dissections that were treated with transcatheter embolization with coils and 1 chronic dissection that experienced spontaneous thrombosis. Ten cases in the acute group and 3 in the chronic group presented with distal perfusion. Five cases in the acute group and 3 cases in the chronic group were found to have secondary entry tears. Secondary endovascular treatment was performed in 1 case in the acute group and 2 in the chronic group. Two cases in the acute group and 1 in the chronic group presented with retrograde type A dissection (RTAD) at postoperative months 1, 8, and 21. All primary entry tears were successfully closed, and all proximal landing zones were >2 cm (Table 4).
Table 4.
Outcomes after TEVAR.
3.3. Survival analysis
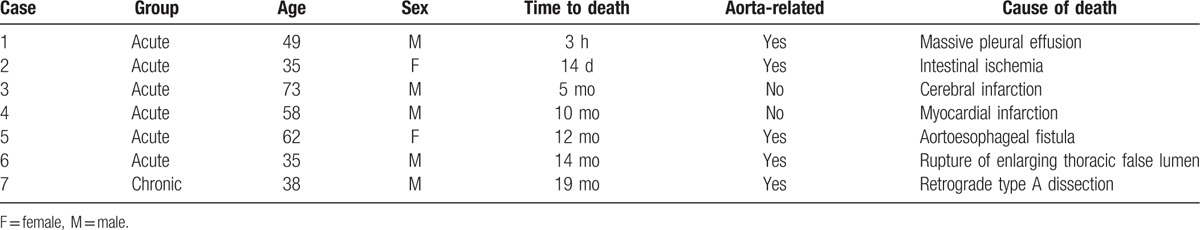
There were 2 early deaths in the acute group and no death in the chronic group. One died from massive pleural effusion 3 hours after TEVAR and 1 died from intestinal ischemia on day 14. Thus, total 30-day mortality was 2.4%. In addition, 3 cases in the acute group died at 10, 12, and 14 months after TEVAR procedure, and 1 patient in the chronic group died at 19 months (Table 5). In the S1 level, 2 mortalities within 30 days after surgery without sign of false lumen thrombosis and another 5 cases died during the longer follow-up. Of the 5 cases, 2 cases died with partial false lumen thrombosis and 3 cases died with patent false lumen, indicating aortic remodeling was essential for successful TEVAR.
Table 5.
Causes of all deaths during the follow-up time.
The mean follow-up duration was 26.4 (interquartile range, 14.6–32.1) months. The cumulative freedom from all-cause mortality at 1 and 3 year were 93.3% and 89.5% (acute) and 100% and 95.2% (chronic) for the 2 groups. The patients in the acute group experienced similar all-cause mortality compared with patients in the chronic setting (P = .308) (Fig. 3A). The freedom from aortic-related mortality at 1 and 3 year was 96.7% and 92.8% (acute) and 100% and 95.2% (chronic) for the 2 groups. Log-rank tests did not reveal a significantly different mortality rate in the acute group versus the chronic group (P = .531) (Fig. 3B).
Figure 3.
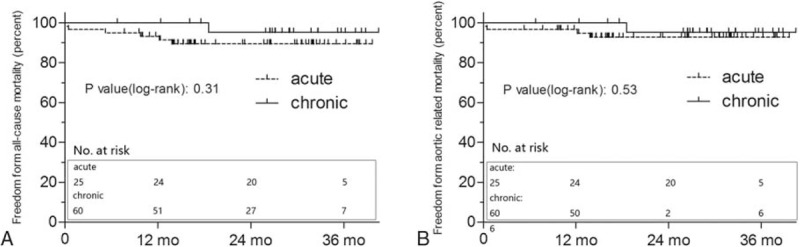
Kaplan–Meier analysis of freedom from all-cause mortality (A) and freedom from aortic-related mortality (B). Vertical bar represents censored data. The number of patients at risk at various time points was given.
3.4. Aortic remodeling
Changes in the average diameters of the true lumen, false lumen, and total aortic lumen were demonstrated in Fig. 4. In the acute group, the TEVAR treatment resulted in a significant increase in TL diameter over time at the level of L1 (from a mean of 16.2–36.7 mm after 36 months, P < .001) and L2 (from a mean of 12.1–26.2 mm after 36 months, P < .001). The FL diameter also significantly decreased at the level of L1 (P < .001) and L2 (P < .001) at 3 years. However, at the level of L3, both TL and FL diameters did not change significantly during the follow-up. In addition, the MD remained stable in all the 3 levels.
Figure 4.
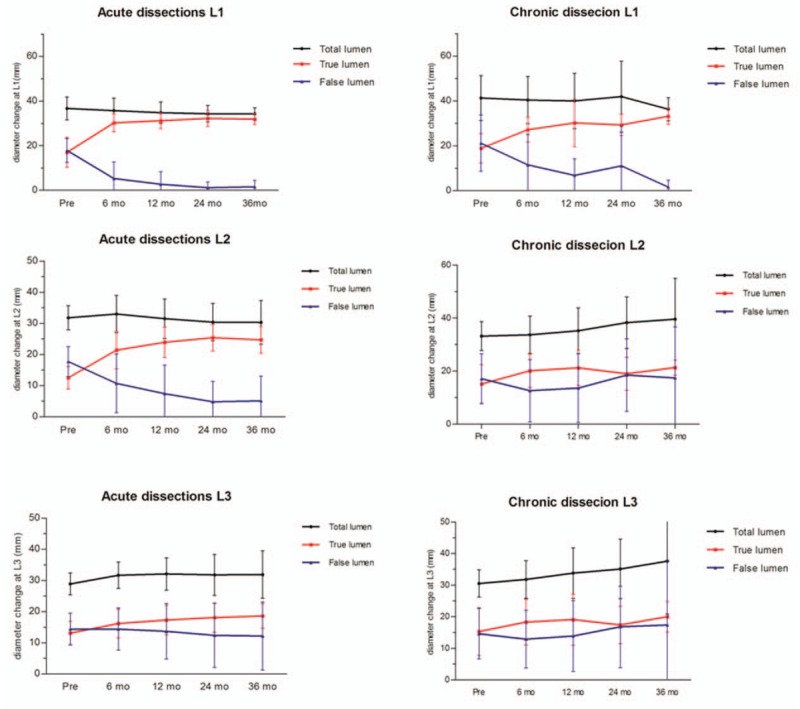
Total aortic diameter, true lumen, and false lumen regression trends at different measured levels, along with the time after thoracic endovascular aortic repair (TEVAR).
In chronic group, TEVAR significantly increases the aortic diameter of TL (P < .01) and decrease the dimension of FL (P < .001) at the level of L1. However, the diameter of TL and FL at the level of both L2 and L3 did not change significantly. The total aortic caliber was also not significantly modified by TAVER in all the 3 levels. Notably, such aortic remodeling occurred early after TEVAR treatment and subsequently changes occurred mostly within 18 months.
Although a patent false lumen was seen more frequently in acute dissections before TEVAR procedure, there was no significant difference in the false lumen status at L1, L2, and L3 levels between acute and chronic dissections during follow-up. All thoracic false lumens were either partially or completely thrombosed after the 30-day follow-ups. In comparison, the rates of complete thrombosis in the abdominal false lumen were lower. Complete thrombosis of the false lumen at the level of L1 was observed in 53 patients (91.4%) in the AD group and 23 patients (92%) in the CD. However, the complete thrombosis rate in the distal abdominal false lumen (L3 level) decreased to 13.4% (8/58) in acute dissection and 16% in chronic dissection, respectively.
4. Discussion
This study demonstrated the feasibility of TEVAR in complicated type B aortic dissection, with acceptable midterm survival rates and relative low complications. All stent grafts were successful deployed and technical success was as high as 100%. The 30-day mortality was low in acute dissection (3.3%) and no perioperative deaths occurred in chronic group. The overall survival was 93.3% and 89.5% (acute) and 100% and 95.5% (chronic) at 1 and 3 years, respectively. FL thrombosis was more evident in AAD within the 1st 12 months after treatment compared with CAD.
TEVAR appears to offer a substantially option for complicated aortic dissection in both acute and chronic setting, and several reports have confirmed this conclusion. Sayer et al[5] reported that the cumulative survival was superior in AAD compared to CAD (93% vs 66.5%, P = .015). In contrast, Böckler et al[19] reported that overall survival rate was significantly better in patients with chronic CAD compared with these with AAD (P = .038). However, some studies did not find significance between the 2 groups.[18,20] Chen et al[20] reported that patients in both acute phase and chronic phase have similar rates of freedom from all-cause mortality (92.4% vs 96.4%, P = .293) and freedom of aortic-specific mortality (89.0% vs 96.4%, P = .102). Our results also revealed similar survival in acute group and chronic group, which was consistent with this study. The discrepancy among various studies was suspected to deprive from the fact that the enrolled patients were heterogeneous and from different medical centers. With more accumulated experience and properly selected patients, the 30-day postoperative mortality has decreased from initial 21%[21] to 0%–7.6%.[22–24] Therefore, TEVAR has been adopted as 1st-line therapy for complicated aortic dissection, and European Society of Cardiology recommended TEVAR as a class I treatment for complicated type B dissections.[25]
As TEVAR has become a popular therapy, deep insights into the related complications will help to alleviate the perioperative morbidity. The most commonly reported complication following TEVAR is endoleak.[26] Type I and type III endoleaks require correction, such as proximal cuff or extension, while type II endoleaks may seal spontaneously in about 50% of cases.[25] Fortunately, most type I endoleaks can be successfully treated by secondary endovascular techniques. Moreover, some type 1 endoleaks may eventually seal without any intervention. Sze et al[27] revealed that coverage of the left subclavian artery, a small radius of aortic arch curvature, and incomplete proximal apposition of the stent graft were significant portending factors for endnoleaks. Therefore, coverage of LSA is often performed to achieve a proximal seal, and this proportion of patients was reported to be as high as 40%.[28] Meanwhile, some studies have demonstrated intentional coverage of the LSA was associated with a higher overall stroke rate (13% vs 2%) and posterior circulation stroke rate (5.5% vs 1.2%) compared with LSA revascularization.[29,30] Therefore, the reconstruction of LSA prior to or during procedure should be carefully evaluated and performed in elective patients.
RTAD during or after TEVAR is a potentially lethal complication unique to thoracic endografting. The incidence of RTAD after TEVAR for acute type B aortic dissection was 8.4% and 3% for chronic dissection[31]; however, the overall mortality of this catastrophic complication could be as high as 33.6% to 57%.[31,32] The occurrence proved to be related with incomplete design of stent-graft system, rough handling, and presence of vascular wall lesions.[33] Indeed, the extent of graft oversizing was associated with an increased risk of RTAD. Increased graft oversizing (>9%) appeared to translate to an increased relative risk of RTAD by 14% for each percent oversize, so many clinicians consider a 10% oversize was appropriate.[31,32,34] Inappropriate endograft oversizing should be avoided, a close surveillance program is recommended and immediately surgical procedure should be performed in case of retrograde type A aortic dissection.[35]
In the last decade, TEVAR has been widely accepted for in clinic for its safety, convenience, and efficiency, but the TEVAR-associated mortality has been a troublesome issue for many surgeons. In this cohort, a total of 7 patients died during the follow-ups, including 6 (10%) acute AD patients and 1 (2.5%) chronic AD patient. Mortality tended to occur more frequently in the acute AD patients without statistical significance due to limited samples (P = .668, Fisher exact test). Two reasons may account for this result. On the one hand, as shown in Table 1, DeBakey IIIA was more frequent in the chronic AD patients compared to acute AD patients. It has been reported that thrombosis of false lumen was more common in DeBakey IIIA, and the thrombosis of false lumen is a protective factor for the better prognosis.[36,37] On the other hand, beta-blocker and calcium channel blocker were more frequently used in the chronic group (Table 1). Previous studies have confirmed that improved survivals were shown in patients both in acute and chronic phase of AD and calcium channel blocker also proved to have a protective effect for acute AD patients.[17,38] Therefore, medical therapy including blood pressure control is an essential part for the management of acute AD patients. Other technology, such as rapid pacing, as an efficient and safe method for lowering blood pressure in selected patients, also facilitates accurate deployment of stent-graft.[39]
TEVAR aims to stable the dissected aorta and prevent late complications by inducing aortic remodeling process. In the INSTEAD trial, the maximum aortic diameter of the patients treated with TEVAR was stable but significantly increased in patients who were medically treated during 5 years.[40] In the present study, TEVAR lead to aortic remodeling by stabling the maximal aortic diameter of 3 levels during an average follow-up of 26.4 months. The complete false lumen thrombosis rate in the thoracic aorta was more than 90% in the acute and chronic group but was relatively low in the abdominal false lumen (Table 4). It has been reported that higher false lumen thrombosis predicted better long-term outcome.[41,42] This finding implied that long-term surveillance after TEVAR is vital, and distal arterial tree should be monitored by imaging examination during follow-ups. Besides, the false lumen complete thrombosis rate tends to be significantly higher in acute group compared to chronic group, which suggests that the capacity for aortic remodeling in CAD is weaker.[43,44] During the chronic phase, the adventitial and intimal flaps become more stable and rigid, thus reducing the capacity of prominent remodeling ability to open up the distal true lumen. However, our results and several other reports indicated that this phenomenon seems to have no effect on the postoperative outcomes.[5,45] Since aortic dissection is a systematic disease with entire aorta and its branches, systemic hypertension, older age, aortic size, and presence of patent false lumen essential predictors of late complications. Therefore, basic anti-impulse drugs, including β blockers, are required to decrease aortic wall, and regular surveillance is necessary to improve prognosis.[34,35] Aortic diameter was also reported to be a risk factor for mortality. In the IRAD study, 18.4% of patients with acute type B aortic dissection present with a descending aortic diameter of 5.5 cm or greater, in-hospital mortality could reach as high as 23.0%, which was significantly higher than these with an aortic diameter less than 5.5 cm (P < .001).[46] In our study, 2 patients died within 30 days after surgery and these patients had aortic diameter of 5.5 cm or greater; therefore, aortic diameter of 55 mm or greater is an indicator for mortality and more attention should be paid.
False lumen (FL) diameter was also an important factor impacting morbidity and mortality. Song et al reported a 22 mm initial false lumen diameter in the upper thoracic descending aorta was a predictor for late aneurysm with a sensitivity of 100% and a specificity of 76% and also demonstrated higher aneurysm or death rate than others.[47] Chang et al[48] reported that false lumen size was an indicator for in-hospital complications after acute type B aortic dissection, and patients with maximal false lumen area (MFLA) ≥922 mm2 showed higher complications than other patients. Therefore, more aggressive procedures should be applied for patients with larger false lumen diameter.
However, there exist some limitations in this study. First, it represents a retrospective analysis of collected data and selection biases may exist. Second, the conclusions were drawn from a single institution and the number of patients was limited, a larger patient cohort with long-term outcomes is still required. Finally, several types of stent grafts were utilized, and this difference may influence the final clinical results to some extent.
In conclusion, TEVER procedure is an effective alternative option for complicated type B aortic dissection, which gains a sufficient proximal landing zone with a healthy aorta for the stent graft to fix and seal. The technical success rate and early and mid-term mortality and morbidity are acceptable in both acute and chronic dissection. However, monitoring of longer-term clinical outcomes and continuous careful surveillance of the entire aorta, especially the distal end of the stent graft, are required, particularly for stainless steel-based stent grafts or any other distal high radial force design.
Footnotes
Abbreviations: AAD = acute aortic dissection, CAD = chronic aortic dissection, CTA = computed tomography angiography, FD = maximal false diameter, MD = maximal aorta diameter, RTAD = retrograde type A dissection, TD = true diameter, TEVAR = thoracic endovascular aortic repair.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- [2].Scott AJ, Bicknell CD. Contemporary management of acute type B dissection. Eur J Vasc Endovasc Surg 2016;51:452–9. [DOI] [PubMed] [Google Scholar]
- [3].Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661–78. [DOI] [PubMed] [Google Scholar]
- [4].Appoo JJ, Bozinovski J, Chu MW, et al. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery Joint Position Statement on Open and Endovascular Surgery for Thoracic Aortic Disease. Can J Cardiol 2016;32:703–13. [DOI] [PubMed] [Google Scholar]
- [5].Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522–9. [DOI] [PubMed] [Google Scholar]
- [6].Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800–11. [DOI] [PubMed] [Google Scholar]
- [7].Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114(1 Suppl):I357–64. [DOI] [PubMed] [Google Scholar]
- [8].Zeng Q, Guo X, Huang L, et al. Single-center experience with simultaneous thoracic endovascular aortic repair and abdominal endovascular aneurysm repair. Vascular 2016;25:157–62. [DOI] [PubMed] [Google Scholar]
- [9].Hajibandeh S, Antoniou SA, Child E, et al. Percutaneous access for endovascular aortic aneurysm repair: a systematic review and meta-analysis. Vascular 2016;24:638–48. [DOI] [PubMed] [Google Scholar]
- [10].Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729–34. [DOI] [PubMed] [Google Scholar]
- [11].Faure EM, Canaud L, Agostini C, et al. Reintervention after thoracic endovascular aortic repair of complicated aortic dissection. J Vasc Surg 2014;59:327–33. [DOI] [PubMed] [Google Scholar]
- [12].Desai ND, Gottret JP, Szeto WY, et al. Impact of timing on major complications after thoracic endovascular aortic repair for acute type B aortic dissection. J Thorac Cardiovasc Surg 2015;149(2 Suppl):S151–6. [DOI] [PubMed] [Google Scholar]
- [13].Fanelli F, Cannavale A, O'Sullivan GJ, et al. Endovascular repair of acute and chronic aortic type B dissections: main factors affecting aortic remodeling and clinical outcome. JACC Cardiovasc Interv 2016;9:183–91. [DOI] [PubMed] [Google Scholar]
- [14].Watanabe Y, Shimamura K, Yoshida T, et al. Aortic remodeling as a prognostic factor for late aortic events after thoracic endovascular aortic repair in type B aortic dissection with patent false lumen. J Endovasc Ther 2014;21:517–25. [DOI] [PubMed] [Google Scholar]
- [15].Jia X, Guo W, Li TX, et al. The results of stent graft versus medication therapy for chronic type B dissection. J Vasc Surg 2013;57:406–14. [DOI] [PubMed] [Google Scholar]
- [16].Shu C, He H, Li QM, et al. Endovascular repair of complicated acute type-B aortic dissection with stentgraft: early and mid-term results. Eur J Vasc Endovasc Surg 2011;42:448–53. [DOI] [PubMed] [Google Scholar]
- [17].Writing C, Riambau V, Böckler D, et al. Editor's Choice – Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- [18].Lombardi JV, Cambria RP, Nienaber CA, et al. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg 2014;59:1544–54. [DOI] [PubMed] [Google Scholar]
- [19].Böckler D, Schumacher H, Ganten M, et al. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg 2006;132:361–8. [DOI] [PubMed] [Google Scholar]
- [20].Chen SL, Zhu JC, Li XB, et al. Comparison of long-term clinical outcome between patients with chronic versus acute type B aortic dissection treated by implantation of a stent graft: a single-center report. Patient Prefer Adherence 2013;7:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546–52. [DOI] [PubMed] [Google Scholar]
- [22].Li DL, Zhang HK, Chen XD, et al. Thoracic endovascular aortic repair for type B aortic dissection: analysis among acute, subacute, and chronic patients. J Am Coll Cardiol 2016;67:1255–7. [DOI] [PubMed] [Google Scholar]
- [23].Vaaramaki S, Suominen V, Pimenoff G, et al. Long-term experience of endovascular repair for thoracic aortic aneurysms and dissections. Vasc Endovasc Surg 2016;50:335–42. [DOI] [PubMed] [Google Scholar]
- [24].Shi Z, Yang J, Fu W, et al. Outcomes and aortic remodelling after proximal thoracic endovascular aortic repair of post type B aortic dissection thoracic aneurysm. Vasa 2016;45:331–6. [DOI] [PubMed] [Google Scholar]
- [25].Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926. [DOI] [PubMed] [Google Scholar]
- [26].Ramdass M. TEVAR for symptomatic Stanford B dissection: a systematic review of 30-day mortality and morbidity. Thorac Cardiovasc Surg 2015;63:97–112. [DOI] [PubMed] [Google Scholar]
- [27].Sze DY, van den Bosch MA, Dake MD, et al. Factors portending endoleak formation after thoracic aortic stent-graft repair of complicated aortic dissection. Circ Cardiovasc Interv 2009;2:105–12. [DOI] [PubMed] [Google Scholar]
- [28].Kotelis D, Geisbusch P, Hinz U, et al. Short and midterm results after left subclavian artery coverage during endovascular repair of the thoracic aorta. J Vasc Surg 2009;50:1285–92. [DOI] [PubMed] [Google Scholar]
- [29].Feezor RJ, Martin TD, Hess PJ, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther 2007;14:568–73. [DOI] [PubMed] [Google Scholar]
- [30].Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433–9. [DOI] [PubMed] [Google Scholar]
- [31].Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg 2014;260:389–95. [DOI] [PubMed] [Google Scholar]
- [32].Kpodonu J, Preventza O, Ramaiah VG, et al. Retrograde type A dissection after endovascular stenting of the descending thoracic aorta. Is the risk real? Eur J Cardiothorac Surg 2008;33:1014–8. [DOI] [PubMed] [Google Scholar]
- [33].Wang G, Zhai S, Li T, et al. Mechanism and management of retrograde type A aortic dissection complicating TEVAR for type B aortic dissection. Ann Vasc Surg 2016;32:111–8. [DOI] [PubMed] [Google Scholar]
- [34].Nauta FJ, Trimarchi S, Kamman AV, et al. Update in the management of type B aortic dissection. Vasc Med 2016;21:251–63. [DOI] [PubMed] [Google Scholar]
- [35].Weber TF, Böckler D, Muller-Eschner M, et al. Frequency of abdominal aortic expansion after thoracic endovascular repair of type B aortic dissection. Vascular 2016;24:567–79. [DOI] [PubMed] [Google Scholar]
- [36].Rodriguez JA, Olsen DM, Lucas L, et al. Aortic remodeling after endografting of thoracoabdominal aortic dissection. J Vasc Surg 2008;47:1188–94. [DOI] [PubMed] [Google Scholar]
- [37].Tanaka A, Sakakibara M, Ishii H, et al. Influence of the false lumen status on clinical outcomes in patients with acute type B aortic dissection. J Vasc Surg 2014;59:321–6. [DOI] [PubMed] [Google Scholar]
- [38].Genoni M, Paul M, Jenni R, et al. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg 2001;19:606–10. [DOI] [PubMed] [Google Scholar]
- [39].Nienaber CA, Kische S, Rehders TC, et al. Rapid pacing for better placing: comparison of techniques for precise deployment of endografts in the thoracic aorta. J Endovasc Ther 2007;14:506–12. [DOI] [PubMed] [Google Scholar]
- [40].Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407–16. [DOI] [PubMed] [Google Scholar]
- [41].Conrad MF, Crawford RS, Kwolek CJ, et al. Aortic remodeling after endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2009;50:510–7. [DOI] [PubMed] [Google Scholar]
- [42].Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149–59. [DOI] [PubMed] [Google Scholar]
- [43].Kusagawa H, Shimono T, Ishida M, et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years’ experience. Circulation 2005;111:2951–7. [DOI] [PubMed] [Google Scholar]
- [44].Resch TA, Delle M, Falkenberg M, et al. Remodeling of the thoracic aorta after stent grafting of type B dissection: a Swedish multicenter study. J Cardiovasc Surg (Torino) 2006;47:503–8. [PubMed] [Google Scholar]
- [45].Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349–59. [DOI] [PubMed] [Google Scholar]
- [46].Trimarchi S, Jonker FH, Hutchison S, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101–7. [DOI] [PubMed] [Google Scholar]
- [47].Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799–804. [DOI] [PubMed] [Google Scholar]
- [48].Chang CP, Liu JC, Liou YM, et al. The role of false lumen size in prediction of in-hospital complications after acute type B aortic dissection. J Am Coll Cardiol 2008;52:1170–6. [DOI] [PubMed] [Google Scholar]


