Abstract
Polycomb group (PcG) proteins are responsible for the stable repression of homeotic (Hox) genes by forming multimeric protein complexes. We show (1) physical interaction between components of the U2 small nuclear ribonucleoprotein particle (U2 snRNP), including Sf3b1 and PcG proteins Zfp144 and Rnf2; and (2) that Sf3b1 heterozygous mice exhibit skeletal transformations concomitant with ectopic Hox expressions. These alterations are enhanced by Zfp144 mutation but repressed by Mll mutation (a trithorax-group gene). Importantly, the levels of Sf3b1 in PcG complexes were decreased in Sf3b1-heterozygous embryos. These findings suggest that Sf3b1-PcG protein interaction is essential for true PcG-mediated repression of Hox genes.
Keywords: Hox genes, Polycomb group, knockout mice, spliceosomal proteins
During mammalian development, spatially and quantitatively appropriate homeotic (Hox) gene expression is essential for the anterior-posterior specification of axial structures (McGinnis and Krumlauf 1992; Duboule and Morata 1994). The maintenance of the Hox gene expressional state is driven by the products of Polycomb group (PcG) and trithorax group (trxG) genes (Satijn and Otte 1999; Francis and Kingston 2001; Simon and Tamkun 2002; Orlando 2003). In general, PcG proteins act as repressors to maintain the silent state, while the trxG proteins are activators that maintain Hox gene transcription. PcG proteins constitute large, chromatin-associated multiprotein complexes, which in mammals can be classified into at least two different classes. The Class I complex, which contains Eed/Ezh2, is associated with histone deacetylase and methyltransferase activity. The PRC1 or Class II complex consists of, for example, Zfp144 (Mel18), Rnf2 (Ring1B), Cbx2/M33, and Phc2/Edr2. The Class II complex, which characteristically includes the products from highly related pairs of genes, has been shown to inhibit nucleosome remodeling by the SWI/SNF complex in vitro (Shao et al. 1999). However, as the inhibition requires preincubation of the Class II with the nucleosomal template (it does not occur when Class II and SWI/SNF products are added together), this suggests that the Class II complex does not interact directly with SWI/SNF, but instead competes for the nucleosome template. Possibly, it is this binding of the Class II complex, which prevents nucleosome remodeling, that silences the genes by blocking the access of transcription activators to cis-regulatory elements such as promoters and enhancers (Francis et al. 2001). Interestingly, recent studies have proposed an alternative mechanism for maintaining gene silence (Breiling et al. 2001; Saurin et al. 2001). Fly Class II complex includes general transcription factors such as TBP and TBP-associated proteins at promoter regions. Using an in vitro assay, King et al. (2002) showed that the fly complex was able to inhibit transcription by RNA polymerase II at particular steps even after activator binding. This raises the possibility that the Class II complex might act directly on the functioning of the transcriptional machinery. However, whether this silencing mechanism is active at Hox loci during mammalian embryo development is not clear. In order to achieve a better understanding of the molecular basis of PcG complex-mediated repressions, we believe that it is important to identify PcG complex-associated non-PcG proteins and subsequently investigate the genetic impact of such proteins on PcG complexes. In this paper, we identified an essential spliceosomal protein Sf3b1 that physically interacts with the Class II PcG proteins and generated Sf3b1 knockout mice. Surprisingly, Sf3b1+/- mice exhibited skeletal phenotypes that are usually observed with PcG mutation. Furthermore, genetic interactions between Sf3b1 and Zfp144 or the trxG gene Mll mutations were also shown. Therefore, it appears that Sf3b1 and the Class II PcG proteins are functionally interacting on the Hox loci in developing embryos.
Results and Discussion
Two partial cDNA clones encoding amino acid 1-489 and 312-777 regions of mouse spliceosomal protein Sf3b1 have been isolated as interactors for the mammalian PcG proteins Zfp144 and Rnf2, respectively, by yeast two-hybrid screening (Fig. 1A). Physical interactions between Sf3b1 and PcG proteins were first examined by glutathione S-transferase (GST) pull-down assay. Nuclear proteins were extracted from mouse embryonic stem (ES) cells and subsequently precipitated by GST-Rnf2 and GST-Zfp144 fusion proteins. The precipitates were subjected to immunoblotting with anti-Sf3b1 antibody. Endogenous Sf3b1 was clearly coprecipitated with GST-Rnf2 and GST-Zfp144 (Fig. 1B). Moreover, assay with Rnf2 truncates revealed that Sf3b1 specifically interacts with the N-terminal region (amino acids 1-188) but not with the C-terminal region (amino acids 189-336). Thus, Sf3b1 probably has the potential to bind directly to Rnf2 and Zfp144.
Figure 1.
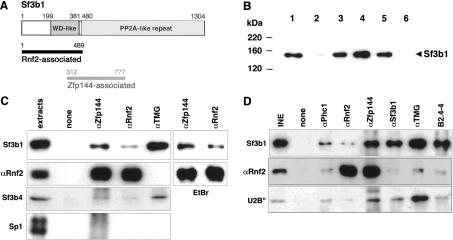
Physical interaction between Sf3b1 and PcG proteins. (A) Primary structure of Sf3b1 (Isono et al. 2001). Sf3b1 has WD-40 like repeats and PP2A repeats (usually termed HEAT motifs) originally identified in the PR65 subunit of protein phosphatase 2A. The N-terminal region (amino acids 1-489) and the internal region (amino acids 312-777), respectively, were associated with Rnf2 and Zfp144 in the yeast two-hybrid system. (B) GST pull-down assays probed with the Sf3b1-specific antibody. Nuclear extracts from ES cells were incubated with GST alone (lane 2) and GST fusions with Zfp144 (lane 3), Rnf2 (lane 4), and the N-terminal (lane 5) or C-terminal (lane 6) regions of Rnf2. In lane 1, one-fifteenth volume of extract sample used in a reaction was applied. (C) Coimmunoprecipitation with whole-cell lysates. Lysate of a mouse embryo at 10.75-11.25 dpc was incubated with or without antibodies to Zfp144, Rnf2, or TMG. Each precipitant was divided into two, and each was used in immunoblotting with Sf3b1 and Rnf2 or Sp1 and Sf3b4 antibodies. Anti-Sp1 was used as a negative control. In each “extract” lane, one-fortieth volume of extract sample used in a reaction was applied. In addition, lysates treated with ethidium bromide (EtBr) were also used to exclude DNA. (D) Coimmunoprecipitation with chromatin-rich lysates. Insoluble nuclear extract from an embryo at 10.75-11.25 dpc was prepared by osmotic shock, solubilized by sonication, and then incubated with antibodies. Phc1 is a mouse homolog to the fly PcG protein, Polyhomeotic (the fly protein homologous to mouse Phc1). B2.4-4 is a polyclonal antibody against the Xenopus Sf3b1, which has an epitope different from the monoclonal antibody (αSf3b1). In the “INE” lane, one-fortieth volume of the insoluble extract used in a reaction was applied.
We examined the in vivo interaction further in wholecell extracts from 11.5 d post-coitus (dpc) embryos in which PcG complexes have been shown to act as repressors of Hox gene expression (Fig. 1C; Akasaka et al. 1996). A significant, but substoichiometric amount, of Sf3b1 was reproducibly coimmunoprecipitated with Zfp144 and Rnf2, as revealed by comparison with anti-Rnf2 immunoblotting. The presence of ethidium bromide did not affect these Sf3b1-Zfp144/Rnf2 interactions, which suggests that they were DNA independent. As Sf3b1 is known to be a component of U2 snRNP (Krämer 1996; Schmidt-Zachmann et al. 1998), we therefore examined other U2 snRNP components, Sf3b4 (SAP49), U2B″, and U snRNAs. In the conventional nuclear extracts, Sf3b4 was also coprecipitated with PcG proteins while anti-2,2,7-trimethylguanosine (TMG) antibody, which reacts to the 5′ cap of U snRNAs characteristic for the U1, U2, U5, and U4/U6 snRNPs (Krämer 1996), failed to coprecipitate Rnf2. It is, however, possible that excess nucleoplasmic spliceosomal proteins or nucleoplasmic interaction between monomeric Sf3b1 and the PcG proteins would obscure experimental outcomes. To exclude these possibilities, we employed nuclear insoluble fraction extracted under high salt condition, in which proteins closely associated to chromatin are presumed to be concentrated. The fractions were solubilized by sonication and used in immunoprecipitation. Coimmunoprecipitation of Rnf2 with Phc1/Rae28 and Zfp144 indicated the presence of PcG multimeric complexes in this fraction. Sf3b1 and U2B″ were coimmunoprecipitated with Phc1, Rnf2, and Zfp144. Concordantly, two different anti-Sf3b1 antibodies were able to coimmunoprecipitate Rnf2, reinforcing the interaction between Sf3b1 and PcG proteins. Notably, significant amounts of Rnf2 as well as Sf3b1 and U2B″ were coimmunoprecipitated by anti-TMG antibody, implying the association of the U2 snRNA to PcG complexes. Importantly, these U2 snRNP components bound to the PcG proteins were detected in similar proportions (Fig. 1C,D). Taken together, these results show that it is likely that PcG complexes associate with at least part of the U2 snRNP rather than Sf3b1 alone.
To examine the biological implications of Sf3b1, we have generated an Sf3b1-mutant allele by replacing four exons with the neo gene in the opposite direction. Sf3b1 null homozygotes died during preimplantation development around the 16- to 32-cell stage. Heterozygotes were externally normal and healthy, although the levels of Sf3b1 mRNA and Sf3b1 were significantly reduced (Supplementary Fig. S1). Since PcG mutants exhibit posterior transformations of the vertebrae (Akasaka et al. 1996; Gould 1997; Suzuki et al. 2002), we examined axial skeletal preparations of Sf3b1+/- newborn pups backcrossed five times (N5) to C57BL/6 background. Significantly, Sf3b1+/- mice exhibited various skeletal alterations along the anterior-posterior axis (Table 1). In the cervical region of Sf3b1+/- mutants, the seventh cervical vertebra (C7) had incomplete ectopic ribs, either unilaterally or bilaterally fused with the first thoracic rib (27%), indicating transformations of C7 toward the first thoracic vertebra (T1) (Fig. 2B). Twenty-nine percent of Sf3b1+/- mutants had a prominent spinous process, characteristic for the second thoracic vertebra (T2), incorrectly associated with T1 (Fig. 2C), suggesting a T1 → T2 transformation. In addition, Sf3b1+/- mice had only 12 ribs (6%) (Fig. 2F) and five lumbar vertebrae (88%) (Fig. 2E-F), indicating T13 → L1 and L6 → S1 transformations, respectively. Thus, Sf3b1+/- mice exhibit skeletal phenotypes similar to PcG mutants although the penetrance is rather low. We investigated next whether these skeletal alterations, seen with Sf3b1+/-, were associated with anterior shifts of Hox gene expression boundaries as well as PcG mutants (Akasaka et al. 1996). The Hoxb8 expression was extended to the seventh prevertebrae in three out of five Sf3b1+/- embryos and from the eighth prevertebra in wild type (Fig. 2L-N), and also Hoxb6 and Hoxc6 were ectopically expressed in the sixth prevertebrae in Sf3b1+/- embryos (Fig. 2I-K,O-Q). Significant changes were not observed for Hoxa5, Hoxb3, Hoxb4, or Hoxd4 expression in the paraxial mesoderm of Sf3b1+/- embryos (Fig. 2R). However, we found a subtle alteration of the Hoxd4 expression in the second branchial arch of Sf3b1+/- embryos (data not shown). In summary, the expressions of several Hox genes were anteriorly extended in the paraxial mesoderm and second branchial arch in Sf3b1+/- embryos. These observations are consistent with the axial skeletal alterations observed in Sf3b1+/- mice. Therefore, we concluded that Sf3b1 mediates the repression of Hox genes.
Table 1.
Summary of skeletal transformations in mutant mice
| Parents
|
B6 × Sf3b1+/− (N4)
|
Mel18+/− × Sf3b1+/− (N4)
|
Mll+/− × Sf3b1+/− (N6)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transformation types | wt (n = 50) | Sf3b1+/− (n = 48) | wt (n = 21) | Mel18+/− (n = 18) | Sf3b1+/− (n = 22) | Mel18+/−Sf3b1+/− (n = 20) | wt (n = 14) | Mll+/− (n = 23) | Sf3b1+/− (n = 25) | Mll+/−Sf3b1+/− (n = 15) |
| C7 → T1 | 0 (0) | 27 (13) | 0 (0) | 6 (1) | 32 (7) | 45 (9), a4/9 | 0 (0) | 0 (0) | 68 (17) | 0 (0) |
| T1 → T2 | 0 (0) | 29 (14) | 0 (0) | 11 (2) | 23 (5) | 75 (15) | 7 (1) | 0 (0) | 60 (15) | 0 (0) |
| T13 → L1 | 0 (0) | 6 (3) | 0 (0) | 28 (5) | 14 (3) | 50 (10) | 0 (0) | 9 (2) | 8 (2) | 13 (2) |
| L6 → S1 | 22 (11) | 88 (42) | 62 (13) | 89 (16) | 100 (22) | 100 (20) | 21 (3) | 61 (14) | 88 (22) | 87 (13) |
Unit: % (n).
Four out of nine C7 → T1 mice exhibited complete first ribs associated with the C7 vertebra (see Fig. 2G).
Figure 2.
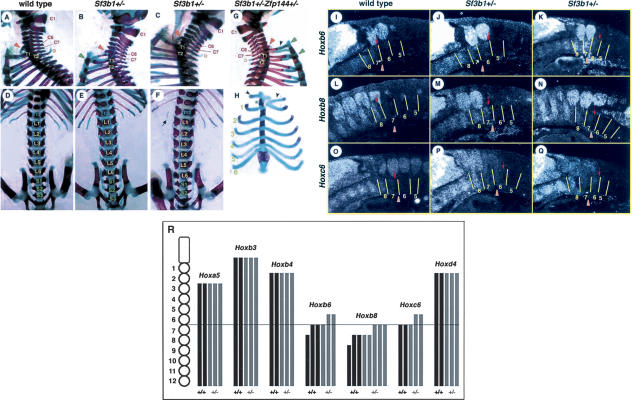
Skeletal abnormalities and ectopic Hox expressions in Sf3b1+/- mice. Cervical-thoracic (lateral view) and thoracic-sacral (ventral view) regions of wild-type (A,D) and two independent Sf3b1+/- (B,C,E,F) newborns are shown. Numbering of vertebrae of mutants makes them consistent with those of wild type. In wild type, C6 and T2 vertebrae have characteristic ventral processes as indicated by arrowheads (orange) and a prominent spinous process (yellow circle), respectively. An arrow indicates the disappearance of the T13 rib in the Sf3b1+/- mutant. (G,H) the lateral view of cervical-thoracic region and the ventral view of rib cage of the Sf3b1+/-Zfp144+/- compound mutant. Ectopic ribs with C7 are represented by black arrowheads. An asterisk indicates an additional ossification center. Ribs numbered as 1-6 associate with T1-T6 vertebrae, respectively. The right ectopic rib with C7 separates from the rib with T1 on the sternum, as shown by green arrowheads. (I-Q) RNA in situ hybridization of Hox genes in embryos at 11.5 dpc. Boundaries of prevertebrae in lateral view are indicated by lines and the prevertebrae are numbered from prospective C1. Vertebral arteries are indicated by arrowheads and anterior boundaries of expressions are indicated by arrows. In Sf3b1+/- embryos, anterior boundaries of Hoxb6, Hoxb8, and Hoxc6 expressions are shown to shift by sixth, seventh, and sixth prevertebrae, respectively. (R) Summary of Hoxa5, Hoxb3, Hoxb4, Hoxb6, Hoxb8, Hoxc6, and Hoxd4 expressions in the paraxial mesoderm is depicted schematically. Each black (wild type) or gray (Sf3b1+/-) bar represents expressional regions and a number of bars show individual embryos. Segment numbers are counted from the prospective C1.
Individual PcG mutations have been shown to mutually enhance their phenotypes (Bel et al. 1998; Akasaka et al. 2001; Suzuki et al. 2002). To investigate whether this Sf3b1-mediated repression involves PcG complexes, we examined the genetic interactions between Zfp144 and Sf3b1 mutations. In Sf3b1+/-Zfp144+/- double heterozygotes, the formation of an additional ossification center in the sternum, and the detachment of the ribs of T7 from the sternum, represented an anterior shift of the sternum of one segment width (Fig. 2H). The ectopic ribs associated with C7 mimicked perfect ribs and formed joints with the anteriorly shifted sternum (Fig. 2G). These alterations were observed in four (44%) out of nine double mutants that displayed perfect C7 → T1 transformation but were not observed in either of the single mutants (Table 1). In addition to the ectopic ribs, the frequencies of other transformations were much higher in the double mutants. In particular, the T1 → T2 transformation occurred at a much higher penetrance: 75% in double mutants versus only 11% or 23% in either single mutant, suggesting that the single phenotypes of respective mutations were enhanced in the presence of each other. We further examined the impact of the Sf3b1 mutation on the mutation at the mixed lineage leukemia (Mll) gene (Yu et al. 1995), a mammalian homolog of the trithorax gene of Drosophila melanogaster, whose product is antagonistic to PcG mutations (Hanson et al. 1999). Posterior transformations at the cervico-thoracic transitional zone caused by Sf3b1 mutation were completely restored by Mll mutation (Table 1; Yagi et al. 1998). Therefore, it is likely that Sf3b1 has an antagonistic relationship to trxG proteins as well as PcG complexes. Taken together, these results indicate that Sf3b1 is functionally involved in PcG repressive complexes.
Since Sf3b1 is involved essentially in pre-mRNA splicing, the basal expression levels of genes, including PcG or Hox genes, may be profoundly altered in Sf3b1+/- mice, leading to homeotic transformations. To investigate this, a basal activity of splicing was examined with nuclear extracts from 10.5 dpc Sf3b1+/- embryos in which the Sf3b1 level was reduced up to ∼75% but Rnf2 accumulated at normal level (data not shown). Despite such lower quantities of Sf3b1, their splicing activity was equal because of the same ratio of their spliced forms (Fig. 3A). Furthermore, while expression levels of Sf3b1 were reduced by half in Sf3b1+/- embryos, those of five PcG genes, Zfp144, Rnf2, Eed, Cbx2/M33, and Phc2/Edr2; three Hox genes, Hoxb3, Hoxb6, and Hoxb8; and a metabolic gene, Hprt, were not (Fig. 3B). Thus, homeotic transformations in Sf3b1+/- mice appear to be independent of the alteration of general gene expression. Next, we investigated the amount of Sf3b1 associated with PcG proteins in Sf3b1+/- embryos. While Zfp144- or Rnf2-associated Sf3b1 were clearly decreased in Sf3b1+/- embryos, Rnf2 was precipitated equally in both embryos (Fig. 3C). It was also notable that the anti-TMG precipitates contained almost equal levels of Sf3b1, suggesting that the majority of U2 snRNP were not significantly altered in Sf3b1+/- embryos. This finding agrees well with the normal expression of other genes described above. Therefore, the heterozygous loss of Sf3b1 gene results in significant reduction of Sf3b1 associated with PcG complexes, strongly suggesting a repressive mechanism of Hox genes via interaction with PcG complexes.
Figure 3.
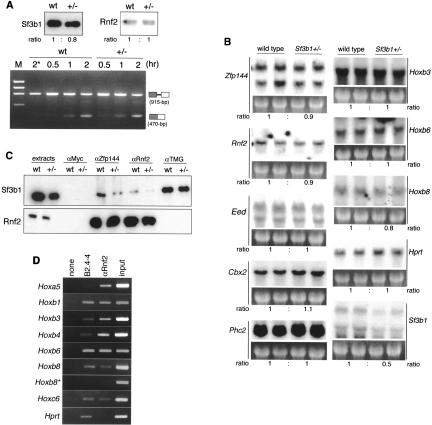
Comparison of wild-type and Sf3b1+/- embryos. Genotyping of embryos was performed with total DNA from yolk sacs. (A) In vitro splicing assay. The soluble fraction of a nuclear extract was prepared from wild-type or Sf3b1+/- embryos at 10.5 dpc. Equal amounts (60 μg) of total protein were confirmed by Western blotting with anti-Sf3b1 and anti-Rnf2 antibodies (upper panels) and subjected to a splicing assay (lower panel). One-step RT-PCR revealed that unspliced forms consisting of two exons and an intron (upper bands) as the RNA substrates were converted into spliced forms (lower bands) by the splicing reaction. Band intensities were measured by the image-processing program Image J and the ratios of Sf3b1+/- to wild type were obtained.(B) Northern analysis of total RNAs (15 μg each) from independent embryos at 12.5 dpc. Ethidium-bromide-stained 28S rRNAs prior to transfer to membranes are displayed below their blots. Intensity of each detected band was normalized by their rRNAs and then the ratios of Sf3b1+/- to wild type were calculated (indicated below their panels). (C) Immunoprecipitation assay with embryos at 11.5 dpc. Three independent embryos with the same genotype were sonicated individually, and the soluble lysates were mixed and then divided into four. Each was immunoprecipitated with a specific antibody. (D) ChIP assay with ES cells. Cross-linked chromatins were immunoprecipitated with or without indicated antibodies and subjected to amplification of Hox and Hprt regions (260-480 bp) by PCR. The amplified regions were detected in 1.5% agarose gels.
We finally examined whether Sf3b1 is closely associated to Hox genomic regions together with PcG proteins by chromatin immunoprecipitation (ChIP) assay. Various Hox cluster genes, including Hoxa5, Hoxb1, Hoxb3, Hoxb4, Hoxb6, Hoxb8, Hoxb9, and Hoxc6, were depressed in Rnf2-/- ES cells, indicating the involvement of PcG proteins in regulation of Hox expressions in ES cells (de Napoles et al. 2004; K. Isono and H. Koseki, unpubl.). Accordingly, we found obvious immunoprecipitation of 5′ genomic regions (260-480 bp) of Hoxa5, Hoxb1, Hoxb3, Hoxb4, Hoxb6, Hoxb8, and Hoxc6 in wild-type ES cells by anti-Rnf2 antibody but not Hprt gene, which is not derepressed in Rnf2-/- ES cells (Fig. 3D). Likewise, we found association of Zfp144 to these Hox genes (K. Isono and H. Koseki, unpubl.). Anti-Sf3b1 antibody also precipitated a significant amount of 5′ genomic regions of these Hox genes while either anti-Rnf2 or anti-Sf3b1 antibody failed to precipitate the second exonic region of Hoxb8 gene (indicated by Hoxb8★ in Fig. 3D). These findings indicate that the Sf3b1 and PcG proteins colocalize locally on the 5′ regions of individual Hox genes. It is also noteworthy that the relative degree of Sf3b1 association in comparison with the Rnf2 is greater at the Hoxb6, Hoxb8, and Hoxc6 genomic regions than the Hoxa5, Hoxb3, and Hoxb4 genes. This is in good agreement with another observation in Sf3b1+/- embryos that the expression of Hoxb6, Hoxb8, and Hoxc6 was derepressed but not the Hoxa5, Hoxb3, or Hoxb4 (summarized in Fig. 2R). Taken together, these results show that it is likely that a mechanistic link between Sf3b1 and PcG complexes regulates Hox gene expression via direct binding to Hox genomic regions.
Our results show a significant and novel mechanistic link between Sf3b1 (together probably with other U2 snRNP components) and PcG repressive complexes on Hox loci. This idea is strongly supported by our recent observation that heterozygous mutant mice for Sf3b2, another U2 snRNP component, exhibited skeletal abnormality similar to Sf3b1 phenotypes: 10 out of 14 targeting mice (71%) had L6 to S1 transformation compared with two out of 18 wild-type mice (11%) (K. Isono and H. Koseki, unpubl.). However, although with respect to the PcG-mediated repression in the transcriptional-competent regions, our findings are in general accord with previous reports (Breiling et al. 2001; Saurin et al. 2001; King et al. 2002; Cmarko et al. 2003; de Graaff et al. 2003; Dellino et al. 2004), nevertheless they indicate the presence a different gene silencing mechanism. Evidence that the level of Sf3b1 in PcG complexes affects the expressional boundary of Hox genes implies that Sf3b1 supports the activity of PcG complexes. The simplest explanation is that Sf3b1/U2 snRNP might be a PcG protein and could form repressive PcG complexes together with other PcG proteins. A more interesting hypothesis is that this interaction constitutes part of a mechanism that is designed to maintain the amount of Hox transcripts required to confer the appropriate positional identities. Regulation of Hox expressions in the vicinity of their boundaries is thought to be loose, because even wild type occasionally exhibits homeotic transformations (Table 1 and Akasaka et al. 1996). RNAs, mistranscribed beyond loose repression, may be tethered by Sf3b1/U2 snRNP bound to PcG complex, leading to the arrest of splicing and a normal Hox boundary as a consequence. However, in Sf3b1+/- cells, because of the decrease of PcG complex-bound Sf3b1, such mistranscribed RNAs become easily associated with splicing-active nucleoplasmic Sf3b1/U2 snRNP. This association leads to the achievement of a splicing reaction, which results in the anterior shift of Hox expression. In support of this model is the important evidence that the Mll mutation completely suppressed Sf3b1 phenotypes, indicating that the PcG-like function of Sf3b1 is very susceptible to levels of Mll; in other words the acting points of both proteins are spatially very close. Of further note is the fact that the human MLL supercomplex includes the 116-kDa protein specific to the U5 snRNP, which acts on pre-mRNA following the U2 snRNP (Nakamura et al. 2002). Finally, it appears that there are multiple interacting surfaces between PcG complexes and gene expression machineries. It might be that, through this interaction, PcG complexes act as a part of the modules that sense the transcriptional status in transcriptionalcompetent regions of the Hox cluster.
Materials and methods
Yeast two-hybrid screening, immunoprecipitation and GST pull-down assays, ChIP analysis, in vitro splicing assay, and knockout mice are described in the Supplemental Material.
Acknowledgments
We thank Drs. Miguel Vidal and Tsukasa Oda for providing Rnf2 cDNA and pBTM116, respectively. In addition, we thank Drs. Takeshi Akasaka and Tomomi Kaneko, Ms. Kazumi Nemoto, Minako Ogawa, Misao Uchida, and Sanae Takeda for help with this work, and Drs. Achim Gossler and Miguel Vidal for their critical reading of the manuscript. This study was supported by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1284605.
References
- Akasaka T., Kanno, M., Balling, R., Mieza, M.A., Taniguchi, M., and Koseki, H. 1996. A role for Mel18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122: 1513-1522. [DOI] [PubMed] [Google Scholar]
- Akasaka T., van Lohuizen, M., van der Lugt, N., Mizutani-Koseki, Y., Kanno, M., Taniguchi, M., Vidal, M., Alkema, M., Berns, A., and Koseki, H. 2001. Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development 128: 1587-1597. [DOI] [PubMed] [Google Scholar]
- Bel S., Core, N., Djabali, M., Kieboom, K., van der Lugt, N., Alkema, M.J., and van Lohuizen, M. 1998. Genetic interactions and dosage effects of Polycomb group genes in mice. Development 125: 3543-3551. [DOI] [PubMed] [Google Scholar]
- Breiling A., Turner, B. M., Bianchi, M. E., and Orlando, V. 2001. General transcription factors bind promoters repressed by Polycomb group proteins. Nature 412: 651-655. [DOI] [PubMed] [Google Scholar]
- Cmarko D., Verschure, P.J., Otte, A.P., van Driel, R., and Fakan, S. 2003. Polycomb group gene silencing proteins are concentrated in the perichromatin compartment of the mammalian nucleus. J. Cell Sci. 116: 335-343. [DOI] [PubMed] [Google Scholar]
- de Graaff W., Tomotsune, D., Oosterveen, T., Takihara, Y., Koseki, H., and Deschamps, J. 2003. Randomly inserted and targeted Hox/reporter fusions transcriptionally silenced in Polycomb mutants. Proc. Natl. Acad. Sci. 100: 13362-13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellino G.I., Schwartz, Y.B., Farkas, G., McCabe, D., Elgin, S.C.R., and Pirrotta, V. 2004. Polycomb silencing blocks transcription initiation. Mol. Cell 13: 887-893. [DOI] [PubMed] [Google Scholar]
- de Napoles M., Mermoud, J.E., Wakao, R., Tang, Y.A., Endoh, M., Appanah, R., Nesterova, T.B., Silva, J., Otte, A.P., Vidal, M., et al. 2004. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7: 663-676. [DOI] [PubMed] [Google Scholar]
- Duboule D. and Morata, G. 1994. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 10: 358-364. [DOI] [PubMed] [Google Scholar]
- Francis N.J. and Kingston, R.E. 2001. Mechanisms of transcriptional memory. Nat. Rev. Mol. Cell Biol. 2: 409-421. [DOI] [PubMed] [Google Scholar]
- Francis N.J., Saurin, A.J., Shao, Z., and Kingston, R.E. 2001. Reconstitution of a functional core Polycomb repressive complex. Mol. Cell 8: 545-556. [DOI] [PubMed] [Google Scholar]
- Gould A. 1997. Functions of mammalian Polycomb group and trithorax group related genes. Curr. Opin. Genet. Dev. 7: 488-494. [DOI] [PubMed] [Google Scholar]
- Hanson R.D., Hess, J.L., Yu, B.D., Ernst, P., van Lohuizen, M., Berns, A., van der Lugt, M.N.T., Shashikant, C.S., Ruddle, F.H., Seto, M., et al. 1999. Mammalian Trithorax and Polycomb-group homologues are antagonistic regulators of homeotic development. Proc. Natl. Acad. Sci. 96: 14372-14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K., Abe, K., Tomaru, Y., Okazaki, Y., Hayashizaki, Y., and Koseki, H. 2001. Molecular cloning, genetic mapping, and expression of the mouse Sf3b1 (SAP155) gene for the U2 snRNP component of spliceosome. Mamm. Genome 12: 192-198. [DOI] [PubMed] [Google Scholar]
- King I.F.G., Francis, N.J., and Kingston, R.E. 2002. Native and recombinant Polycomb group complexes establish a selective block to template accessibility to repress transcription in vitro. Mol. Cell. Biol. 22: 7919-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A. 1996. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu. Rev. Biochem. 65: 367-409. [DOI] [PubMed] [Google Scholar]
- McGinnis W. and Krumlauf, R. 1992. Homeobox genes and axial patterning. Cell 68: 283-302. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Mori, T., Tada, S., Krajewski, W., Rozovskaia, T., Wassell, R., Dubois, G., Mazo, A., Croce, C.M., and Canaani, E. 2002. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10: 1119-1128. [DOI] [PubMed] [Google Scholar]
- Orlando V. 2003. Polycomb, epigenomes, and control of cell identity. Cell 112: 599-606. [DOI] [PubMed] [Google Scholar]
- Satijn D.P.E. and Otte, A.P. 1999. Polycomb group protein complexes: Do different complexes regulate distinct target genes? Biochim. Biophys. Acta 1447: 1-16. [DOI] [PubMed] [Google Scholar]
- Saurin A.J., Shao Z., Erdjument-Bromage, H., Tempst, P., and Kingston R.E. 2001. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412: 655-660. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann M.S., Knecht, S., and Krämer, A. 1998. Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components. Mol. Biol. Cell 9: 143-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z. Raible, F., Mollaaghababa, R., Guyon, J.R., Wu, C.-T., Bender, W., and Kingston, R.E. 1999. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98: 37-46. [DOI] [PubMed] [Google Scholar]
- Simon J.A. and Tamkun, J.W. 2002. Programming off and on states in chromatin: Mechanisms of Polycomb and trithorax group complexes. Curr. Opin. Genet. Dev. 12: 210-218. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Mizutani-Koseki, Y., Fujimura, Y., Miyagishima, H., Kaneko, T., Takada, Y., Akasaka, T., Tanzawa, H., Takihara, Y., Nakano, M., et al. 2002. Involvement of the Polycomb-group gene Ring1B in the specification of the anterior-posterior axis in mice. Development 129: 4171-4183. [DOI] [PubMed] [Google Scholar]
- Yagi H., Deguchi, K., Aono, A., Tani, Y., Kishimoto, T., and Komori, T. 1998. Growth disturbance in fetal liver hematopoiesis of MLL-mutant mice. Blood 92: 108-117. [PubMed] [Google Scholar]
- Yu B.D., Hess, J.L., Horning, S.E., Brown, G.A., and Korsmeyer, S.J. 1995. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378: 505-508. [DOI] [PubMed] [Google Scholar]


