Abstract
Background:
SEPP1 encodes selenoprotein P, which involved in oxidative stress and plays an important role in the development of preeclampsia (PE). The aim of this study was to investigate the association between PE and genetic variants of SEPP1 in Chinese Han women.
Methods:
In all, 2434 unrelated pregnant women were recruited, including 1034 PE cases and 1400 normal pregnant controls. TaqMan allelic discrimination real-time PCR method was used to genotype the 2 polymorphisms of rs7579 and rs230813 in SEPP1.
Results:
No statistically significant difference in genotypic or allelic frequencies were found at the 2 genetic variants in SEPP1 between PE patients and controls (rs7579: genotype χ2 = 2.417, P = .299 and allele χ2 = 0.197, P = .761, odds ratio 1.049, 95% confidence interval 0.744–1.151; rs230813: genotype χ2 = 3.273, P = .195 and allele χ2 = 0.252, P = .615, odds ratio 0.971, 95% confidence interval 0.864–1.091). There were also no statistically significant differences in genetic distributions between mild/severe PE or early/late-onset PE and control subgroups.
Conclusion:
Our data indicate that the 2 genetic variants of rs7579 and rs230813 in SEPP1 may not play a role in the pathogenesis of PE in Chinese Han Women.
Keywords: preeclampsia, SEPP1, single-nucleotide polymorphism, susceptibility
1. Introduction
As a multifactorial disease, preeclampsia (PE) characterized by hypertension after 20th week gestation and de novo proteinuria, affects about 2% to 8% of all pregnancies in the world[1] and carries a severe morbidity and mortality risk for both mother and fetus. Numerous studies[2] including immune maladaptation, placental ischemia, inflammation, and endothelial dysfunction about the mechanisms of PE have been investigated; however, it is not fully elucidated. Furthermore, increasing evidence indicates that oxidative stress, which results from abnormal placetation and ischemia injury,[3] may contribute to the pathophysiology of PE.[4,5] On the basis of this hypothesis, several related candidate genes, such as GSTZ1, eNOS, and COMT,[6–8] have been investigated whether the genetic polymorphisms in antioxidant enzymes influence the formation of PE. But the results are inconsistent, because of different race and different sample size. Therefore, other studies related to oxidative stress candidate genes involved in PE still remain to be identified.
As 1 of the genes related to oxidative stress, SEPP1 located on chromosome 5q31, encodes selenoprotein P, which contains a selenocysteine residue. The selenocysteine residue C-terminal confers redox function and metal-binding function, acting as antioxidants to decrease oxidative stress and as transport of selenium.[9] Additionally, the up-regulation of selenoprotein P may protect the tissue from the effects of oxidative stress or inflammation.[10] Previous animal study indicated a selenium-free diet caused a PE-like syndrome in pregnant rats, including significantly increased blood pressure, proteinuria, and placental oxidative stress.[11] Moreover, significantly lower levels of the selenoenzymes reductase have been found in placental in PE patients compared with healthy pregnancy controls and lower plasma selenium concentrations in PE patients,[12–14] which were validated in UK pregnant women.[15–17] Epidemiological investigation showed that selenium supplementation may be beneficial in reducing oxidative stress in women at risk of PE among 45 countries.[18]
As a complex multifactorial disorder, PE is the consequence of interactions between genetic and environmental risk factors. More and more studies[19] supported that genetic factors play an important role in the maintenance of PE. Hence, genetic variants in SEPP1 may affect the activity of these selenoproteins, and subsequently oxidative stress and disease risk. Although the impact of SEPP1 polymorphisms (rs7579 and rs230813) on multiple complex diseases such as prostate cancer,[20,21] breast cancer,[22] and colorectal cancer[23] has previously been investigated, few studies have focused on the association between SEPP1 polymorphisms and PE in the Han Chinese population. Therefore, in the present study, we selected the 2 single-nucleotide polymorphisms (SNPs) of SEPP1 and designed a case-control study to explore their relationship with PE risk in Han Chinese women.
2. Materials and methods
2.1. Subjects
A total of 2434 Chinese Han women (1034 cases, mean age ± SD = 30.69 ± 4.47 years and 1400 controls, mean age ± SD = 30.98 ± 3.37 years) were recruited from the Affiliated Hospital of Qingdao University, Binzhou Medical University Hospital, Yantaishan Hospital, and Liaocheng People's Hospital between January 2012 and November 2015. The present study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and informed consent was obtained from all participants.
The inclusion criteria of PE were according to the American College of Obstetricians and Gynecologists (ACOG) 2013 criteria.[24] It defined as de novo hypertension (above 140 mm Hg systolic blood pressure or above 90 mm Hg diastolic blood pressure on 2 or more occasions at least 6 hours apart) and detectable urinary protein (above 300 mg/24 h, above 30 mg/dL or above a positive urine dipstick) after 20th gestational weeks. Women with PE associated with chronic hypertension, multiple pregnancies, cancer, cardiovascular, autoimmune, renal, and hepatic diseases were excluded. The control group is composed of singleton normal pregnant women, which is in the third trimester of normal pregnancy and without any fetal disorder, or pathological states. To further investigate the association between SEPP1 variants and PE, all PE patients were divided into 2 subgroups: mild PE (n = 181) and severe PE (n = 853). Severe PE was diagnosed if any of the following symptoms appear on case subjects, such as blood pressure above 160/110 mm Hg, or progressive renal insufficiency (proteinuria above 5 g/24 h), new-onset cerebral or visual disturbances, pulmonary edema, and impaired liver functions. Furthermore, we also divided the case into early-onset PE (before 34 weeks of gestation, n = 529) and late-onset PE (after 34 weeks of gestation, n = 505).
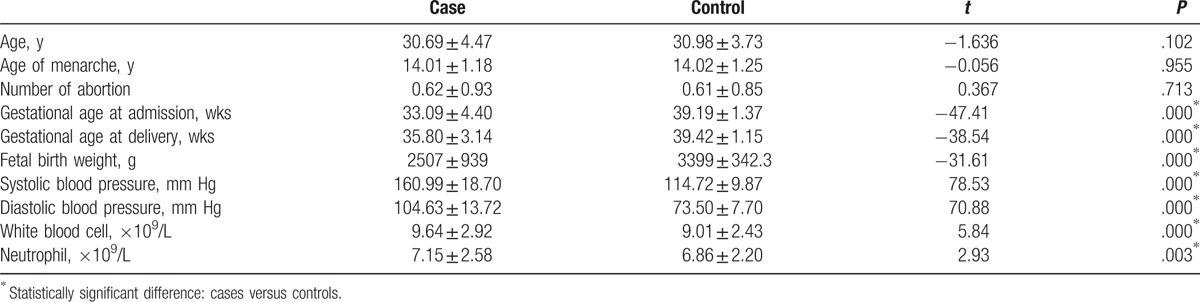
Demographic and clinical characteristics of all participates, such as maternal age, gravidity times, abortion number, menarche age, gestational week, blood pressure, and results of laboratory examinations, were shown in Table 1.
Table 1.
Demographic and clinical characteristics of case and control groups.
2.2. Genotyping
DNA was extracted from venous blood, which were collected on EDTA from all participates, using Qiagen DNA extraction kits (Qiagen, Hilden, Germany). SEPP1 genotyping was carried out by the TaqMan allelic discrimination real-time PCR. The rs7579 primers were 5′-CCTTCAAACTAAATATTTAAAATAG-3′ (forward) and 5′- ACATACTCCCCAATTTAGTCTAGAC-3′ (reverse); rs230813 primers were 5′- GCCTCAAAGTTCCTGCAGAAAGCTA-3′ (forward) and 5′- GTGAGGTTTTCTTCCTTGACTGTTT-3′ (reverse), which were synthesized by Applied Bio-systems of Life Technologies (ABI, NY). The total volume of the reaction mixture was 25 μL and contained 1.25 μL 20 × SNP Genotyping Assay, 12.5 μL 2 × PCR Master Mix, and 11.25 μL DNA and DNase-free water. The amplification condition is 95°C for 3 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute, and then the fluorescent signals from VIC/FAM-labeled probes were detected by each cycle. Amplifications were carried out in C1000?thermal cycler and CFX96?real-time system (Bio-Rad, CA), and discrimination of genotypes was conducted using Bio-Rad CFX manager software 3.0.
2.3. Statistical analysis
Statistical analysis was carried out by SPSS 22.0 (SPSS Inc., Chicago, IL). Hardy–Weinberg equilibrium using the goodness-of-fit chi-square test was tested in control group. Comparisons between 2 groups were made by Student t test for clinical characteristics, and were described by the mean ± standard error (SE) or percentage. Allele and genotype frequencies between the 2 groups were analyzed by Pearson chi-square test. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to express the risk between case and control groups. Statistical significance was assumed at the P value <.05 level. The power analysis was calculated using the program Power and Sample Size Calculations (PS, Version 3.1.2).
3. Results
3.1. Demographic and clinical characteristics
The comparison of demographic and clinical characteristics between cases and controls were shown in Table 1. No statistically significant differences were found in age, age of menarche, and abortion numbers between the 2 groups (all P > .05). However, PE group had earlier admission gestational age, delivery gestational age, lower fetal birth weight, higher blood pressure, and higher levels of white blood cell and neutrophil (all P < .001).
3.2. Analysis of genotypic and allelic frequencies
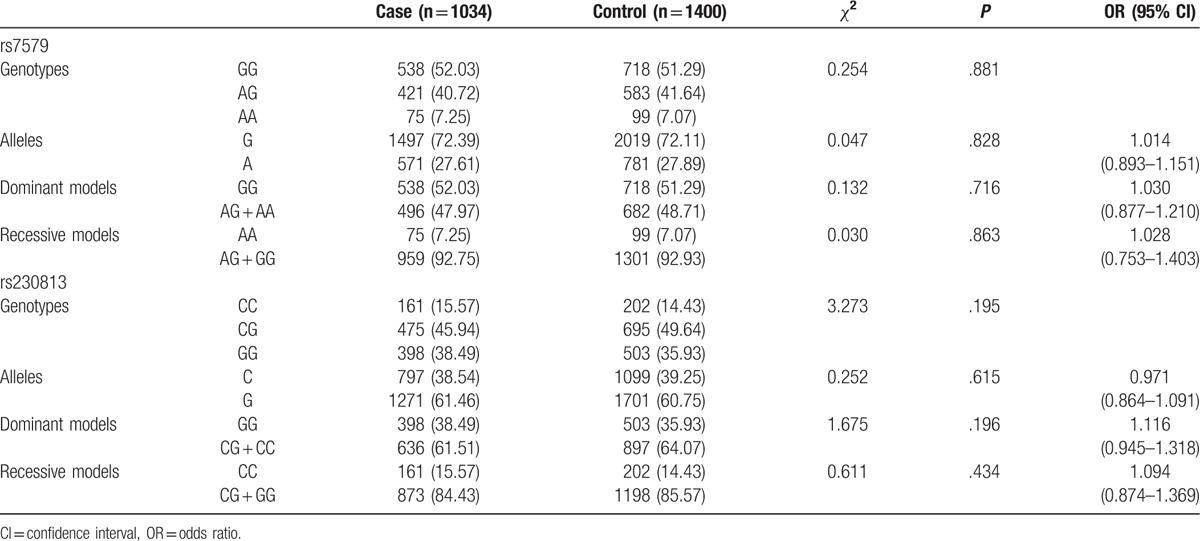
The control groups in our study were in accordance with the Hardy–Weinberg equilibrium (for 7579, χ2 = 1.738, P = .187; for rs230813, χ2 = 2.351, P = .125). The genotypic and allelic distributions of rs7579 and rs230813 between cases and controls were presented in Table 2. There were no statistically significant differences in the genotype and allele frequencies of rs7579 and rs230813 between cases and controls (for rs7579, χ2 = 2.417, P = .299 by genotype; χ2 = 0.197, P = .761, OR 1.049, 95% CI 0.744–1.151 by allele; GG vs AG + AA, χ2 = 0.132, P = .716, OR 1.030, 95% CI 0.893–1.151; AA vs AG + GG, χ2 = 0.197, P = .030, OR 1.028, 95% CI 0.753–1.1403); for rs230813, χ2 = 3.273, P = .195 by genotype; χ2 = 0.252, P = .615, OR 0.971, 95% CI 0.864–1.091 by allele; GG vs CG + CC, χ2 = 1.675, P = .196, OR 1.116, 95% CI 0.945–1.318; CC vs CG + GG, χ2 = 0.611, P = .434, OR 1.094, 95% CI 0.874–1.369).
Table 2.
Genotypic and allelic distributions in case and control groups.
To further investigate the association between SEPP1 variants and PE, all PE patients were divided into mild PE (n = 181) and severe PE (n = 853) groups. Table 3 shows no statistically significant difference in mild/severe PE and controls (mild PE vs control: for rs7579, χ2 = 2.417, P = .299 by genotype; χ2 = 0.197, P = .761, OR 1.049, 95% CI 0.744–1.206 by allele. For rs230813, χ2 = 5.757, P = .056 by genotype; χ2 = 0.038, P = .846, OR 1.022, 95% CI 0.817–1.279 by allele; severe PE vs control: for rs7579, χ2 = 0.938, P = .626 by genotype; χ2 = 0.177, P = .674, OR 1.029, 95% CI 0.900–1.178 by allele. For rs230813, χ2 = 1.553, P = .460 by genotype; χ2 = 0.423, P = .516, OR 0.960, 95% CI 0.848–1.086 by allele). We also divided cases early-onset PE (529 cases) and late-onset PE (505 cases). Table 4 shows no statistically significant difference in early-onset/late-onset PE and controls (early-onset PE vs control: for rs7579, χ2 = 1.277, P = .528 by genotype; χ2 = 0.332, P = .564, OR 0.955, 95% CI 0.817–1.117 by allele. For rs230813, χ2 = 1.467, P = .480 by genotype; χ2 = 0.000, P = .989, OR 0.999, 95% CI 0.846–1.155 by allele; late-onset PE vs control: for rs7579, χ2 = 0.271, P = .873 by genotype; χ2 = 0.902, P = .342, OR 1.082, 95% CI 0.920–1.273 by allele; for rs230813, χ2 = 3.076, P = .215 by genotype; χ2 = 0.637, P = .425, OR 0.941, 95% CI 0.812–1.092 by allele).
Table 3.
Genotypic and allelic distributions in mild/severe PE and control groups.
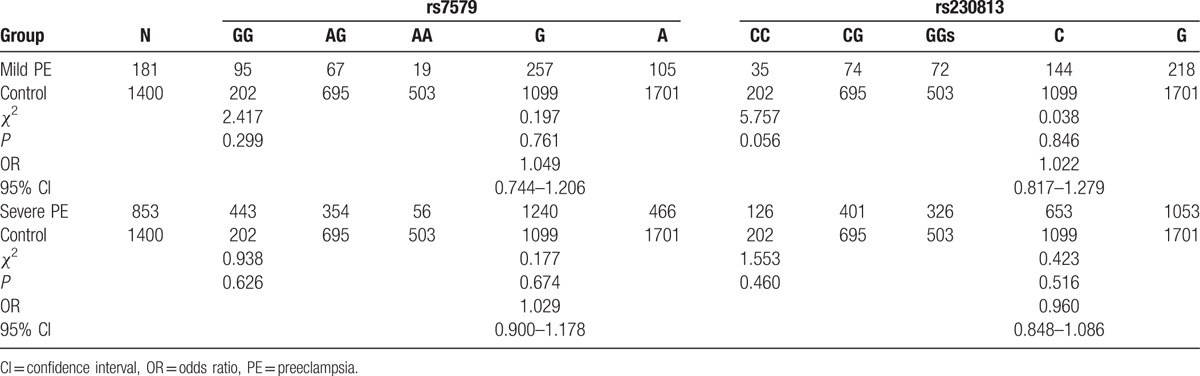
Table 4.
Genotypic and allelic distributions in early-onset/late-onset PE and controls.

4. Discussion
Preeclampsia is 1 of the most common and severe pregnancy-specific syndrome, and remains a severe morbidity and mortality risk for both mother and fetus worldwide, especially in low-income and middle-income countries.[1] Furthermore, PE women have a greater risk of developing hypertension, stroke, cardiovascular disease (CVD), and chronic kidney disease in their later life.[25,26] More importantly, numerous strategies to prevent and treat PE have been investigated, but the effect is not satisfactory and the mechanisms of PE are still not fully elucidated. However, more and more evidence supports that placental and systemic oxidative stress plays a crucial role in the development of PE.[3–5] As we all know, SEPP1 is 1 of the candidate genes that relate to oxidative stress.
SEPP1 locates on chromosome 5q31 and encodes selenoprotein P, which is 1 of the major selenoprotein in plasma, acting as a selenium transport protein and antioxidant.[9,27] Selenoproteins have previously been associated with risk of various cancers and redox-related diseases, such as prostate,[20,21] lung,[28] breast,[22] and colorectal[23] cancer. It is reported that oxidative stress causes endothelial dysfunction, which may lead to hypertension through lipid peroxidation and leukocyte activation.[29] As ischemia or reperfusion of placental, PE patients created a hypoxic environment which favors oxidative stress, which can result in the formation of unbalanced free radical, lipid peroxidation, and endothelial dysfunction.[29] Thus, it is important to study genetic variations of the candidate genes that result in susceptibility to oxidative stress. Previous study has suggested that rs7579 in the 3′-untranslated region of SEPP1 has a functional effect which modulates the selenoprotein transport and enzyme activities in the plasma.[30] This variant also influences the proportion of the protein isoform.[31] Previous studies have investigated the association between the rs7579 and risk of many diseases. For instance, Strauss et al demonstrated that rs7579 is associated with aggressive-growing abdominal aortic aneurysm (AAA) and aortoiliac occlusive disease (AIOD).[32] Steinbrecher et al[21] showed a borderline significant association between rs7579 (AA vs GG) and prostate cancer risk in European men. As another tag-SNP of SEPP1, rs230813 locates in the intron variant. It has been reported that it has a relationship with many disorders such as breast cancer.[22] Takata et al found that rs230813 was significantly associated with malondialdehyde (MDA) concentration, which is a marker of oxidative stress.[33]
On the contrary, SEPP1 is suggested as selenium transport and has a relationship with the content of selenium in body. Selenium is a micronutrient essential for human health, and has the capacity to reduce the risk of PE through selenoproteins/selenoenzymes.[16,34] Previous studies indicated that PE patients have lower selenium status in toenail or circulating selenium concentrations during pregnancy.[16,17] Hence, it is possible that genetic variations of SNPs in SEPP1 have the potential to modulate the relationship between selenoprotein and diseases, which may through alters the synthesis of protein isoform. Therefore, we evaluated 2 SEPP1 SNPs that have been associated with oxidative stress and PE. To the best of our knowledge, it is the first study on the relationship between SEPP1 and PE susceptibility in Chinese Han women.
In the present study, we conducted the genotypes of 1034 PE patients and 1400 age-matched normal pregnant women, but we did not find any statistically significant difference in genotypic and allelic frequencies of rs7579 and rs230813 in SEPP1 between PE and control groups in Chinese Han population. To further understand the relationship between SEPP1 and PE, we divided the PE patients into mild/severe and early/late-onset subgroups, but found no statistically significant difference. In conclusion, our data suggest that rs7579 and rs230813 in SEPP1 do not play a crucial role in the risk of PE in Chinese Han women.
Although the sample size of our study was large enough and post hoc power calculations (for rs7579 and rs230813 are 5.4% and 6.2%, respectively) to draw credible conclusions, there were several limitations that should be noted. Firstly, all the cases and controls were recruited from Shandong Province in China; the results may not be representative of other regions or ethnics. Secondly, other SNPs in the SEPP1 may affect the risk of PE; only 2 SNPs (rs7579 and rs230813) were investigated in our study. Finally, PE is a complex multifactorial disease, which is the consequence of interaction between genetic and environment risk factors and their interaction. Hence, several genetic variants in SEPP1 might not influence gene expression; other genes or environmental factors such as diet, obesity, and stress may contribute to the development of PE. Therefore, studies with more SNPs and functions are needed to be verified in different races and regions to explore the association between SEPP1 polymorphisms and PE.
Acknowledgment
We are grateful to all participants who completed this study.
Footnotes
Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer
Abbreviations: CI = confidence intervals, OR = Odds ratios, PE = preeclampsia.
H.W. and X.J. contributed equally to this work.
Funding: This work was supported by the National Natural Science Foundation of China (81371499 and 30971586).
The authors report no conflicts of interest.
References
- [1].Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. [DOI] [PubMed] [Google Scholar]
- [2].Williams PJ, Pipkin FB. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv 2005;60:807–16. [DOI] [PubMed] [Google Scholar]
- [4].Ozan H, Ilcol Y, Kimya Y, et al. Plasma anti-oxidant status and lipid profile in non-gravida women with a history of pre-eclampsia. J Obstet Gynaecol Res 2002;28:274–9. [DOI] [PubMed] [Google Scholar]
- [5].Chamy VM, Lepe J, Catalan A, et al. Oxidative stress is closely related to clinical severity of pre-eclampsia. Biol Res 2006;39:229–36. [DOI] [PubMed] [Google Scholar]
- [6].Smith-Jackson K, Hentschke MR, Poli-de-Figueiredo CE, et al. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta 2015;36:607–10. [DOI] [PubMed] [Google Scholar]
- [7].Saadat M, Anvar Z, Namavar-Jahromi B, et al. Genetic polymorphisms of glutathione S-transferase Z1 (GSTZ1) and susceptibility to preeclampsia. Mol Biol Rep 2012;39:8995–8. [DOI] [PubMed] [Google Scholar]
- [8].Roten LT, Fenstad MH, Forsmo S, et al. A low COMT activity haplotype is associated with recurrent preeclampsia in a Norwegian population cohort (HUNT2). Mol Hum Reprod 2011;17:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 2009;1790:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bosschaerts T, Guilliams M, Noel W, et al. Alternatively activated myeloid cells limit pathogenicity associated with African trypanosomiasis through the IL-10 inducible gene selenoprotein P. J Immunol 2008;180:6168–75. [DOI] [PubMed] [Google Scholar]
- [11].Vanderielie J, Venardos K, Perkins AV. Selenium deficiency as a model of experimental pre-eclampsia in rats. Reproduction 2004;128:635–41. [DOI] [PubMed] [Google Scholar]
- [12].Mistry HD, Wilson V, Ramsay MM, et al. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension 2008;52:881–8. [DOI] [PubMed] [Google Scholar]
- [13].Walsh SW, Wang YP. Deficient glutathione-peroxidase activity in preeclampsia is associated with increased placental production of thromboxane and lipid peroxides. Am J Obstet Gynecol 1993;169:1456–61. [DOI] [PubMed] [Google Scholar]
- [14].Vanderlelie J, Venardos K, Clifton VL, et al. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005;26:53–8. [DOI] [PubMed] [Google Scholar]
- [15].Rayman MP, Searle E, Kelly L, et al. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. Brit J Nutr 2014;112:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rayman MP, Bode P, Redman CWG. Low selenium status is associated with the occurrence of the pregnancy disease preeclampsia in women from the United Kingdom. Am J Obstet Gynecol 2003;189:1343–9. [DOI] [PubMed] [Google Scholar]
- [17].Rayman MP, Bath SC, Westaway J, et al. Selenium status in UK pregnant women and its relationship with hypertensive conditions of pregnancy. Brit J Nutr 2015;113:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vanderlelie J, Perkins AVA. Selenium and preeclampsia: a global perspective. Pregnancy Hypertens 2011;1:213–24. [DOI] [PubMed] [Google Scholar]
- [19].Gynecol ACO. Diagnosis and management of preeclampsia and eclampsia: number 33, January 2002. Int J Gynecol Obstet 2002;77:67–75. [PubMed] [Google Scholar]
- [20].Geybels MS, van den Brandt PA, Schouten LJ, et al. Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J Natl Cancer Inst 2014;106: dju003. [DOI] [PubMed] [Google Scholar]
- [21].Steinbrecher A, Meplan C, Hesketh J, et al. Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiol Biomarkers Prev 2010;19:2958–68. [DOI] [PubMed] [Google Scholar]
- [22].Pellatt AJ, Wolff RK, John EM, et al. SEPP1 influences breast cancer risk among women with greater native American ancestry: the Breast Cancer Health Disparities Study. PloS One 2013;8: (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meplan C, Hughes DJ, Pardini B, et al. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis 2010;31:1074–9. [DOI] [PubMed] [Google Scholar]
- [24].Roberts JM, August PA, Bakris G, et al. Hypertension in pregnancy report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- [25].Ahmed R, Dunford J, Mehran R, et al. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 2014;63:1815–22. [DOI] [PubMed] [Google Scholar]
- [26].Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. Brit Med J 2003;326:845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhuo P, Diamond AM. Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta 2009;1790:1546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gresner P, Gromadzinska J, Jablonska E, et al. Expression of selenoprotein-coding genes SEPP1, SEP15 and hGPX1 in non-small cell lung cancer. Lung Cancer 2009;65:34–40. [DOI] [PubMed] [Google Scholar]
- [29].Muetze S, Rudnik-Schoeneborn S, Zerres K, et al. Genes and the preeclampsia a syndrome. J Perinat Med 2008;36:38–58. [DOI] [PubMed] [Google Scholar]
- [30].Meplan C. Selenium and chronic diseases: a nutritional genomics perspective. Nutrients 2015;7:3621–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Meplan C, Nicol F, Burtle BT, et al. Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, Se intake, and cancer status. Antioxid Redox Signal 2009;11:2631–40. [DOI] [PubMed] [Google Scholar]
- [32].Strauss E, Oszkinis G, Staniszewski R. SEPP1 gene variants and abdominal aortic aneurysm: gene association in relation to metabolic risk factors and peripheral arterial disease coexistence. Sci Rep 2014;4:7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takata Y, King IB, Lampe JW, et al. Genetic variation in GPX1 is associated with GPX1 activity in a comprehensive analysis of genetic variations in selenoenzyme genes and their activity and oxidative stress in humans. J Nutr 2012;142:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brigelius-Flohe R, Banning A, Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal 2003;5:205–15. [DOI] [PubMed] [Google Scholar]


