Abstract
Monocytosis is associated with chronic infections such as tuberculosis or endocarditis as well as rheumatic and myeloproliferative disorders. Monocytes are also involved in the pathogenesis of atherosclerosis, coronary artery disease, and stroke. The value of monocytosis as a prognostic marker in different diagnostic groups in the emergency setting, however, has not been investigated so far.
The aim of the article is to study monocytosis as an outcome factor in the emergency setting.
In a Swiss register study, we analyzed monocyte counts in 4238 patients aged >18 years who were admitted to the emergency department of a regional tertiary care hospital. Monocytosis was defined as 0.8×109 cells/L. Diagnoses were grouped into infection, cardiovascular, neurological, metabolic, gastrointestinal, pulmonary, or other. Thirty-day mortality was defined as the primary endpoint
A total of 1217 patients with monocytosis were identified. Patients with monocytosis at admission suffered more frequently from respiratory symptoms (17.7% vs 8.9%, P <.001) and infection as the final diagnosis (20.8% vs 10.3%, P <.001) while neurological diagnoses were significantly lower in the monocytosis group (15.3% vs 30.9%, P <.001). Patients with monocytosis suffered from more comorbidities such as congestive heart failure, chronic obstructive pulmonary disease, tumor, diabetes, or renal failure but not dementia. When adjusted for age, gender, comorbidities, and main diagnosis, the 30-day mortality (P = .002) and length of stay (P = .001) were significantly higher in patients with monocytosis. The 30-day mortality in patients with monocytosis was most notably influenced by a cardiological diagnosis (odds ratio 3.91).
An increased monocyte count predicts adverse outcome in patients admitted to the emergency department. Mechanistic studies will be necessary to specify the potentially detrimental role of monocytosis in critical illness.
Keywords: cardiovascular, emergency department, infection, monocytosis, mortality, outcome, primary care
1. Introduction
Monocytes represent about 5% of all leukocytes in the peripheral blood.[1] After circulating for several days in the bloodstream, monocytes usually undergo extravasation. In the tissue they differentiate into macrophages or dendritic cells[2] and are involved in cytokine expression, antigen presentation, or phagocytosis.[3] “Patrolling” monocytes constantly migrate along the endothelium in blood vessels serving as vascular innate immune system.[4] Monocytes can be specified into different subsets such as CD16high14− monocytes which produce high amounts of inflammatory cytokines such as tumor necrosis factor or a more regulatory CD16low14+ monocyte subset.[5,6]
As widely known monocytosis occurs in chronic infection such as tuberculosis, endocarditis, granulomatous disease, or in myeloproliferative disorders. Other disorders that can be associated with increased monocyte counts are the metabolic syndrome[7] and autoimmune disorders including rheumatoid arthritis.[8] The underlying pathophysiology leading to monocytosis is not fully understood. Chemokines such as monocyte chemoattractant protein-1 and growth factors trigger monocyte recruitment and homeostasis.[9] Smoking also leads to increased monocyte numbers.[10]
Monocytosis is associated with artherosclerosis and its consequences such as coronary artery disease, cerebrovascular disease, or kidney artery stenosis, for example, as a source of foam cells.[11,12] Increased monocyte counts after acute myocardial infarction (AMI) were associated with left ventricular dysfunction, left ventricular aneurysm, and other cardiac events.[13] Another study showed similar effects to the nonrecovery of the left ventricular function after reperfused AMI.[14] To this end, monocytosis has been identified as an independent risk factor for myocardial infarction or cerebral arterial disease.[15] The level of the National Institutes of Health Stroke Scale on stroke patients correlates with the amount of monocytes.[16]
So far, the prognostic value of monocytosis in the emergency setting has not been investigated although monocyte numbers usually are assessed in routine blood tests. In this Swiss register study we have analyzed monocytes counts in patients admitted to the emergency department as a predictive factor for survival and hospital stay.
2. Methods
2.1. Study design and setting
This is an observational, prospective cohort study. Between March 2013 and February 2014, consecutive adult medical patients were included upon hospital admission in the emergency department into the quality-control TRIAGE project. This project's main aim is to optimize the triage and patient flow of adult patients with medical emergency.[17]
As an observational quality control study, the Institutional Review Board (IRB) of the Canton of Aargau has approved the study and waived the need for informed consent (EK 2012/059).
2.2. Patient population and management
Adult in-patients with an acute medical illness were included in this study; children and surgical patients were excluded. We collected pertinent clinical information, including sociodemographic characteristics, main medical diagnosis, and comorbidities at hospital admission using the information routinely gathered from the hospital electronic medical system for coding of diagnosis-related group codes. This already available information supported the reliable assessment of baseline characteristics and different patient outcomes. Clinical information and patient outcomes were assessed until hospital discharge and structured patient interviews were conducted via telephone 30 days after hospital admission to assess information about different clinical and functional outcome measures such as location after discharge, quality of life, performance of activities of daily living, hospital readmission, and mortality. If a patient could not be reached, we contacted the family or the general practitioner to assess vital status.
2.3. Main diagnosis and comorbidities
Patients were divided into main diagnosis groups including infections, cardiovascular diseases, metabolic diseases, cancer, neurological disorders, digestive tract diseases, pulmonary diseases, and other disease. We also defined the following comorbidity groups: congestive heart failure, chronic obstructive pulmonary disease (COPD), dementia, diabetes mellitus, tumor, renal failure, and obesity.
2.4. Outcomes
Our primary outcomes were 30-day mortality, in-hospital mortality, length of stay, intensive care unit (ICU) admission, and rate of 30-day readmission assessed during the hospital stay and by telephone interviews at day 30.
Secondary outcomes included functional impairment and quality of life. Performance of daily living was measured by the Barthel index. We defined functional impairment as a Barthel index <95 points. In order to assess quality of life, we used the standardized measure of health EQ-5D including a descriptive system with 5 dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression). These results were displayed as 2 levels, “impairments” or “no impairments.”
2.5. Assessment of monocyte count and definition of monocytosis
Monocytes were counted using the automated hematology analyzer Sysmex XN or by hand in case of discrepancy. The Sysmes XN uses fluorescence and the SAFLAS method (Sysmes adaptive Flagging Algorithm based on Shape-recognition) for monocyte recognition.
The cut off for monocytosis was defined as 0.8 × 109/L blood, which is according to common literature. Monocytopenia was defined as 0.3 × 109 cells/L blood. Both thresholds were tested in this cohort regarding the 30-day mortality.
2.6. Statistical analysis
Categorical variables are expressed as percentages and counts or vice versa and continuous variables as medians (interquartile ranges: 25th–75th percentiles), unless stated otherwise. Frequency comparison was done by the χ2 test. For all binary endpoints, logistic models with odds ratios (OR) and 95% confidence intervals (95% CI) were used. For time to hospital discharge, Cox regression models with hazard ratios (HR) were calculated. To adjust for possible confounds, we used 3 statistical models: model 1 for age and gender; model 2 for age, gender, and comorbidities; and model 3 for age, gender, comorbidities, and main diagnosis.
We evaluated the association between monocyte count and outcomes in the overall population as well as within different predefined subgroups based on gender, age (cut off 75 years) and main medical diagnosis. Evidence of effect modification within these subgroups was assessed by including interaction terms into the statistical models. A P value <.05 (for a 2-sided test) was considered statistically significant. All statistical analyses were performed with STATA 12.1 (Stata Corp, College Station, TX).
3. Results
3.1. Patient characteristics and comorbidities
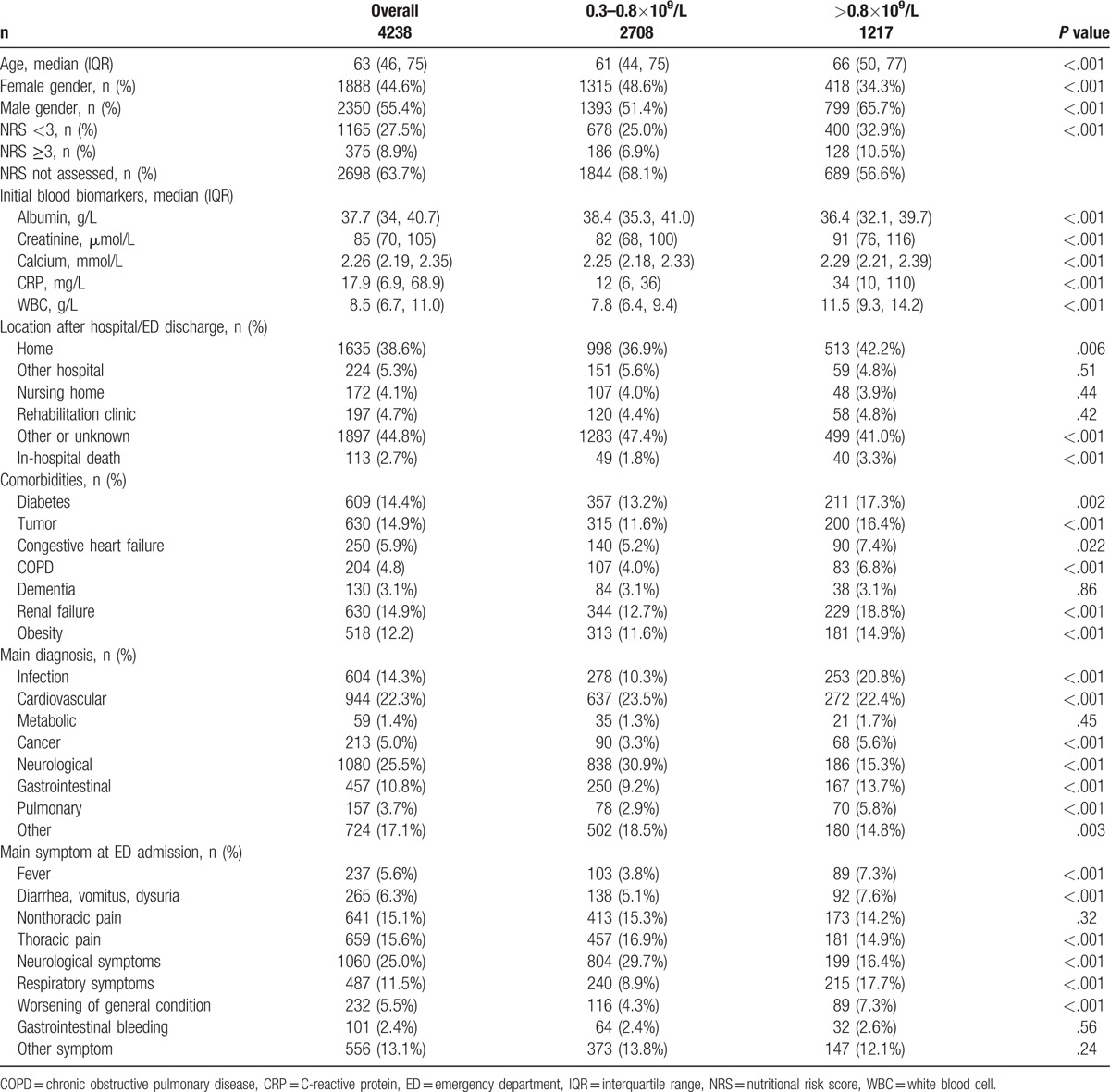
The mean age in patients with monocytosis was higher compared to patients with normal monocyte counts (66 vs 61 years, P <.001, Table 1) and there were more male patients in the monocytosis group (65.7% vs 51.4%, P <.001). The nutritional risk status was higher in patients with monocytosis (P = .001) and accordingly, patients with monocytosis had lower albumin values (P = .001). Serum creatinine (P = .001), CRP (P = .001), and blood leukocyte count were also higher in the monocytosis group (P = .001). Diabetes (P = .002), tumor (P <.001), heart failure (P = .022), COPD (P <.001), renal failure (P <.001), and obesity (P <.001) were more prevalent in patients with monocytosis. Conversely, dementia was not more frequently observed in monocytosis (P = .086).
Table 1.
Patient characteristics overall and according to monocyte count (counts per liter blood stated).
3.2. Symptoms and diagnosis
At admission, neurological symptoms (16.4% vs 29.7%, P <.001) and thoracic pain (14.9% vs 16.9%, P <.001) were lower in the monocytosis group whereas respiratory symptoms were more frequent (17.7% vs 8.9%, P <.001). Nonthoracic pain (14.2% vs 15.3%, P = .32) pain was similar. Worsening of the general condition (7.3% vs 4.3%, P <.001) and fever (7.3% vs 3.8%, P <.001) were also more likely in the monocytosis group.
In terms of diagnosis which let to hospital admission, neurologic disorders were identified in 15.3% versus 30.9% (P <.001) of the cases. Cardiovascular diagnosis as a reason for admission was similar in monocytosis in 22.4% versus 23.5% (P <.001) in patients with a normal monocyte count. The most notable increase was observed in the diagnosis of infection (20.8% vs 10.3%, P <.001). Gastrointestinal (13.7% vs 9.2%, P <.001), pulmonary (5.8% vs 2.9%, P <.001), or cancer (5.6% vs 3.3%, P <.001) diagnosis were higher in the monocytosis group.
3.3. Mortality, length of hospitalization and functional impairment
We studied mortality and length of hospitalization in different models (Table 2). Adjusted for age and gender, 30-day mortality (P <.001), length of stay (P <.001), and ICU admission (P = .020) were significantly higher in patients with monocytosis while in-hospital mortality (P = .088) and rate of 30-day admission (P = .100) were similar. When adjusted for age, gender, comorbidities, and main diagnosis, 30-day mortality (P = .002) and length of stay (P = .001) remained significant. In a subgroup analysis, the 30-day mortality was mostly influenced by cardiologic diagnosis (OR 3.91, Table 3 ) but without a significant effect modification. Conversely, there were no differences of clinical functional impairment in the monocytosis versus normal monocyte count group (Table 4).
Table 2.
Primary outcomes baseline overall and according to monocyte count.
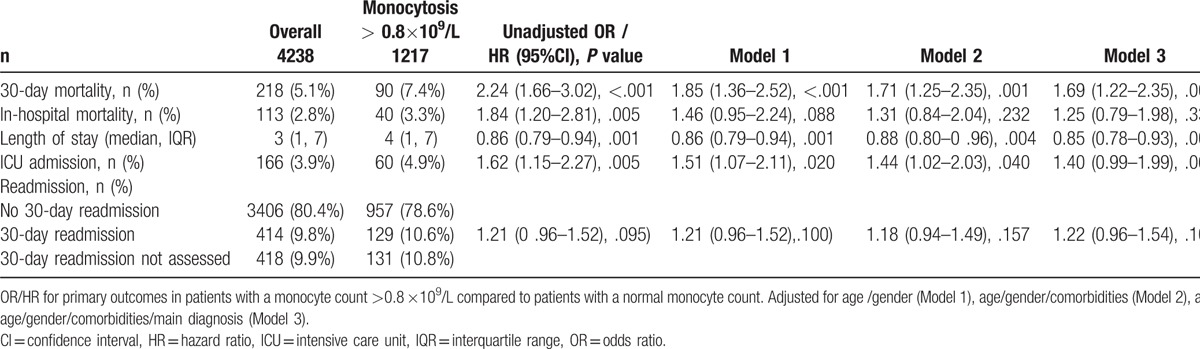
Table 3.
Subgroup analysis.
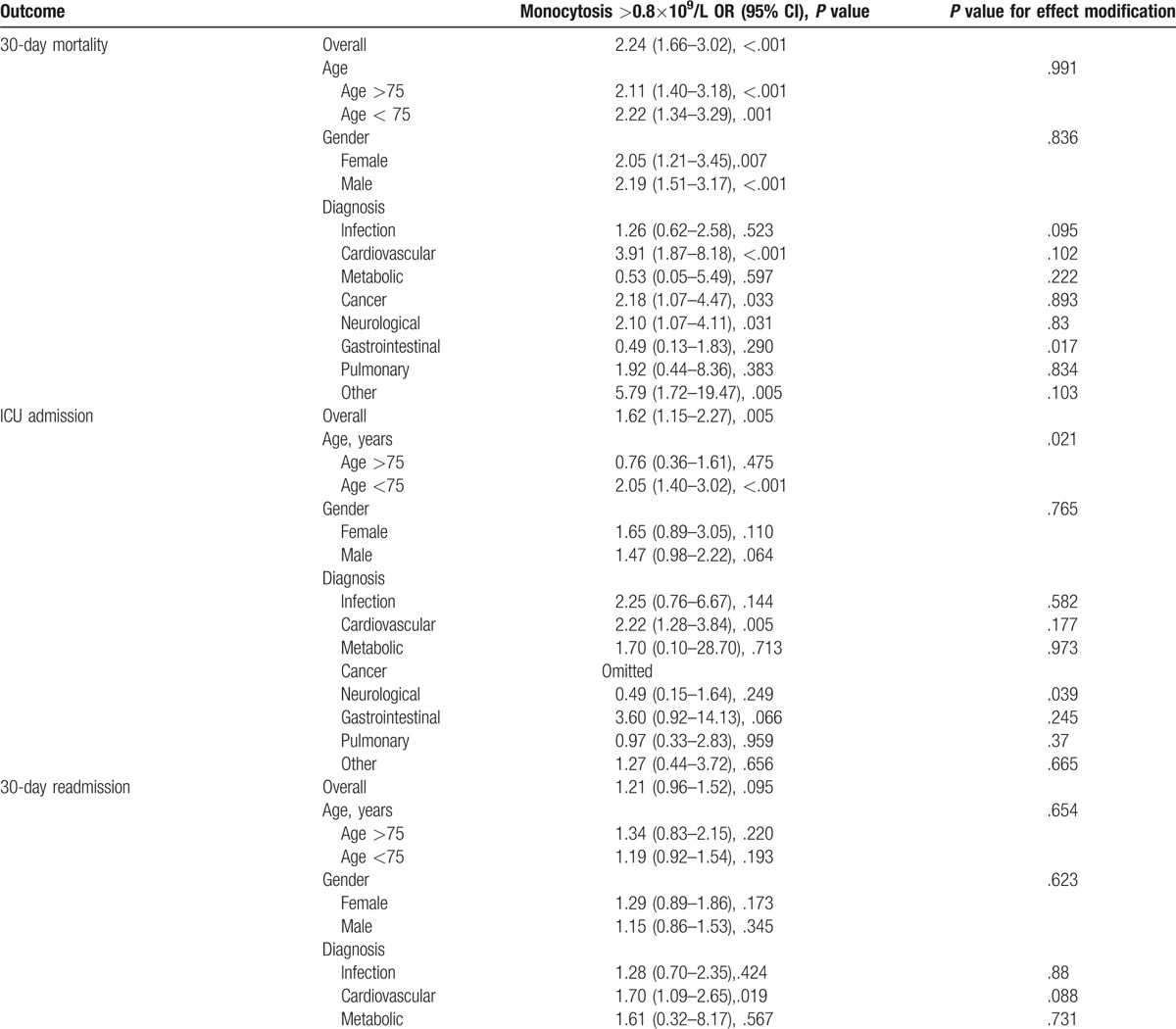
Table 3 (Continued).
Subgroup analysis.
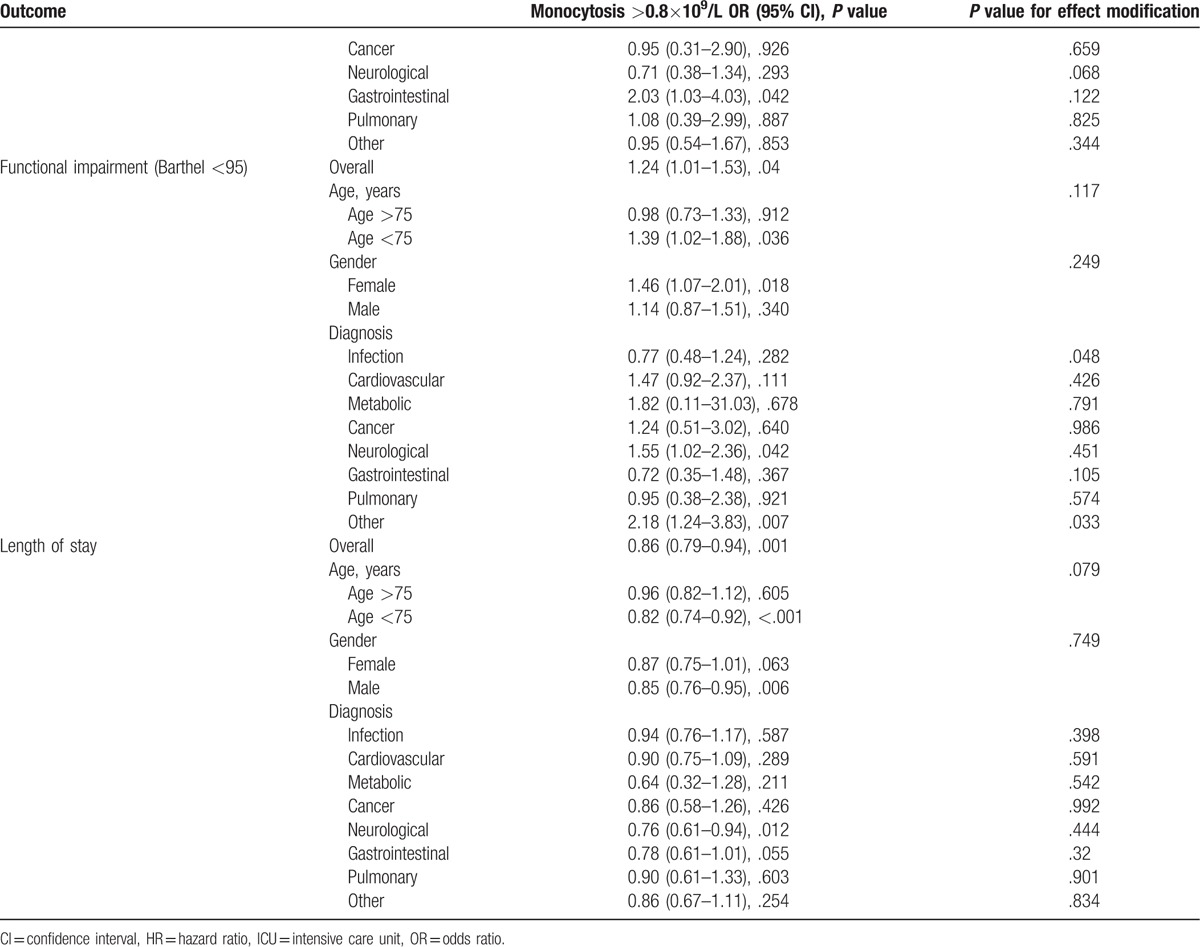
Table 4.
Secondary outcomes baseline overall and according to monocyte count. OR/HR for primary outcomes in patients with a monocyte count >0.8×109/L compared to patients with a normal monocyte count. Adjusted for age /gender (Model 1), age/gender/comorbidities (Model 2), and age/gender/comorbidities/main diagnosis (Model 3).
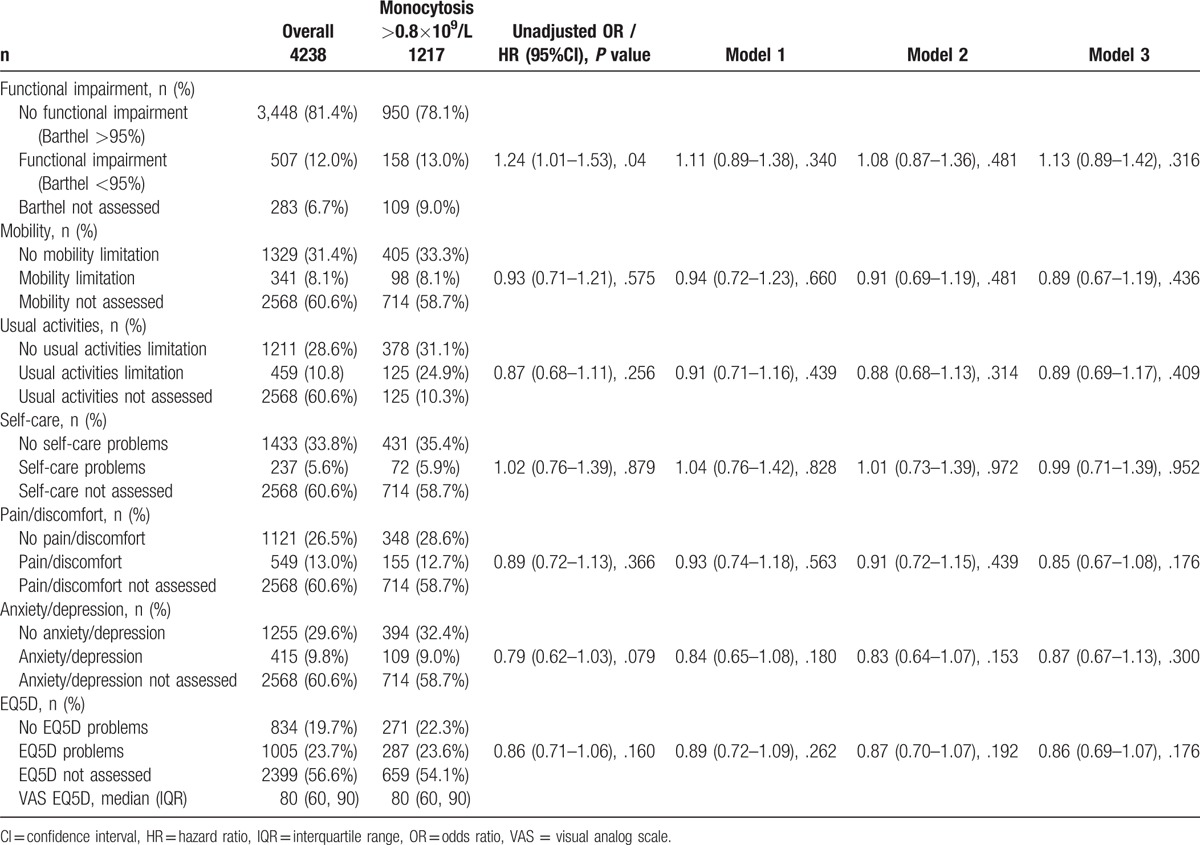
3.4. Functional impairment of patients
No differences were found in patients with monocytosis regarding functional impairment in terms of mobility (P = .575), usual activities (P = .256), self care (P = .879), pain or discomfort (P = .366), or anxiety (P = .079) (Table 4).
4. Discussion
Despite the profound knowledge in monocyte biology, surprisingly little is known about monocytosis in the clinical setting. In this large survey, we identified peripheral blood monocytosis as a negative prognostic marker in the emergency setting. This is in line with a plethora of previous studies showing that activation of the innate immune system may be detrimentally associated with critical illness.[18] Monocytes are a major source of oxidative stress and thus can trigger organ damage under certain circumstances.[19] Unfortunately, we could not specify the monocyte subsets in this study. The role of the ‘inflammatory’ CD14++CD16− monocyte subset would be interesting and important in order to understand the mechanism of monocytes in critical illness.[20] Patients with monocytosis had more often respiratory symptoms and suffered from infection than individuals with normal monocyte counts. In part this might be related to the higher number of COPD patients in this group and indicates that smoking, which was not assessed in this study, triggers monocytosis. It can however be postulated that lung impairment, most likely due to infection, is a main stimulator of monocytosis. Fever, which was also associated with monocytosis in this study, further indicates that a potentially unspecific systemic inflammatory response is involved in monocytosis. Why neurologic diagnosis inversely correlated with monocytosis is unclear and surprising. Prior studies have shown an association between monocytes and cerebral vascular disease.[16] Potentially, patrolling monocytes at the inner side of the vessel wall behave differently in blood–brain barrier than in the rest of the circulation.
In contrast, cardiovascular diagnoses were the strongest influence for the 30-day mortality in patients with monocytosis. This is in line with previous studies showing that monocytosis is also involved in the pathogenesis of atherosclerosis. Apart from the brain, we postulate that monocytosis is notably toxic to organs affected from atherosclerosis, for example, by increased extravasation or release of cytokines and oxidative stress. Monocytopenia was also associated with an increased 30-mortality (data not shown) in this survey but this mainly affected hematological disorders and was not influenced by cardiovascular diagnoses. Clearly, this study is observational and has several limitations. In this survey we cannot draw conclusions about mechanistic processes and we cannot answer the question whether monocytosis is the cause or just a consequence of adverse outcome. There was no negative effect of monocytosis on functional outcomes such as mobility or pain. We therefore conclude that in case of monocytosis, the activated innate immune system affects organ function, notably in patients with an already impaired cardiovascular system. Mechanistic studies are necessary in order to understand the negative role of monocytosis in critical care and to identify potential new treatment targets such as a monocyte-based immune modulation in critical care.
Footnotes
Abbreviations: AMI = acute myocardial infarction, CAD = coronary artery disease, CHF = congestive heart failure, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, ED = emergency department, HR = hazard ratio, ICU = intensive care unit, IQR = interquartile range, NRS = nutritional risk score, OR = odds ratio, WBC = white blood cell.
PS and TH both contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Nichols BA, Bainton DF, Farquhar MG. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol 1971;50:498–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets 2011;11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages and dendritic cells. Science 2010;327:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lauvau G, Loke P, Hohl TM. Monocyte-mediated defense against bacteria, fungi, and parasites. Semin Immunol 2015;27:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Serbina NV, Jia T, Hohl TM, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008;26:421–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol [Internet] 2015;35:1066–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol Rev 2014;262:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klimek E, Mikolajczyk T, Sulicka J, et al. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int 2014;2014:736853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res 2009;29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Corre F, Lellouch J, Schwartz D. Smoking and leucocyte-counts. Lancet 1971;2:632–4. [PubMed] [Google Scholar]
- [11].Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chapman CML, Beilby JP, McQuillan BM, et al. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke 2004;35:1619–24. [DOI] [PubMed] [Google Scholar]
- [13].Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241–6. [DOI] [PubMed] [Google Scholar]
- [14].Hong YJ, Jeong MH, Ahn Y, et al. Relationship between peripheral monocytosis and nonrecovery of left ventricular function in patients. Circ J 2007;71:1219–24. [DOI] [PubMed] [Google Scholar]
- [15].Abrahão Afiune Neto, Antonio de Pádua Mansur SDA, Everly PSG, et al. Monocytosis is an independent risk marker for coronary artery disease. Arq Bras Cardiol 2006;86:240–4. [DOI] [PubMed] [Google Scholar]
- [16].Kaito M, Araya SI, Gondo Y, et al. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS One 2013;8:e69409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schuetz P, Hausfater P, Amin D, et al. Optimizing triage and hospitalization in adult general medical emergency patients: the triage project. BMC Emerg Med 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wiersinga WJ, Leopold SJ, Cranendonk DR, et al. Host innate immune responses to sepsis. Virulence 2014;5:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2014;112:1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol [Internet] 2011;11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


