Abstract
Patients with drug-susceptible tuberculosis (TB) show good tolerance of the medications used and have few side effects. However, medications used to treat multidrug-resistant tuberculosis (MDR-TB) have many known side effects. Here, we studied the occurrence of side effects due to treatment of patients with MDR-TB.
We conducted a retrospective and consecutive review of the medical records of 256 patients who received treatment for MDR-TB between January 2006 and December 2011.
One or more side effects were observed in 95 (37.1%) of the 256 patients. These side effects led to the suspension of the use of 1 or more drugs from the regimen of individualized treatment prescribed for 44 of the patients (17.2%). The side effects observed most frequently included gastrointestinal disturbance (18.4%), psychiatric disorder (5.5%), arthralgia (4.7%), hepatitis (3.9%), peripheral neuropathy (3.1%), hypothyroidism (2.3%), epileptic seizures (2%), dermatological effects (2%), ototoxicity (1.6%), and nephrotoxicity (1.2%). The treatment was successful in 220 (85.9%) patients with MDR-TB.
Our study may help in formulating strategies for the timely and aggressive management of drug side effects. This may reduce the suspension of therapy and increase the rate of clinical success.
Keywords: drug-resistant tuberculosis, side effects, tuberculosis
1. Introduction
Multidrug-resistant tuberculosis (MDR-TB) is caused by strains of Mycobacterium tuberculosis with resistance to both isoniazid (INH) and rifampicin (RFP). Approximately 132,120 cases of MDR-TB were detected in 2015, 124,990 of whom received treatment.[1] Despite a global effort to control MDR-TB, the success rate of treatment is still unsatisfactory. The target for treatment success is 75% or higher for MDR-TB patients, but the latest treatment outcome data show that the treatment success rate is 52%.[1] One of the most important independent factors underlying treatment failure is side effects of medications. However, there is not enough information regarding the relationship between side effects and outcomes in patients with MDR-TB in South Korea. We thus performed a retrospective study to investigate the occurrence of side effects of treatment in patients with MDR-TB.
2. Methods
This study included 256 consecutive patients with MDR-TB who were treated at the National Masan Hospital between January 2006 and December 2011. The National Masan Hospital, which is affiliated with Korea's Ministry of Health and Welfare, was established to contribute to curing TB and improving the health of the nation. The hospital's operations depend entirely on national funds, and no treatment fees are borne by patients seeking healthcare. All patients were diagnosed with MDR-TB at the National Masan Hospital or other health facilities referring patients to the MDR-TB Clinic at the National Masan Hospital. This unit provides long-term hospitalization and provides all drugs required for the treatment free of charge to patients with MDR-TB. All patients were initially treated as inpatients, although a few patients were followed up as outpatients after hospitalization. We reviewed the medical records of patients and obtained data on demographics, presence of comorbidities, drug susceptibility testing (DST) results, side effects, drugs discontinued due to side effects, treatment regimens, and clinical outcomes. The study design was approved by the ethics committee of the National Masan Hospital (Korean National Institute of Health).
All patients provided sputum specimens for M tuberculosis DST at the time of diagnosis. DST was performed using Lowenstein–Jensen agar slants and the following drugs: INH (0.2 mg/mL), RFP (40 mg/mL), ethambutol (2.0 mg/mL), streptomycin (10 mg/mL), kanamycin (40 mg/mL), ofloxacin (2.0 mg/mL), ethionamide (40 mg/mL), cycloserine (CS) (30 mg/mL), and para-aminosalicylic acid (PAS) (1 mg/mL).[2]
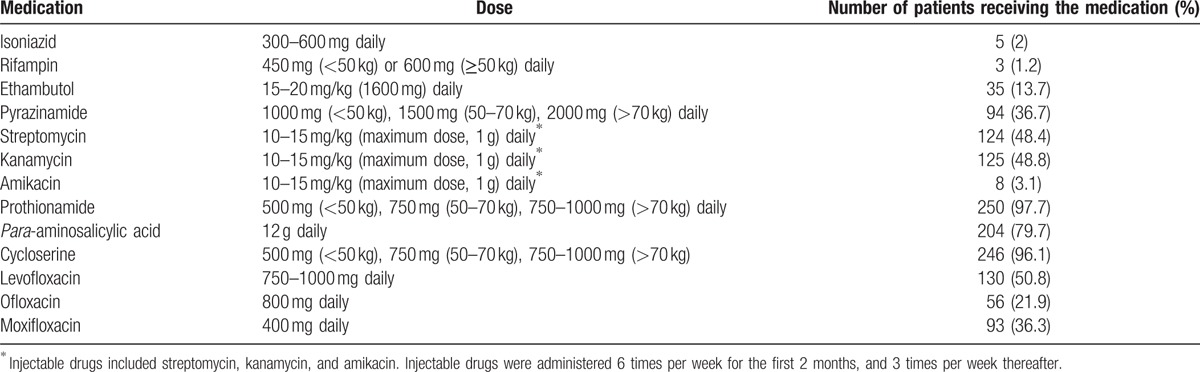
If possible, the patients were started on an individualized regimen consisting of at least 5 drugs, which included 1 parenteral drug (aminoglycoside). Four oral drugs were administered over the entire duration of treatment and the injectable drug was administered for 8 months. The injectable drug was administered 6 times per week for the first 2 months, and 3 times per week thereafter. All patients were hospitalized at least for the duration of the parenteral treatment. The drugs and doses used in the regimens are shown in Table 1. The duration of treatment was 8 to 24 months after the first negative sputum culture. All patients received 100 mg of pyridoxine supplementation daily throughout their treatment.
Table 1.
Antituberculous medications and dosage.
We obtained a complete blood count, performed electrolyte analysis, and determined the levels of serum creatinine, blood urea nitrogen, serum transaminases, serum total bilirubin, and alkaline phosphatase for all patients. We repeated these tests every month during the hospitalization and every 2 months after discharge. Audiometric and thyroid function tests were performed for patients with symptoms indicating the need for these tests and if the clinician deemed them necessary. A team of physicians and nurses monitored the patients daily for side effects during hospitalization. Before discharge, the patients were informed of the side effects of MDR-TB treatment. After discharge, we monitored the patients for side effects of MDR-TB treatment once per month. We recommended that the patients schedule appointments before their routine visit if any symptoms developed.
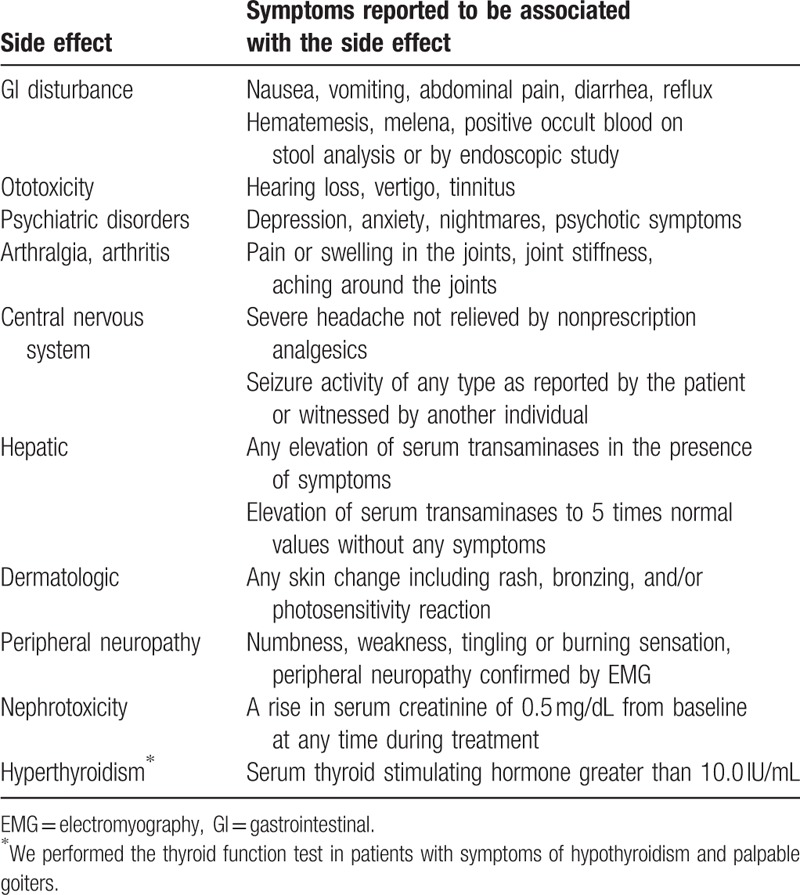
The drugs were discontinued whenever ototoxicity, nephrotoxicity, hypothyroidism, uncontrolled hepatotoxicity, uncontrolled psychiatric disorders, epileptic attacks, or uncontrolled gastrointestinal (GI) disorders developed. Side effects that were not controlled with additional medications or changes in drug dosage or drug intervals, and those that required 1 or more drugs to be discontinued temporarily or permanently by the clinician, were defined as uncontrolled side effects. If the side effect was controlled by discontinuation, or changes in dosage or administration interval, the side effect was deemed to be associated with the individual drug whose regimen was altered.[3] The definitions used to determine the presence of side effects are shown in Table 2. The clinical outcomes of MDR-TB followed the international consensus guidelines.[4] A “cured” patient was defined as having completed the treatment as recommended by the national policy, without evidence of failure, and 3 or more consecutive TB-negative cultures, taken at least 30 days apart. A patient classified as “completed” was defined as having completed treatment as recommended by the national policy without evidence of failure, but with no record of 3 or more consecutive TB-negative cultures. Patients classified as “treatment failed” had their treatment terminated or needed a regimen change affecting at least 2 anti-TB drugs due to: lack of culture conversion (from TB-positive to TB-negative) by the end of the intensive phase, bacteriological reversion in the continuation phase after conversion to TB-negative, evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs, or adverse drug reactions. Patient mortality was classified under the category “died.” Successful outcomes were defined as the combination of “cured” and “completed treatment,” while “defaulted,” “treatment failed,” and “died” were used as a composite variable for poor outcome. All statistical analyses were performed using SPSS software, version 18.0 (IBM Corp., Armonk, NY).
Table 2.
Definitions of side effects.
3. Results
In total, 256 patients had received treatment for MDR-TB at the time of this analysis, comprising 192 men (75%) and 64 women (25%). The mean age of the patients was 42.1 ± 14.2 years and the mean body mass index was 19.7 ± 3.3. Regarding risk factors for resistance development, 165 patients (64.5%) had previously received treatment for TB. Comorbidities were present in 85 patients (33.2%). Fifty-eight patients (22.6%) had diabetes mellitus, 10 (3.9%) had viral hepatitis, 2 (0.8%) had liver cirrhosis, 9 (3.5%) had hypertension, 10 (3.9%) had respiratory disease, 1 (0.4%) were infected with human immunodeficiency virus, and 13 (5.1%) had psychotic disorders, such as insomnia, mood disorder, major depressive disorder, and bipolar disorder (Table 3).
Table 3.
The characteristics of patients with MDR-TB (n = 256).

The patients received different initial treatments. The most commonly used drugs were prothionamide (PTH), CS, PAS, levofloxacin, pyrazinamide, ethambutol, moxifloxacin, and ofloxacin. Kanamycin and streptomycin were commonly used as the parenteral drugs (Table 1). The treatment regimens included a mean of 5.4 ± 0.8 drugs. The mean treatment duration was 21.2 ± 5.1 months. DST at the time of initial treatment determined that there was resistance to a mean of 4 ± 1.6 drugs (Table 3). The resistance patterns in the 256 patients are reported in Table 4, and Fig. 1.
Table 4.
Antituberculosis drug resistance of MDR-TB patients (n = 256).

Figure 1.
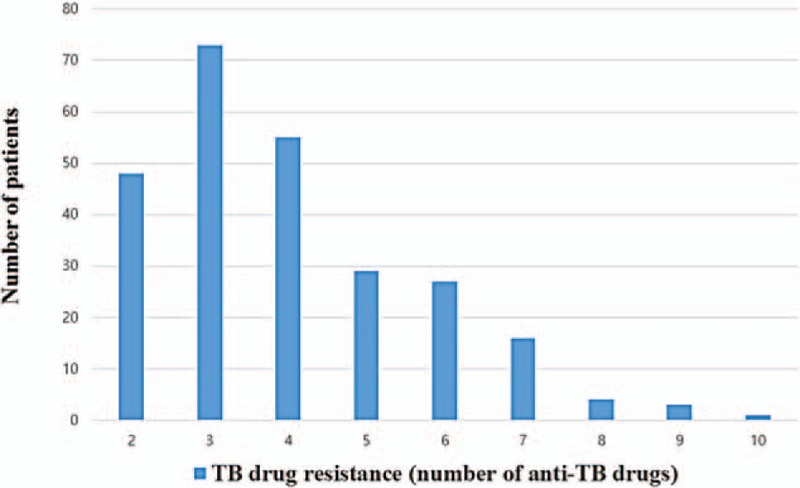
Frequency of resistance at treatment initiation (n = 256). TB = tuberculosis.
At least 1 side effect developed in 95 patients (37.1%). One or more drugs were withdrawn from the regimen in 54 patients (21.1%) due to uncontrolled side effects (Table 5). The most common side effect was GI disorder, which was detected in 47 patients (18.4%) and occurred at a mean of 5.9 months into treatment. PAS was withdrawn in 29 patients (11.3%) due to uncontrolled diarrhea, nausea, or vomiting. PAS was suspended in 18 patients (7%) until symptoms were relieved. H2 receptor blockers or antiemetic agents were added to the regimen for 42 patients (18%).
Table 5.
Side effects of drugs (n = 256).
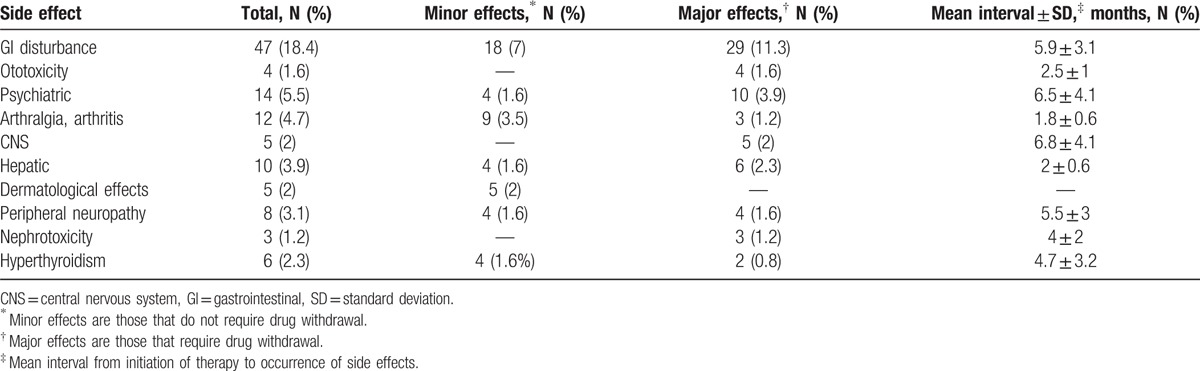
Ototoxicity was observed in 4 patients (1.6%). This side effect occurred at mean of 4.7 months into treatment. Ototoxicity led to the withdrawal of kanamycin in 2 patients (0.8%), while amikacin was withdrawn in 2 patients (0.8%).
Psychiatric symptoms were observed in 14 patients (5.5%). These symptoms included depression, anxiety, nightmares, and psychotic symptoms. These symptoms were controlled with additional medication or by tapering the drug dose in 4 patients (1.6%). An antidepressant was added to the drug regimen for 3 patients (1.2%), and an anticonvulsant was added to the drug regimen for 1 patient. CS was withdrawn in 10 patients (3.9%) at a mean time of 6.5 months after treatment onset. In these patients, the psychiatric symptoms were not controlled by additional medications.
Twelve patients (4.7%) complained of arthralgia or arthritis. In 9 patients (3.5%), the symptoms were relieved with the use of nonsteroidal antiinflammatory drugs (NSAIDs), although pyrazinamide was withdrawn in 2 patients, due to uncontrolled acute gout attack. Central nervous system (CNS) effects were observed in 5 patients (2%). These symptoms were not controlled by antiepileptic agents in any of the patients with CNS side effects. As a result, CS was withdrawn in these patients.
Hepatitis developed in 10 patients (3.9%) at a mean time of 2 months into treatment. Two patients (0.8%) had previously had viral hepatitis, while 8 patients did not. It was controlled by withdrawing pyrazinamide or PAS. Treatment was interrupted until hepatitis was relieved in 4 patients. In these patients, the full drug regimen was restarted and treatment was successfully completed.
Dermatological side effects were observed in 5 patients (2%). Rash occurred in 4 patients and itching sensation occurred in 1 patient. None of the drugs was withdrawn in these patients. Hyperthyroidism developed in 6 patients (2.3%). Nephrotoxicity was found in 3 patients (1.2%) after a mean of 4 months of treatment. Aminoglycosides (streptomycin, kanamycin, or amikacin) were withdrawn in these patients.
The overall success rate of MDR-TB treatment was approximately 85.9% (220 patients), with 14.1% (36 patients) of the patients experiencing poor treatment outcomes. Two-hundred five participants (80.2%) were “cured,” 15 (5.8%) “completed” the treatment, 29 (11.3%) had “failed treatment,” and 7 (2.7%) died.
4. Discussion
Among patients with drug susceptible TB, the success rate of treatment with short-course chemotherapy is expected to be at least 85%. Patients showed good tolerance to the treatments and exhibited few side effects.[5] However, MDR-TB therapy has a long treatment period (18–24 months) and the number of drugs used for treatment is relatively higher than for drug susceptible TB, and thus many side effects are known.[6–8] In our study, we evaluated side effects observed during the treatment of MDR-TB.
Most side effects occurred relatively infrequently in our study compared to that observed in previous reports.[9,10] Goble et al[10] reported that 30% of patients had side effects requiring withdrawal of 1 or more TB medications. In our study, side effects were noted in 95 of the 256 patients (37.1%), and treatment had to be modified due to uncontrolled side effects in 54 patients (21.1%); this is lower than that observed in previous studies. The lower frequency of certain side effects and of the discontinuation of therapy may be attributable to a number of factors. First, almost all of the patients were treated for MDR-TB in the hospital. This permitted close monitoring of side effects and prompt implementation of management strategies designed to minimize these effects. Second, our team educated our patients regarding the severity of their disease and the absence of options for cure.
The most common side effects were complaints of GI disturbance after MDR-TB treatment (18.4%). This is consistent with previous reports. GI disturbance was most pronounced after treatment with ethambutol and PAS. Most GI side effects can be managed without stopping the drug by escalating the dose, dividing the dose, or with the use of antiemetics. If the GI symptoms were not severe, we added H2 receptor blockers or antiemetic agents to the regimen. However, if symptoms such as nausea, vomiting, or loss of appetite were severe, PAS was withdrawn.[11]
Some drugs used for MDR-TB treatment cause psychiatric disorder. Psychosis has been reported as a side effect of INH, ethambutol, fluoroquinolones, and CS. Depression psychosis has been reported as side effect primarily associated with CS.[12–16] In response to depression and psychosis, we initially used antidepressant or anticonvulsant therapy after the case was discussed with a specialist physician. In cases where additional medication failed to control psychiatric side effects, we withdrew or suspended CS until the symptoms were resolved. In our study, psychiatric disorders (5.5%) were the second most common reason leading to drug withdrawal. Nine out of all the patients with depression (3.5%) were no longer depressed after 6 months of the above-mentioned therapy.
Hepatotoxicity is the most common factor associated with the discontinuation or suspension of the use of MDR-TB drugs. The manifestations of drug-induced hepatotoxicity are highly variable, and range from asymptomatic elevation of liver enzymes to fulminant hepatic failure. It is also important to consider the possibility that TB itself can invade the liver and result in abnormal liver function. In this case, treatment of TB can improve liver function.[3,11] When there is hepatotoxicity associated with the use of a drug, the drug leading to hepatotoxicity is suspended until liver function tests return to baseline. Gradually introducing the drugs by administering them in increasing numbers and dosages is recommended. The drugs most commonly associated with hepatotoxicity should be added last.
Fluoroquinolones now play an important role in the treatment of TB, particularly MDR-TB. Fluoroquinolones inhibit DNA gyrases and thus prevent bacterial DNA synthesis. They thus have substantial in vitro activity against M tuberculosis. Ciprofloxacin and ofloxacin have been used for the treatment of TB, although recent studies have shown that levofloxacin and moxifloxacin, which are quinolones, are more effective.[11,15] We thus preferred to retain fluoroquinolones in the drug regimens while controlling their side effects.
Somashekar et al have reported the occurrence of drug-induced hypothyroidism during treatment for MDR-TB. Among the 133 patients who received regimens containing ethambutol, 42 (32%) developed hypothyroidism. Among the 17 patients that received regimens containing PAS, 6 (35%) developed hypothyroidism.[16] Furin et al[9] have reported a hypothyroidism incidence of approximately 10% in their study, while hypothyroidism was found in 2.3% of our patients. Hypothyroidism might be underestimated in our study, as thyroid stimulating hormone (TSH) was measured only in symptomatic patients. To prevent hypothyroidism, if possible, routine TSH testing should be considered for all patients receiving treatment for MDR TB.
Pyrazinamide is the most common cause of arthralgia, arthritis, and gout. These side effects may develop as multiple joint pain in the shoulders or knees within the first 2 months after using the drug. Arthralgia and arthritis developed at a mean time of 2 months into the treatment. Symptoms of pyrazinamide-induced arthralgia may be reduced by concomitant treatment with NSAIDs, although pyrazinamide should be withdrawn if acute gout attacks occur.[11,17,18] In our study, these symptoms developed at a mean duration of 1.8 months into the treatment, which is consistent with previous reports. These symptoms disappeared with NSAID use in 7 of 9 patients, but pyrazinamide was withdrawn in 2 patients due to uncontrolled acute gout attacks.
There are several limitations to our study. First, the study was retrospective. Second, we only evaluated patients from a single TB referral hospital, which may have introduced selection bias; this also limits the extrapolation of our findings to the entire population of South Korean patients. In spite of these limitations, we believe that our study provides important information regarding the side effects of second-line anti-TB drugs in resource-poor settings.
The various side effects of anti-TB drugs can lead to withdrawal or suspension of the use of these drugs in patients with MDR-TB. The appropriate treatments for drug side effects in MDR-TB are important key determining factors for the success or failure of treatment. Our study might thus help to develop strategies for the timely and aggressive management of drug side effects, which may then reduce suspension of therapy and increase rates of cure.
Acknowledgments
We acknowledge the outstanding contributions of the technicians and nursing staff at the National Masan Tuberculosis Hospital. We greatly thank Dr. Dae Yeon Kim, Director of Department of Chest Medicine, National Masan Tuberculosis Hospital, South Korea for his leadership.
Footnotes
Abbreviations: CNS = central nervous system, CS = cycloserine, DST = drug susceptibility testing, GI = gastrointestinal, INH = isoniazid, MDR-TB = multidrug-resistant tuberculosis, NSAIDs = nonsteroidal antiinflammatory drugs, PAS = para-aminosalicylic acid, PTH = prothionamide, RFP = rifampicin, TB = tuberculosis, TSH = thyroid stimulating hormone.
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization (WHO). Global tuberculosis report 2016. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 12 May 2017. [Google Scholar]
- [2].Park HO, Kim SW, Moon SH, et al. Association between body mass index and sputum culture conversion among South Korean patients with multidrug resistant tuberculosis in a tuberculosis referral hospital. Infect Chemother 2016;48:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization (WHO). Definitions and reporting framework for tuberculosis—2013 revision. Available at: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf. Accessed 6 Jun 2017. [Google Scholar]
- [4].Torun T, Gungor G, Ozmen I, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Teberc Lung Dis 2005;9:1373–7. [PubMed] [Google Scholar]
- [5].American Thoracic Society/Centers for Disease Control, Prevention/Infectious Disease Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med 2003;167:603–62. [DOI] [PubMed] [Google Scholar]
- [6].Iseman MD. Treatment of multidrug-resistant tuberculosis. N Engl J Med 1993;329:784–91. [DOI] [PubMed] [Google Scholar]
- [7].Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 2004;363:474–81. [DOI] [PubMed] [Google Scholar]
- [8].Tahaoğlu K, Törün T, Sevim T, et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med 2001;345:170–4. [DOI] [PubMed] [Google Scholar]
- [9].Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2001;5:648–55. [PubMed] [Google Scholar]
- [10].Goble M, Iseman MD, Madsen LA, et al. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med 1993;328:527–32. [DOI] [PubMed] [Google Scholar]
- [11].Park WS, Kang HY, Kim SJ, et al. Notified tuberculosis status in Korea, 2015. Public Health Weekly Report, KCD 2015;9:342–5. [Google Scholar]
- [12].Leston JM, Rey JC, Gonzalez Montaner LJ, et al. Psychosomatic reactions to cycloserine in the treatment of tuberculosis. Scand J Respir Dis 1970;71:231–4. [PubMed] [Google Scholar]
- [13].Vega P, Sweetland A, Acha J, et al. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004;8:749–59. [PubMed] [Google Scholar]
- [14].Parsad R, Rajiv G, Sanjay KV. Isoniazid- and ethambutol-induced psychosis. Ann Thorac Med 2008;3:149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 2000;117:744–51. [DOI] [PubMed] [Google Scholar]
- [16].Somashekar M, Singarajipura A, Balaji N, et al. Drug-induced hypothyroidism during anti-tuberculosis treatment of multidrug-resistant tuberculosis: notes from the field. J Tuberc Res 2016;4:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qureshi W, Hassan G, Kadri SM, et al. Hyperuricemia and arthralgia during pyrazinamide therapy in patients with pulmonary tuberculosis. Lab Med 2007;38:495–7. [Google Scholar]
- [18].Pham AQ, Doan A, Andersen M. Pyrazinamide-induced hyperuricemia. P T 2014;39:695–7. [PMC free article] [PubMed] [Google Scholar]


