Abstract
To determine cerebrovascular risk factors for patients with cerebral watershed infarction (CWI) from Southwest China.
Patients suffering from acute ischemic stroke were categorized into internal CWI (I-CWI), external CWI (E-CWI), or non-CWI (patients without CWI) groups. Clinical data were collected and degrees of steno-occlusion of all cerebral arteries were scored. Arteries associated with the circle of Willis were also assessed. Data were compared using Pearson chi-squared tests for categorical data and 1-way analysis of variance with Bonferroni post hoc tests for continuous data, as appropriate. Multivariate binary logistic regression analysis was performed to determine independent cerebrovascular risk factors for CWI.
Compared with non-CWI, I-CWI had higher degrees of steno-occlusion of the ipsilateral middle cerebral artery, ipsilateral carotid artery, and contralateral middle cerebral artery. E-CWI showed no significant differences. All the 3 arteries were independent cerebrovascular risk factors for I-CWI confirmed by multivariate binary logistic regression analysis. I-CWI had higher degrees of steno-occlusion of the ipsilateral middle cerebral artery compared with E-CWI. No significant differences were found among arteries associated with the circle of Willis.
The ipsilateral middle cerebral artery, carotid artery, and contralateral middle cerebral artery were independent cerebrovascular risk factors for I-CWI. No cerebrovascular risk factor was identified for E-CWI.
Keywords: carotid artery, cerebral infarction, circle of Willis, computed tomography angiography, middle cerebral artery, risk factor
1. Introduction
Cerebral watershed infarction (CWI) (also called border zone infarction) is an ischemic lesion that involves the junction between 2 adjacent arterial territories. It was first reported by Pozzi in 1884. Zulch and Behrend determined its typical locations and hypothesized its pathogenesis in the 1960s. CWI is well known for its particular morphological characteristics and locations. It is reported to account for approximately 12.7% of all cerebral infarcts[1] and may occur in situations involving severe systemic hypotension, such as when a person rises from the supine position, excess exercise, Valsalva's maneuvers, administration of antihypertensive drugs, bleeding, and anemia.[2] The prognosis of CWI is reportedly different from other types of cerebral infarction after intravenous thrombolysis therapy.[3]
The pathogenesis of CWI has been studied for more than 50 years and still remains controversial. It is reported that CWI, especially internal CWI (I-CWI), is typically caused by systemic or local hypoperfusion.[1,4–6] A positron emission tomography study reported that metabolic activity levels, a sign of blood perfusion, significantly decreased around CWI,[7] and an autopsy study reported cholesterol microemboli in external CWI (E-CWI) patients.[8] Some researchers think that CWI is an interplay between microemboli, from the heart or atherosclerotic artery, and a reduction in perfusion.[9] Microemboli favors the watershed zone, but cannot be cleared because of reduced perfusion, blocking distal arteries and resulting in a cerebral infarction.[10–12] Extracranial and intracranial atherosclerotic arteries cannot only reduce blood perfusion through severe vascular stenosis, but also occlude small arteries when unstable atherosclerotic plaques detach into the bloodstream.[13] Cerebrovascular changes may be critical risk factors in CWI patients.
Increasingly, researchers are realizing the importance of severe cerebrovascular changes for CWI.[14] Vascular variations (including vascular tortuosity and hypoplasticity) and collateral circulation (including the circle of Willis [CoW], reversed flow in the ophthalmic artery, and collateral flow via the leptomeningeal arteries) may also play important roles in CWI. The CoW is the primary and most important collateral circulation, and a considerable number of CoW variations have been reported among Chinese populations.[15] Yet, no systemic research has looked at all the cerebrovascular changes in CWI patients.
Computed tomography angiography (CTA) is capable of delineating major intracranial vessels, very small arteries, and whole cerebral vasculature.[16] It is a noninvasive vascular examination that does not rely on hemodynamics, and can clearly depict both the intracranial and extracranial vasculature. The sensitivity and specificity of CTA are widely accepted.[17–20]
To better understand the pathogenesis of CWI and provide a basis for its treatment and prevention, we examined the cerebral vasculature of patients with cerebral infarctions and determined cerebrovascular risk factors for CWI using CTA.
2. Methods
2.1. Participants
A total of 1989 consecutive patients with acute ischemic infarctions at the Department of Neurology, the First Affiliated Hospital of Chongqing Medical University between June 2011 and May 2016 met inclusion criteria and were enrolled in the study. Inclusion criteria were clinically diagnosed acute ischemic infarction; magnetic resonance imaging performed within 1 week after the stroke, including a diffusion weighted imaging sequence; and CTA examination was performed within 1 week after the stroke. There were 926 patients who met exclusion criteria and who were excluded. Exclusion criteria were no new infarctions, infarctions in both hemispheres, subtentorial infarctions, or encephalomalacia focus; history of stroke, surgery, injury, or coexisting cerebral diseases (including tumor, vascular malformation, aneurysm, Moyamoya disease, or venous sinus thrombosis); underwent a neurointerventional procedure (including injecting tissue plasminogen activator, mechanical clot removal, angioplasty, or stenting); and poor CTA image quality that prevented visualization of the whole cerebral vasculature.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and written informed consent was obtained from patients or their guardians. The research protocol was performed in accordance with relevant ethical guidelines and regulations for human studies.
2.2. Demographic characteristics
Patients were categorized into either I-CWI, E-CWI, or non-CWI (patients without CWI) groups according to the criteria defined by Mangla et al[21] (Fig. 1) based on localization determined from diffusion-weighted imaging sequences. Briefly, I-CWI was defined as an infarction of the lenticulostriate-middle cerebral artery (MCA), lenticulostriate-anterior cerebral artery (ACA), Heubner-ACA, anterior choroidal-MCA, and anterior choroidal-posterior cerebral artery (PCA) territories. E-CWI was defined as an infarction located in the frontal cortex (between the ACA and MCA), occipital cortex (between the MCA and PCA), or paramedian white matter (between the ACA and MCA). Demographic characteristics of all patients were recorded by a third-party neurological physician. All definitions and diagnoses met international criteria.[22,23]
Figure 1.
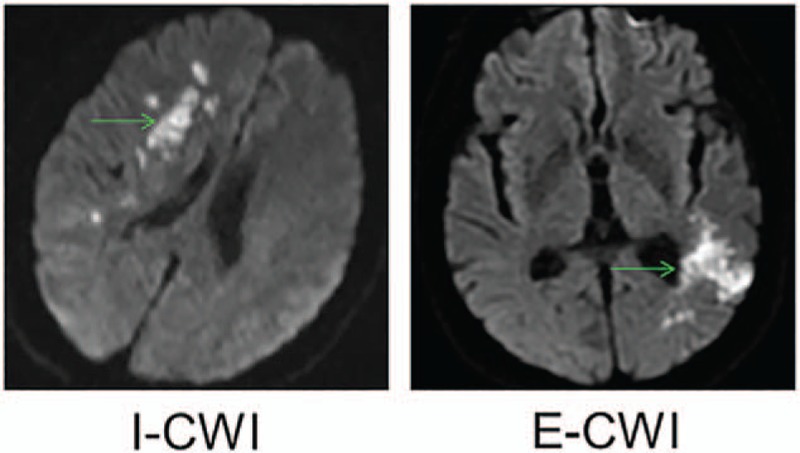
Typical internal and external cerebral watershed infarction showed in diffusion-weighted imaging sequences. E-CWI = infarction localized to the area between the middle cerebral artery and posterior cerebral artery on the left, I-CWI = infarction localized to the area between the lenticulostriate and middle cerebral artery on the right.
2.3. Image acquisition
CTA examinations were performed using a 64-row multidetector computed tomography scanner (Light-Speed VCT; GE Healthcare, Milwaukee, WI). Scanning parameters were tube voltage, 100 or 120 kV; tube current, 300 mA; pitch, 0.531; matrix, 512 × 512; section thickness, 5 mm; and reconstruction slice thickness, 0.625. Patients were in the supine position on a computed tomography table with a head holder. Contrast material (80 mL) was injected at 4 mL/s using a power injector through an intravenous cannula. Synchronization of data acquisition was achieved using the real-time bolus tracking method. Images were reconstructed and transferred to a workstation for postprocessing. Images were reformatted as maximum intensity projections and volume-rendered images to evaluate the CoW. Two observers independently reviewed all CTA data. When disagreements occurred, they were resolved via discussion until a consensus was reached.
2.4. Vascular data
The percentage of stenosis was used to score arteries,[24] and was calculated as: percentage of stenosis = 1 − Dstenosis/Dnormal, where Dstenosis indicated the diameter of the most severe site of the artery and Dnormal was the diameter of a proximal normal artery. A score of 0 was given for stenosis percentage <50%, a score of 1 was given for stenosis percentage ≥50% and <70%, a score of 2 was given for stenosis percentage ≥70% and <100%, and a score of 3 was given when the artery was occluded (Fig. 2). The following arteries were examined in bilateral hemispheres: internal carotid artery or common carotid artery, vertebral artery, basilar artery, ACA, MCA, and PCA. Arteries associated with the CoW were categorized as absent, hypoplastic, or normal. Hypoplastic indicated that the diameter was <1 mm and had a string-like appearance. The anterior communicating artery and the posterior communicating arteries (PComA) were regarded as normal upon observation if their diameters were small. The following cerebral vessels associated with CoW were examined: anterior communicating artery, PComA, precommunicating segments of the ACA (ACA-A1), and precommunicating segments of the PCA (PCA-P1). Fetal-type PCA (FTP) was defined as an artery that originated at the internal carotid artery and continued as a PCA (Fig. 3). Uni-FTP was defined as a unilateral FTP and bi-FTP as a bilateral FTP.
Figure 2.
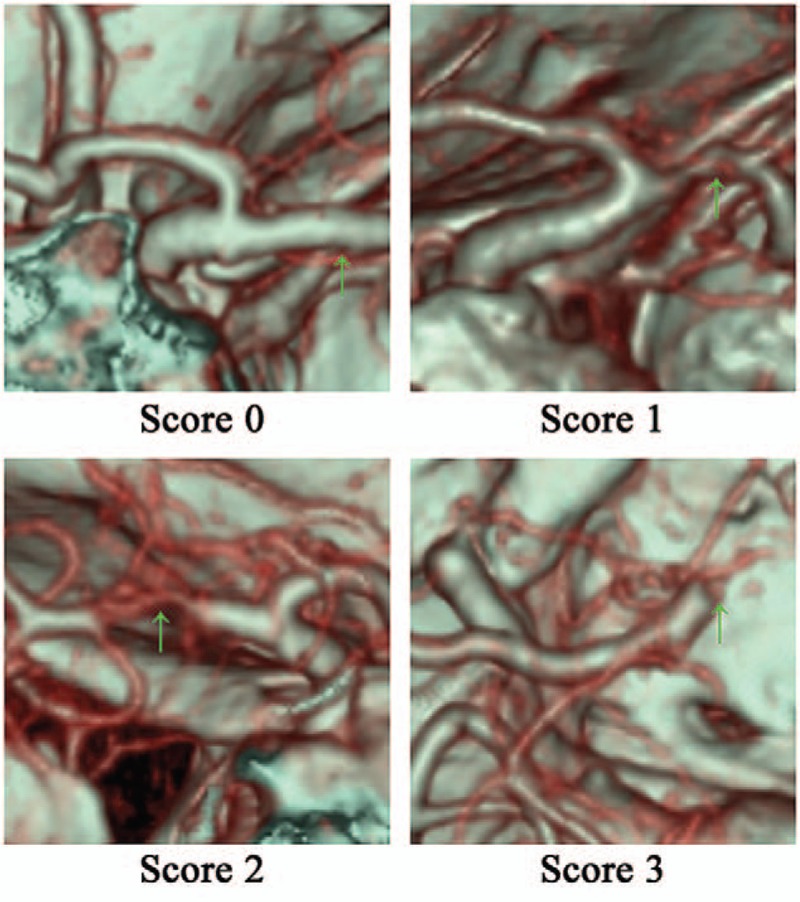
Scores based on the steno-occlusion degree of the MCA. MCA = middle cerebral artery, Score 0 = the right MCA is nearly normal or <50% occluded, Score 1 = the right MCA was ≥50% but <70% occluded, Score 2 = the left MCA was ≥70% but was not completely occluded, Score 3 = the right MCA was completely occluded.
Figure 3.
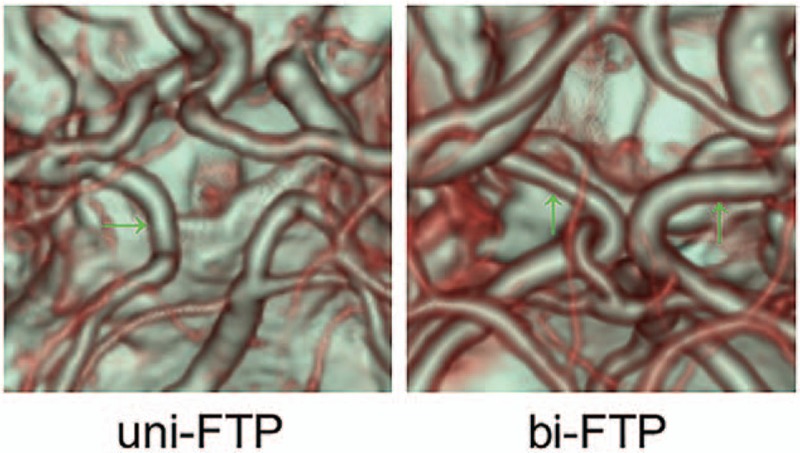
Fetal-type PCA showed in computed tomography angiography. bi-FTP = patient had a bilateral FTP with the PCA-P1 absent on both sides, PCA = posterior cerebral artery, uni-FTP = patient had a unilateral FTP with an absent PCA-P1 on the left.
2.5. Statistical analysis
Statistical analyses were performed using a commercially available software package (SPSS, ver. 22.0; IBM, New York, NY). Categorical data are exhibited as absolute numbers and percentage (%) and continuous data are exhibited as mean ± standard error. Data were compared among groups using Pearson chi-squared tests or Fisher exact tests for categorical data and 1-way analysis of variance with Bonferroni post hoc tests for continuous data, as appropriate. Multivariate binary logistic regression analysis was performed to determine independent cerebrovascular risk factors for CWI. A P value < .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
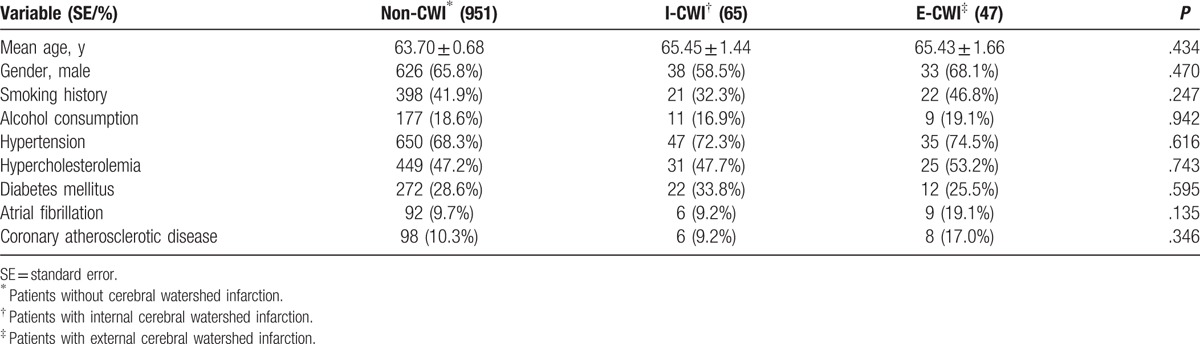
In total, 1063 patients with cerebral infarction were included. CWI was present in 112 (10.5%) patients, which is approximately equal to the 10% reported in the literature.[1] Forty-seven patients had E-CWI and 65 had I-CWI. Demographic characteristics are shown in Table 1. The 3 groups were sex and age matched (P > .05). There were no significant differences in demographic characteristics among the groups (P > .05).
Table 1.
Demographic characteristics of patients with different types of cerebral infarction.
3.2. Vascular steno-occlusion
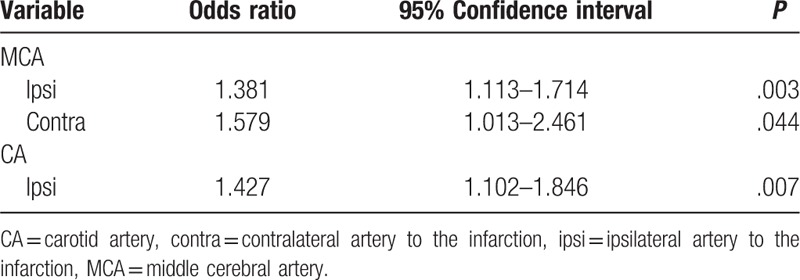
Vascular steno-occlusion data are shown in Table 2. Compared with non-CWI, I-CWI had higher degrees of steno-occlusion of the ipsilateral MCA, contralateral MCA, and ipsilateral carotid artery. There was no statistical significant difference with E-CWI (P > .05). Multivariate binary logistic regression analysis indicated that all 3 arteries were independent cerebrovascular risk factors for I-CWI (P < .05) (Table 3). I-CWI showed more severe degrees of steno-occlusion of the ipsilateral MCA compared with E-CWI (P < .05).
Table 2.
Vascular steno-occlusion degree of the mainly supplying arteries in different types of cerebral infarction.
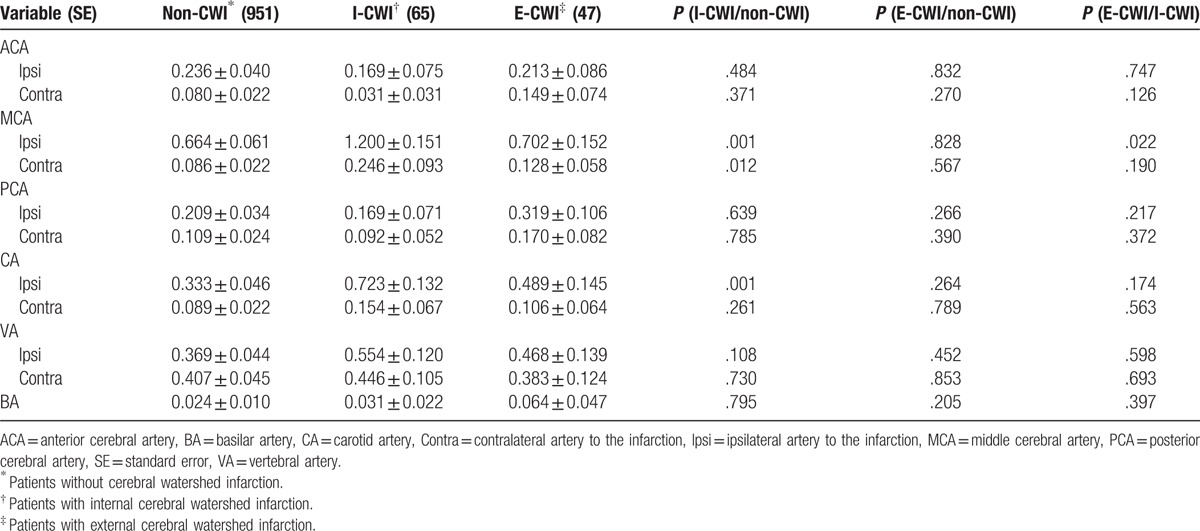
Table 3.
Multivariate binary logistic regression analysis of cerebrovascular risk factors for internal cerebral watershed infarction.
3.3. Variations in arteries associated with the CoW
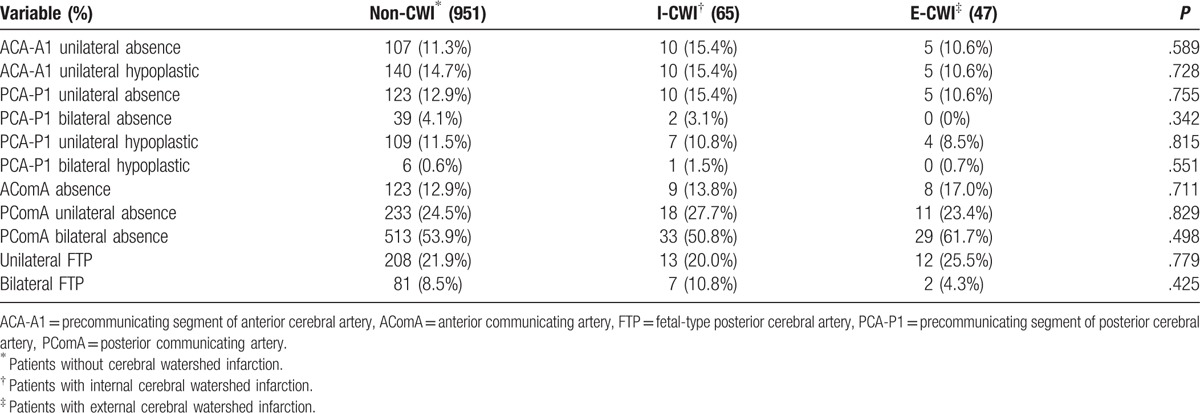
The variations in arteries associated with the CoW are shown in Table 4. There were no significant differences among groups (P > .05). FTP was common among the Chinese population with cerebral infarction (uni-FTP = 21.9%, bi-FTP = 8.5%) but there were no differences among the groups (P > .05).
Table 4.
Variations of the arteries associated with circle of Willis.
4. Discussion
CWI is recognized for its particular morphological characteristics and locations. It usually occurs in specific situations and is a result of unique risk factors. In the present study, we did not find any risk factors for CWI from the demographic characteristics. This may be because we only examined persistent risk factors rather than transient inducements. Transient inducements, including rising from a supine position, Valsalva's maneuvers, administration of antihypertensive drugs, and bleeding, for example, may be critical risk factors for CWI.
A study from the Netherlands used magnetic resonance angiography combined with magnetic resonance spectroscopy and found that CWI patients with severe carotid stenosis had a significantly reduced blood flow in both the common carotid artery and MCA, and decreased N-acetyl aspartate/choline ratios in noninfarct regions,[25] indicating that severe carotid stenosis might be an important risk factor for CWI in western populations. To the best of our knowledge, western populations are more susceptible to extracranial vascular diseases, while intracranial atherosclerotic diseases are more common among Asians.
In the present study, we confirmed that the ipsilateral carotid artery was an independent risk factor for I-CWI. However, we also found that both ipsilateral and contralateral MCA were independent cerebrovascular risk factors for I-CWI. Ipsilateral MCA has been confirmed as a risk factor in a positron emission tomography study from Japan[4] and a magnetic resonance angiography study from South Korea[26] in Asian populations. The involvement of the contralateral MCA, however, is a novel finding of the present study. It is thought that the contralateral MCA may indicate extensive cerebrovascular changes in I-CWI, leading to persistent hypoperfusion of brain tissue through collateral circulation at different levels, with I-CWI arising if any transient inducements occur during such conditions.
We did not identify any cerebrovascular risk factors for E-CWI. E-CWI patients shared the same degrees of cerebrovascular steno-occlusion compared with non-CWI. The degree of steno-occlusion of the ipsilateral MCA in E-CWI was found to be milder than in I-CWI. This may be because E-CWI is mainly associated with potential cardiac embolic disease while I-CWI is mainly associated with atherosclerotic MCA disease, a suggestion consistent with a magnetic resonance angiography study from South Korea.[27]
Not all severe vascular steno-occlusions lead to ischemic stroke. Many patients with severe common carotid artery or MCA stenosis do not show any clinical symptoms until they undergo a physical examination. This is because collateral circulation in the brain plays an important role in preventing stroke.[28]
The CoW is the primary and most important collateral circulation, and understanding its involvement in stroke is very important. The absence of PComA was common in the population of the present study. PComA is reported to prevent infarction upon occlusion of the common carotid artery.[29] However, no significant differences were found among the arteries associated with the CoW in the present study. The CoW did not seem to have any unique compensatory functions in CWI patients from Southwest China. A probable explanation for this result is that CWI in people from Southwest China is mainly related to MCA while MCA is distal to the CoW. Considering ethnic differences, the function of the CoW in western populations with CWI requires further research as extracranial atherosclerotic disease is more common these populations.[30,31]
This study had some limitations. First, we did not investigate the clinical symptoms in CWI patients and their relationship to the cerebrovascular changes. Second, we only included patients from 1 hospital in Southwest China. A multicenter study is required. Third, CTA is reported to overestimate vascular stenosis compared with digital subtraction angiography; therefore, the epidemiological results of cerebrovascular changes should be treated with caution.
5. Conclusions
Ipsilateral MCA, carotid artery, and contralateral MCA were independent cerebrovascular risk factors for I-CWI. No cerebrovascular risk factor was identified for E-CWI. The CoW had no relationship with CWI.
Footnotes
Abbreviations: ACA = anterior cerebral artery, ACA-A1 = precommunicating segment of the ACA, CoW = circle of Willis, CTA = computed tomography angiography, CWI = cerebral watershed infarction, E-CWI = external CWI, FTP = fetal-type PCA, I-CWI = internal CWI, MCA = middle cerebral artery, PCA = posterior cerebral artery, PCA-P1 = precommunicating segment of the PCA, PComA = posterior communicating artery.
This work was supported by the National Key Clinical Specialties Construction Program of China, and Chongqing Science and Technology Commission (No. cstc2016jcy jA0423).
The authors have no conflicts of interest to disclose.
References
- [1].Yong SW, Bang OY, Lee PH, et al. Internal and cortical border-zone infarction: clinical and diffusion-weighted imaging features. Stroke 2006;37:841–6. [DOI] [PubMed] [Google Scholar]
- [2].D’Amore C, Paciaroni M. Border-zone and watershed infarctions. Front Neurol Neurosci 2012;30:181–4. [DOI] [PubMed] [Google Scholar]
- [3].Liu D, Scalzo F, Starkman S, et al. DWI lesion patterns predict outcome in stroke patients with thrombolysis. Cerebrovasc Dis 2015;40:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamauchi H, Nishii R, Higashi T, et al. Hemodynamic compromise as a cause of internal border-zone infarction and cortical neuronal damage in atherosclerotic middle cerebral artery disease. Stroke 2009;40:3730–5. [DOI] [PubMed] [Google Scholar]
- [5].Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke 2005;36:567–77. [DOI] [PubMed] [Google Scholar]
- [6].Siemund R, Cronqvist M, Andsberg G, et al. Cerebral perfusion imaging in hemodynamic stroke: be aware of the pattern. Interv Neuroradiol 2009;15:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamauchi H, Kudoh T, Kishibe Y, et al. Selective neuronal damage and borderzone infarction in carotid artery occlusive disease: a 11C-flumazenil PET study. J Nucl Med 2005;46:1973–9. [PubMed] [Google Scholar]
- [8].Masuda J, Yutani C, Ogata J, et al. Atheromatous embolism in the brain: a clinicopathologic analysis of 15 autopsy cases. Neurology 1994;44:1231–7. [DOI] [PubMed] [Google Scholar]
- [9].Maki T, Wakita H, Mase M, et al. Watershed infarcts in a multiple microembolic model of monkey. Neurosci Lett 2011;499:80–3. [DOI] [PubMed] [Google Scholar]
- [10].Pollanen MS, Deck JH. The mechanism of embolic watershed infarction: experimental studies. Can J Neurol Sci 1990;17:395–8. [DOI] [PubMed] [Google Scholar]
- [11].Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82. [DOI] [PubMed] [Google Scholar]
- [12].Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol 2002;52:74–81. [DOI] [PubMed] [Google Scholar]
- [13].Moustafa RR, Izquierdo-Garcia D, Jones PS, et al. Watershed infarcts in transient ischemic attack/minor stroke with > or = 50% carotid stenosis: hemodynamic or embolic? Stroke 2010;41:1410–6. [DOI] [PubMed] [Google Scholar]
- [14].Howard R, Trend P, Russell RW. Clinical features of ischemia in cerebral arterial border zones after periods of reduced cerebral blood flow. Arch Neurol 1987;44:934–40. [DOI] [PubMed] [Google Scholar]
- [15].Li Q, Li J, Lv F, et al. A multidetector CT angiography study of variations in the circle of Willis in a Chinese population. J Clin Neurosci 2011;18:379–83. [DOI] [PubMed] [Google Scholar]
- [16].Li Q, Lv F, Wei Y, et al. Automated subtraction CT angiography for visualization of the whole brain vasculature: a feasibility study. Acad Radiol 2013;20:1009–14. [DOI] [PubMed] [Google Scholar]
- [17].van der Lugt A, Buter TC, Govaere F, et al. Accuracy of CT angiography in the assessment of a fetal origin of the posterior cerebral artery. Eur Radiol 2004;14:1627–33. [DOI] [PubMed] [Google Scholar]
- [18].Waaijer A, van Leeuwen MS, van der Worp HB, et al. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidetector row CT angiography. Cerebrovasc Dis 2007;23:267–74. [DOI] [PubMed] [Google Scholar]
- [19].Wintermark M, Meuli R, Browaeys P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology 2007;68:694–7. [DOI] [PubMed] [Google Scholar]
- [20].Ovesen C, Christensen AF, Havsteen I, et al. Prediction and prognostication of neurological deterioration in patients with acute ICH: a hospital-based cohort study. Bmj Open 2015;5:e008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mangla R, Kolar B, Almast J, et al. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics 2011;31:1201–14. [DOI] [PubMed] [Google Scholar]
- [22].Hu L, Dong MX, Zhao H, et al. Fibulin-5: a novel biomarker for evaluating severity and predicting prognosis in patients with acute intracerebral haemorrhage. Eur J Neurol 2016;23:1195–201. [DOI] [PubMed] [Google Scholar]
- [23].Dong MX, Hu QC, Shen P, et al. Recombinant tissue plasminogen activator induces neurological side effects independent on thrombolysis in mechanical animal models of focal cerebral infarction: a systematic review and meta-analysis. PLoS ONE 2016;11:e0158848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barnett HJM, Taylor DW, Haynes RB, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53. [DOI] [PubMed] [Google Scholar]
- [25].van der Grond J, van Everdingen KJ, Eikelboom BC, et al. Assessment of borderzone ischemia with a combined MR imaging-MR angiography-MR spectroscopy protocol. J Magn Reson Imaging 1999;9:1–9. [DOI] [PubMed] [Google Scholar]
- [26].Kim DE, Lee KB, Roh H, et al. Association of internal border zone infarction with middle cerebral artery steno-occlusion. Eur Neurol 2010;64:178–85. [DOI] [PubMed] [Google Scholar]
- [27].Lee PH, Oh SH, Bang OY, et al. Isolated middle cerebral artery disease: clinical and neuroradiological features depending on the pathogenesis. J Neurol Neurosurg Psychiatry 2004;75:727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liebeskind DS. Collateral circulation. Stroke 2003;34:2279–84. [DOI] [PubMed] [Google Scholar]
- [29].Hendrikse J, Hartkamp MJ, Hillen B, et al. Collateral ability of the circle of Willis in patients with unilateral internal carotid artery occlusion: border zone infarcts and clinical symptoms. Stroke 2001;32:2768–73. [DOI] [PubMed] [Google Scholar]
- [30].Lee PH, Oh SH, Bang OY, et al. Infarct patterns in atherosclerotic middle cerebral artery versus internal carotid artery disease. Neurology 2004;62:1291–6. [DOI] [PubMed] [Google Scholar]
- [31].Del Sette M, Eliasziw M, Streifler JY, et al. Internal borderzone infarction: a marker for severe stenosis in patients with symptomatic internal carotid artery disease. For the North American Symptomatic Carotid Endarterectomy (NASCET) Group. Stroke 2000;31:631–6. [DOI] [PubMed] [Google Scholar]


