Abstract
Pre-eclampsia (PE) is one of the leading causes of maternal and neonatal morbidity and mortality. In recent years, many studies have shown that microRNAs (miRNA) play important roles in the development of PE. However, the molecular pathogenesis of PE remains unknown.
In the present study, we performed a case–control study to verify the differential expression of 4 candidate miRNAs (miR-210, miR-155, miR-125b-5p, and miR-125a-5p) in 20 PE pregnancies and 20 healthy pregnancies. The real-time quantitative reverse transcriptase-polymerase chain reaction has been utilized to estimate the Ct values in both groups.
Our results have shown that miR-210 and miR-155 were upregulated in serum of PE pregnancies, which suggest a potential association between these 2 miRNAs and the pathogenesis of PE. Further studies showed that the area under the receiver operating characteristic curve (AUC) of miR-210 and miR-155 were 0.750 and 0.703, respectively. The AUC of the expression ratio of miR-210 (serum/urine) and miR-155 (serum/urine) were 0.761 and 0.718, respectively. Moreover, 24-hour urine proteins have positive correlation with urine miR-210 and miR-155.
Our findings indicated that serum miR-210 and miR-155 could be 2 sensitivity and specificity biomarkers for the diagnosis of PE while urine miR-210 and miR-155 both could be used to evaluate the severity of kidney injury. Using these miRNAs may provide a novel diagnosis method for identifying pregnant women who are at risk for developing PE.
Keywords: diagnostic markers, microRNAs, pre-eclampsia, serum, urine
1. Introduction
Pre-eclampsia (PE) is a pregnancy-specific syndrome associated with hypertension, proteinuria, thrombocytopenia, or renal insufficiency.[1] It affects 2% to 5% of pregnancies worldwide and causes 10% to 15% of maternal deaths.[2,3] The occurrence, development, and diagnosis of PE have become a challenging issue in clinical research today. However, current diagnosis still depends on the results of measuring blood pressure, blood tests, and urine analysis (proteinuria). Therefore, to discover specific biomarkers for early diagnosis of PE has become a hot topic in present studies. In recent years, many studies have been designed to identify putative biomarkers for accurate and timely diagnosing PE. Previous studies have demonstrated that the dysregulation of the specific plasma-related miRNAs expression (miR-210, miR-155, miR-125b-5p, and miR-125a-5p) is associated with the development of PE.[4–7] In the present study, we investigated the expression level of miR-210, miR-155, miR-125b-5p, and miR-125a-5p in the serum and urine of PE pregnancies compared with healthy pregnancies in order to find novel candidates that bear a greater potential to identify women at risk for PE. Analyzing these miRNAs may improve our understanding of the pathophysiological mechanisms of PE.
2. Materials and methods
2.1. Pregnant women and samples collection
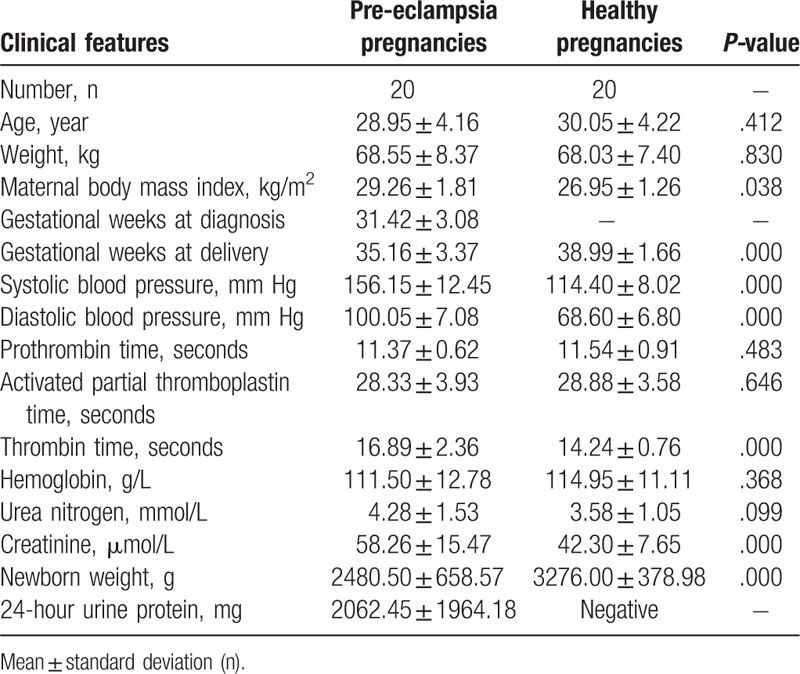
A total of 40 serum and urine samples were collected from pregnant women who gave birth in the First Affiliated Hospital of Jinan University between November 2015 and October 2016. The serum and urine specimens were collected on admission. According to the clinical data, 20 singleton pregnant women who delivered in the hospital had PE complication, and 20 healthy pregnant women without complications were selected as the control based on similar maternal age at delivery and the similar weight at delivery. A total of 20 pregnant women enrolled in our study were diagnosed with PE. Briefly, PE pregnancies had a systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥110 mm Hg on at least 2 occasions, accompanying severe proteinuria (≥2 g/24 hours) at 20 to 34 weeks of gestation. For the healthy pregnancies, women with any other complications during pregnancy were excluded from this study. As presented in Table 1, there are no significant variations in age and weight between PE pregnancies and healthy pregnancies. However, the systolic blood pressure and the diastolic blood pressure of PE pregnancies are significantly higher than those of the controls. This study met the requirements of the Regional Committee for Medical and Health Research Ethics of the First Affiliated Hospital of Jinan University, and written informed consent was achieved from all pregnant women. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki of 1975, as revised in 2000.
Table 1.
Clinical characteristics and laboratory results.
2.2. Serum and urine collection and RNA preparation
Serum was collected from 20 PE pregnancies and 20 healthy pregnancies after fasting by centrifugation under sterile conditions. A total volume of 30 to 50 mL of midstream morning urine was collected from each subject in a sterile cup and stored at 4°C until processing (<3 hours). Urothelial cells were pelleted from the total urine sample by centrifugation (600×g, 4°C, and 5 minutes), rinsed in phosphate buffer solution, pelleted again, and frozen for storage at −80°C. RNA was extracted from 250 μL serum and the urothelial cells using the Trizol LS reagent (Invitrogen, CA) following the manufacturer's instructions. The RNAs were dissolved in 14 μL RNase-free water. To minimize DNA contamination, we treated the eluted RNA with 5 μL of DNase I (Promega, Shanghai, China) for 30 minutes at 37°C. The RNA concentration and purity were confirmed by the spectrophotometric ratio using absorbance measurements at wavelengths of 260 and 280 nm on a Beckman DU 640UV spectrophotometer (Beckman, Fullerton, CA). The A (260/280) absorbance ratio of isolated RNA was 1.8 to 2.0, demonstrating that the RNA fraction was pure and could be used for analysis.
2.3. Reverse transcription reactions
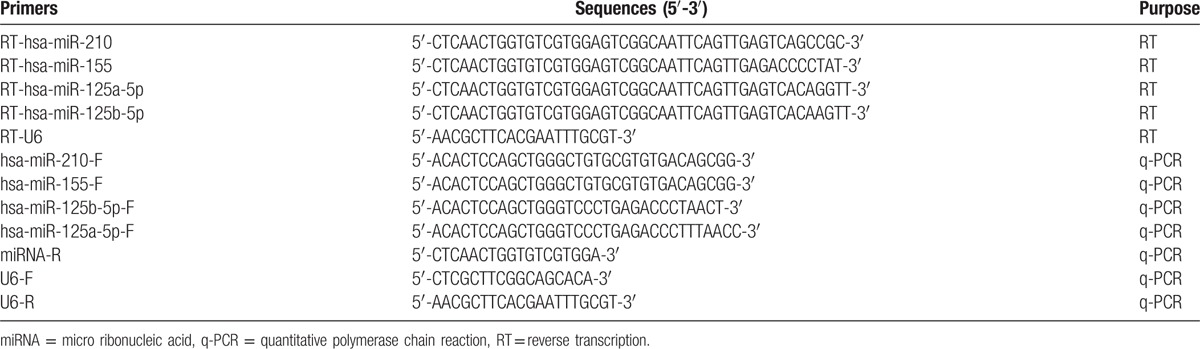
MiR-210, miR-155, miR-125b-5p, miR-125a-5p, and RNA U6-specific cDNA sequences were synthesized from total RNA using gene-specific primers, according to miRBASE database. Reverse transcription (RT) reactions contained 1 μg of total RNA, 50 nmol stem-loop RT primer (Table 2), 4 μL of 5×RT buffer, 2 μL dNTP (10 mmol/L), 5.0 μL 5×buffer, 1 μL of RNase inhibitor, and 1 μL Moloney Murine Leukemia Virus. The 20 μL reaction mixture was initially incubated at 85°C for 5 minutes, 42°C for 60 minutes, and 85°C for 10 minutes, then held at 4°C.
Table 2.
Primers used in the present study.
2.4. Real-time PCR
Expression of miR-210, miR-155, miR-125b-5p, and miR-125a-5p were assessed by the SYBR Green-based real-time polymerized chain reaction (PCR) method. The small nuclear RNA U6 was used as an internal control. Real-time PCR (RT-PCR) analysis was performed using primers 0.5 μL, cDNA template 5 μL, 2×SYBR Green PCR master mix 10 μL and dH2O 4 μL. The analysis was performed using a 7500 Real-Time PCR System (Applied Biosystems, Branchburg, USA). The thermocycling program was of 40 cycles of 95°C for 15 seconds, 60°C for 15 s, and 72°C for 32 seconds with an initial cycle of 95°C for 5 minutes. All PCRs were performed in triplicates. A sample was considered positive if the amplification signal occurred before the 40th threshold cycle. Relative miRNAs expression values were calculated by the 2-ΔCt method.
2.5. Correlation analysis of miRNAs with PE
Univariate and multivariate logistic regressions were performed to observe the correlations between candidate miRNA biomarkers and PE. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic accuracy of selected miRNAs.
2.6. Statistics
All the results were defined as mean ± standard deviation of expression level of miR-210, miR-155, miR-125b-5p, and miR-125a-5p. The one-way analysis of variance test and Bonferroni adjusted Mann–Whitney U test were used to compare miRNAs expression levels between PE pregnancies and healthy pregnancies. Statistical analysis was performed using SPSS 16.0 statistical software (SPSS, Inc, Chicago, IL) and a P-value of <.05 was considered statistically significant.
3. Results
Clinical characteristics of pregnant women
Table 1 shows the main features of PE pregnancies and healthy pregnancies. The median of ages of the pregnant women were 28.95 ± 4.16 (PE pregnancies) and 30.05 ± 4.22 weeks (healthy pregnancies). There were no significant differences between the PE pregnancies and healthy pregnancies with respect to ethnicity, age, weight, partial thromboplastin time, activated partial thromboplastin time, hemoglobin, and urea nitrogen. The days of pregnancy were 246.10 ± 23.60 (PE pregnancies) and 272.95 ± 11.63 weeks (healthy pregnancies) (P <.001).
3.1. Elevation of serum miR-210 level in PE pregnancies
Quantitative RT-PCR was conducted to determine the expression levels of serum and urine miR-210 in PE pregnancies and healthy pregnancies. MiR-210 expression levels were significantly increased in serum of PE pregnancies in comparison with those of controls (t = 2.943, P = .007) (Fig. 1). However, the difference of urine miR-210 expression in PE pregnancies was not significant compared with those of healthy pregnancies (t = –0.408, P = .685) (Fig. 2).
Figure 1.
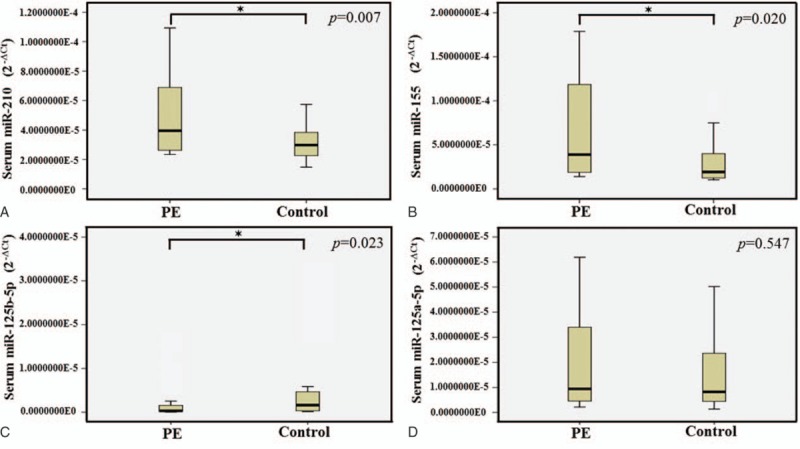
Serum miRNAs selected for verification by real-time PCR in PE pregnancies and healthy pregnancies. The levels of serum miR-210 (A) and miR-155 (B) were significantly higher in the PE pregnancies compared with the controls (P = .007 and P = .020). Serum miR-125b-5p (C) was lower in the PE pregnancies compared with the controls (P = .023), while no significant differences were detected in the expression of miR-125a-5p (D) (P = .547). The expression levels of the miRNAs were normalized to U6 snRNA (2-ΔCt). ∗≤.05. miRNA = micro ribonucleic acid, PE = pre-eclampsia, snRNA = small nuclear ribonucleic acid.
Figure 2.
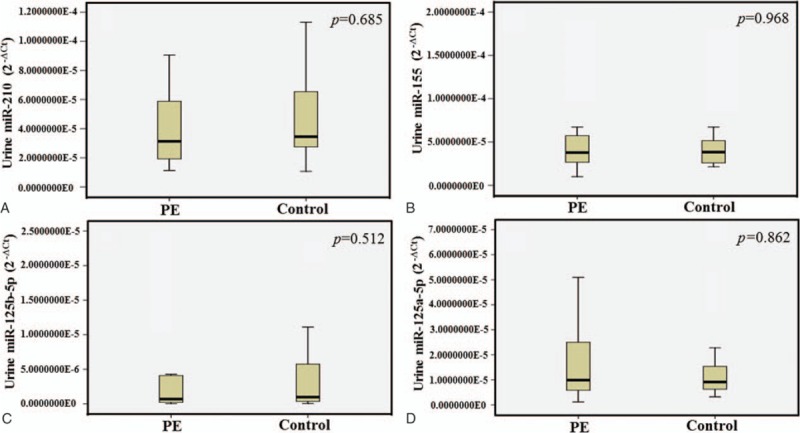
The differential expression levels of urine miRNAs in PE pregnancies versus healthy pregnancies. The levels of urine miR-210 (A) and miR-155 (B) were no different in PE pregnancies and controls (P = .685 and P = .968). Similar results were also obtained from miR-125b-5p and miR-125a-5p. In urine, the levels of miR-125b-5p (C) and miR-125a-5p (D) were no different in PE pregnancies and controls (P = .512 and P = .862). The expression levels of the miRNAs were normalized to U6 snRNA (2−ΔCt). miRNA = micro ribonucleic acid, PE = pre-eclampsia, snRNA = small nuclear ribonucleic acid.
3.2. Elevation of serum miR-155 level in PE pregnancies
To investigate the expression levels of the miR-155 in serum, we measured miR-155 in PE pregnancies and healthy pregnancies. MiR-155 level in serum was significantly increased in PE pregnancies compared to healthy pregnancies (Z = –2.326, P = .020) (Fig. 1), while there was no significant difference in urine between PE pregnancies and healthy pregnancies (Z = –0.054, P = .968) (Fig. 2).
3.3. Expression of miR-125b-5p and miR-125a-5p
Our data showed the expression level of miR-125b-5p in serum is decreased in PE pregnancies compared to controls (Z = –2.272, P = .023) (Fig. 1). However, miR-125a-5p in serum were not different in PE pregnancies and healthy pregnancies (Z = –0.622, P = .547) (Fig. 1). In Fig. 2, the expressions of these 2 miRNAs in urine were not different in PE pregnancies compared with the controls.
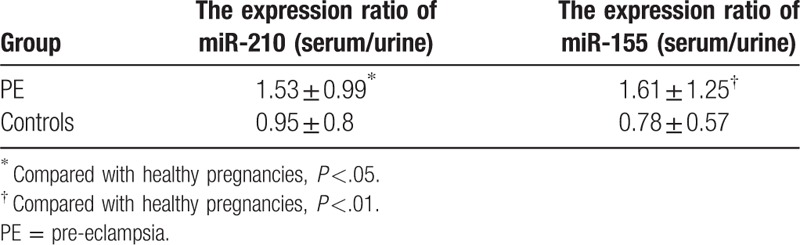
3.4. The expression ratio of miR-210 and miR-155 in serum and urine
The expression ratio of miR-210 (serum/urine) and miR-155 (serum/urine) in PE pregnancies were significantly increased, and the difference was statistically significant (P = .046 and P = .01). However, the expression ratio of miR-210 (serum/urine) and miR-155 (serum/urine) in healthy pregnancies were not different, as shown in Table 3.
Table 3.
The expression ratio (serum/urine) of miR-210 and miR-155.
3.5. Specificity and sensitivity of miR-210 and miR-155 for PE pregnancies
In order to study the diagnostic accuracy of miR-210, miR-155, or miR-125b-5p as biomarkers for PE, a ROC curve was calculated (Fig. 3). The value of the area under the ROC curve (AUC) between 0.5 and 1 is considered having diagnostic significance. The AUC of miR-210 was 0.750 (95%, CI 0.592–0.908, P = .01). The AUC of miR-155 was 0.703 (95%, CI 0.539–0.868, P = .036). The AUC of the expression ratio of miR-210 (serum/urine) was 0.761(95%, CI 0.605–0.917, P = .005). The AUC of the expression ratio of miR-155 (serum/urine) was 0.718 (95% CI 0.560–0.875, P = .019). However, the AUC of miR-125b-5p was 0.290.
Figure 3.
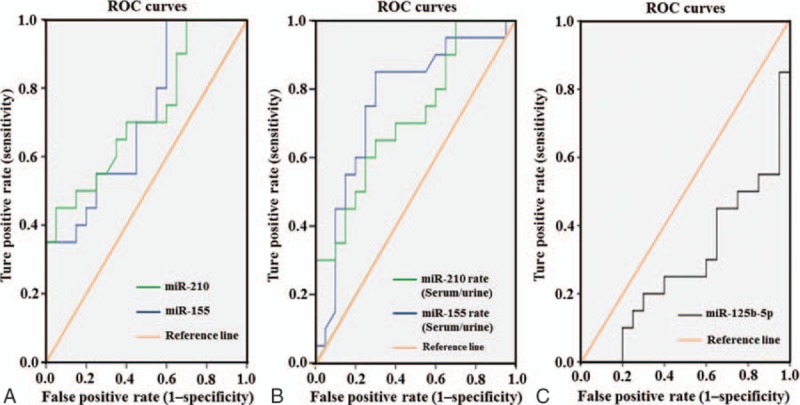
ROC curve analysis for candidate miRNAs between the cases of PE pregnancies and healthy pregnancies. The ROC display the true positive rates versus the false positive rates (i.e., “sensitivity” versus “1–specificity”). The reference line represents the ROC curve for a statistical test with the controls. The AUC (areas under the ROC curve) of miRNA-210 and miR-155 were 0.724 (P = .015, CI = 0.567–0.881) and 0.715 (P = .020, CI = 0.556–0.874), respectively (A). The AUC of the expression rates of miRNA-210 (serum/urine) and miRNA-155 (serum/urine) were 0.761 (P = .005, CI = 0.605–0.917 and 0.718 (P = .019, CI = 0.560–0.875), respectively (B). However, the AUC of miR-125b-5p was 0.290 (C). The AUC between 0.5 and 1 was considered having diagnostic significance. AUC = area under the curve, CI = confidence interval, miRNA = micro ribonucleic acid, PE = pre-eclampsia, ROC = receiver operating characteristic.
3.6. Correlation between the clinical data and miR-210 or miR-155
Urine miR-210 and 24-hour urine protein of PE pregnancies was positively correlated (r = 0.560, P = .010). Similar results were also obtained from miR-155 (r = 0.552, P = .012), as shown in Fig. 4.
Figure 4.
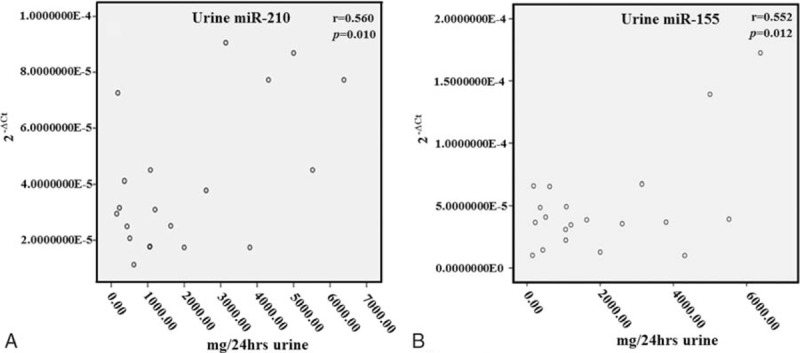
Comparison of the expression levels of urine miRNA-210 and miRNA-155 with urine protein in PE pregnancies. The expression level of miRNA-210 (A) and miRNA-155 (B) both have a positive correlation with the 24-hour urine total proteins. miRNA = micro ribonucleic acid, PE = pre-eclampsia.
4. Discussion
In the present study, we confirmed that miR-210 and miR-155 were upregulated in the serum of PE pregnancies by a case–control study compared with healthy pregnancies. MiR-210 has been involved in various pathophysiological pathways, such as cancer, oxidative stress, and apoptosis.[8,9] Recently miR-210 has been found to be upregulated in PE placentas compared with normal placentas.[10] In PE pregnancies, the oxygen tension at the feto–maternal interface was much lower than healthy pregnancies due to the reconstruction of maternal blood vessels by trophoblasts.[11,12] Upregulated miR-210 was associated with the placental mitochondrial dysfunction by targeting several mitochondria-related proteins, such as ISCU, NDUFA4, THSD7A, and KCMF1.[8,13,14] These proteins are critical for mitochondrial oxidation–reduction reactions. Furthermore, the impairment of trophoblast cell invasion by these proteins aggravate the hypoxic condition at the feto–maternal interface.[11,15] Such an injurious feedback loop between oxygen tension, miR-210 expression, and trophoblast cell invasion play an important role in the pathogenesis of PE.[11] By using the gene microarray, Mayor-Lynn et al[16] found that over 100 gene expressions are regulated by miR-210 in PE. Indeed, many reports have demonstrated that the hypoxia/NF-κB/mir-210/EFNA3 pathway played an important role in the pathogenesis of PE.[8,17] The luciferase assay confirmed that both HIF-1α and NF-κBp50 bound to the miR-210 promoter and induced its expression.[17] Overexpression of miR-210 decreased STAT6 and IL-4, suggesting that TLR3 activation induced placental miR-210 through HIF-1α and NF-κBp50 leading to decreased STAT6 and IL-4 levels and this contributed to the development of PE.[17] In recent years, ISCU and KCMF1 have been found to contribute the occurrence of PE. After miR-210 binding to ISCU mRNA 3’ UTR, the decreased expression of ISCU resulted in autophagosomal and siderosomal iron accumulation and a fourfold decrease of Matrigel invasion. This finding suggested that ISCU downregulation by miR-210 perturbing trophoblast iron metabolism was associated with defective placentation.[8] In addition, 1 recent report indicated that aberrant miR-210 expression contributed to the occurrence of PE by interfering with KCMF1-mediated signaling in the human placenta.[15]
MiR-155 was identified to be an essential regulator of endothelium-dependent vasorelaxation.[18] In 2010, Zhang et al[19] for the first time found that microRNA-155 contributed to PE by downregulating CYR61. Li et al[20] demonstrated a significant increase in the levels of miR-155 and decreased eNOS expression in the severe PE placentas, as compared with the controls. Recently, miR-155 has been found involving in NF-κB-dependent miR-155/eNOS pathway in the pathogenesis of PE.[21] A transwell insert invasion assay demonstrated that miR-155 inhibited cell invasion in trophoblast cells, and the effect was rescued by the overexpression of eNOS. This study revealed that miR-155 has a negative regulatory role in the migratory behavior via modulating eNOS.[18] Furthermore, Kim et al[21] demonstrated that the redox-sensitive NF-κB/miR-155/eNOS axis might be crucial in the pathogenesis of vascular disorders including PE. MiR-125b-5p is a member of miR-125b family. It has been implicated in many pathophysiological pathways, such as hepatitis B infection, gallbladder cancer, or acute myocardial infarction.[22–24] MiR-125b-5p could be used as novel noninvasive biomarkers of hepatitis B virus-related hepatocellular carcinoma.[25] One recent study has shown that downregulation of miR-125b-5p was a common phenomenon shared between gestational hypertension, PE, and intrauterine growth restriction.[6] However, the molecular mechanism of miR-155 and miR-125b-5p in the development of PE remains unclear.
In recent years, miRNAs have been identified to be involved in the pathogenesis of various disease processes, such as rheumatoid arthritis and urologic cancers, by regulating the corresponding gene expression.[26,27] In our study, we demonstrated that miR-210 and miR-155 had the potential to be diagnostic biomarkers for PE because miR-210 and miR-155 in serum were elevated in the PE pregnancies. ROC analysis is a widely accepted method for analyzing and comparing the diagnostic accuracy of body fluid tests. By performing ROC analysis, we found that miR-210 and miR-155 had the diagnostic power with an AUC of 0.750 and 0.703, respectively. These results revealed that serum miR-210 and miR-155 detection was specificity and sensitivity tests for the diagnosis of PE. Recently, several potential proteins markers, such as PAPP-A, s-Flt-1/PlGF, s-Endoglin, and PP13, have been discovered for the prediction or the detection of PE.[28,29] However, the sensitivity and specificity of these markers for PE diagnosis still need to be investigated because these markers could not be commercialized. Compared with the protein markers, the main advantages of miRNAs are the relatively low price, high throughput, and high sensitivity and specificity. Although our report open perspectives for miRNAs as biomarkers for the PE diagnosis, a large amount of work is required in the future. Foremost, it requires a standardization of methods/techniques used in miRNA profiling.
Renal damage is one of the most common complications of PE. At present, proteinuria is a necessary condition for PE diagnosis. A number of studies have confirmed the positive correlation between proteinuria and maternal perinatal outcome.[30,31] Data from our study demonstrated that the expressions of urine miR-210 and miR-155 in the PE group were positively correlated with the 24-hour urine protein, suggesting that urine miR-210 and miR-155 could be used as an index of evaluating the severity of kidney injury in PE pregnancies. Screening PE pregnancies with these 2 miRNAs makers in urine can reduce the risk of kidney injury.
In conclusion, our study identified that an elevation of miR-210 and miR-155 expression in the serum has been found to be associated with the development of PE. In the further study, these 2 markers, miR-210 and miR-155, still need to be investigated by increasing the sample size. Moreover, more studies are needed to clarify the function of the elevated miR-210 and miR-155 in PE and how these relate to the etiological and pathophysiological characteristics of the PE. Our findings may provide a novel diagnosis method to identify women who are at risk for developing PE.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, AUC = area under the curve, PE = pre-eclampsia, PT = partial thromboplastin time, qRT-PCR = quantitative reverse transcriptase-polymerase chain reaction, ROC = receiver operating characteristic.
LG and ZL contributed equally to this work.
Funding: This work was supported by the Natural Science Foundation of Shaanxi (2014JQ2–8055, 2016JM8046), Health research projects of Shaanxi-key support project (2014A), Key Scientific Research Plan Project of Education Department of Shaanxi Province (16JS096), and Doctoral research initial foundation of Xi’an Medical University in 2016(2016DOC22).
The authors have no conflicts of interest to disclose.
References
- [1].Mol BW, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet 2016;387:999–1011. [DOI] [PubMed] [Google Scholar]
- [2].Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. [DOI] [PubMed] [Google Scholar]
- [3].Munaut C, Tebache L, Blacher S, et al. Dysregulated circulating miRNAs in preeclampsia. Biomed Rep 2016;5:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu C, Zhou Y, Zhang Z. MiR-210: an important player in the pathogenesis of preeclampsia? J Cell Mol Med 2012;16:943–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng W, Liu T, Jiang F, et al. microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int J Mol Med 2011;27:393–9. [DOI] [PubMed] [Google Scholar]
- [6].Hromadnikova I, Kotlabova K, Hympanova L, et al. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb Res 2016;137:126–40. [DOI] [PubMed] [Google Scholar]
- [7].Yang Q, Lu J, Wang S, et al. Application of next-generation sequencing technology to profile the circulating microRNAs in the serum of preeclampsia versus normal pregnant women. Clinica Chimica Acta 2011;412:2167–73. [DOI] [PubMed] [Google Scholar]
- [8].Muralimanoharan S, Guo C, Myatt L, et al. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond) 2015;39:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gee HE, Camps C, Buffa FM, et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 2010;116:2148–58. [DOI] [PubMed] [Google Scholar]
- [10].Muralimanoharan S, Maloyan A, Mele J, et al. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 2012;33:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luo R, Wang Y, Xu P, et al. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci Rep 2016;6:19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang X, Le QT, Giaccia AJ. MiR-210—micromanager of the hypoxia pathway. Trends Mol Med 2010;16:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen Z, Li Y, Zhang H, et al. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 2010;29:4362–8. [DOI] [PubMed] [Google Scholar]
- [14].Nikuei P, Davoodian N, Tahamtan I, et al. Predictive value of miR-210 as a novel biomarker for pre-eclampsia: a systematic review protocol. BMJ Open 2016;6:e011920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luo R, Shao X, Xu P, et al. MicroRNA-210 contributes to preeclampsia by downregulating potassium channel modulatory factor 1. Hypertension 2014;64:839–45. [DOI] [PubMed] [Google Scholar]
- [16].Mayor-Lynn K, Toloubeydokhti T, Cruz AC, et al. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci 2011;18:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kopriva SE, Chiasson VL, Mitchell BM, et al. TLR3-induced placental miR-210 down-regulates the STAT6/interleukin-4 pathway. PLoS One 2013;8:e67760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun HX, Zeng DY, Li RT, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 2012;60:1407–14. [DOI] [PubMed] [Google Scholar]
- [19].Zhang Y, Diao Z, Su L, et al. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am J Obstet Gynecol 2010;202:466.e1-7. [DOI] [PubMed] [Google Scholar]
- [20].Li X, Li C, Dong X, et al. MicroRNA-155 inhibits migration of trophoblast cells and contributes to the pathogenesis of severe preeclampsia by regulating endothelial nitric oxide synthase. Mol Med Rep 2014;10:550–4. [DOI] [PubMed] [Google Scholar]
- [21].Kim J, Lee KS, Kim JH, et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol Med 2017;104:185–98. [DOI] [PubMed] [Google Scholar]
- [22].Ninomiya M, Kondo Y, Kimura O, et al. The expression of miR-125b-5p is increased in the serum of patients with chronic hepatitis B infection and inhibits the detection of hepatitis B virus surface antigen. J Viral Hepat 2016;23:330–9. [DOI] [PubMed] [Google Scholar]
- [23].Yang D, Zhan M, Chen T, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep 2017;7:43109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jia K, Shi P, Han X, et al. Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol Med Rep 2016;14:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Giray BG, Emekdas G, Tezcan S, et al. Profiles of serum microRNAs; miR-125b-5p and miR223-3p serve as novel biomarkers for HBV-positive hepatocellular carcinoma. Mol Biol Rep 2014;41:4513–9. [DOI] [PubMed] [Google Scholar]
- [26].Ouyang H, Zhou Y, Zhang L, et al. Diagnostic value of microRNAs for urologic cancers: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen XM, Huang QC, Yang SL, et al. Role of micro RNAs in the pathogenesis of rheumatoid arthritis: novel perspectives based on review of the literature. Medicine (Baltimore) 2015;94:e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grill S, Rusterholz C, Zanetti-Dällenbach R, et al. Potential markers of preeclampsia—a review. Reprod Biol Endocrinol 2009;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Anderson UD, Olsson MG, Kristensen KH, et al. Review: biochemical markers to predict preeclampsia. Placenta 2012;33:42–7. [DOI] [PubMed] [Google Scholar]
- [30].Newman MG, Robichaux AG, Stedman CM, et al. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria. Am J Obstet Gynecol 2003;188:264–8. [DOI] [PubMed] [Google Scholar]
- [31].Zhuang X, Chen YY, Zhou Q, et al. Qualitative analysis of diagnostic value of 24-h proteinuria for preeclampsia. Chin Med J (Engl) 2015;128:2998–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]


