Abstract
Background
Alcohol and drug use results in substantial morbidity, mortality, and cost. Individuals with alcohol and drug use disorders are overrepresented in general medical settings. Hospital-based interventions offer an opportunity to engage with a vulnerable population that may not otherwise seek treatment.
Objective
To determine whether inpatient addiction consultation improves substance use outcomes 1 month after discharge.
Design
Prospective quasi-experimental evaluation comparing 30-day post-discharge outcomes between participants who were and were not seen by an addiction consult team during hospitalization at an urban academic hospital.
Participants
Three hundred ninety-nine hospitalized adults who screened as high risk for having an alcohol or drug use disorder or who were clinically identified by the primary nurse as having a substance use disorder.
Intervention
Addiction consultation from a multidisciplinary specialty team offering pharmacotherapy initiation, motivational counseling, treatment planning, and direct linkage to ongoing addiction treatment.
Main Measures
Addiction Severity Index (ASI) composite score for alcohol and drug use and self-reported abstinence at 30 days post-discharge. Secondary outcomes included 90-day substance use measures and self-reported hospital and ED utilization.
Key Results
Among 265 participants with 30-day follow-up, a greater reduction in the ASI composite score for drug or alcohol use was seen in the intervention group than in the control group (mean ASI-alcohol decreased by 0.24 vs. 0.08, p < 0.001; mean ASI-drug decreased by 0.05 vs. 0.02, p = 0.003.) There was also a greater increase in the number of days of abstinence in the intervention group versus the control group (+12.7 days vs. +5.6, p < 0.001). The differences in ASI-alcohol, ASI-drug, and days abstinent all remained statistically significant after controlling for age, gender, employment status, smoking status, and baseline addiction severity (p = 0.018, 0.018, and 0.02, respectively). In a sensitivity analysis, assuming that patients who were lost to follow-up had no change from baseline severity, the differences remained statistically significant.
Conclusions
In a non-randomized cohort of medical inpatients, addiction consultation reduced addiction severity for alcohol and drug use and increased the number of days of abstinence in the first month after hospital discharge.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-017-4077-z) contains supplementary material, which is available to authorized users.
KEY WORDS: addiction, substance use disorder, addiction consultation, hospitalized patients, post-discharge abstinence
INTRODUCTION
Addiction has emerged as a public health crisis in America. There are 22 million people in the United States with a substance use disorder (SUD).1 Unintentional overdose is the leading cause of accidental death, killing more Americans than car accidents.2 From 2000 to 2014, nearly half a million people in this country died from drug overdose, with 47,055 deaths in 2014 alone.3 Each year only 10% of people with an SUD receive treatment.1 Among hospitalized patients the prevalence of SUD is higher than the general population.4 Hospital discharge is a vulnerable time for individuals with SUD when the risk of overdose is increased, highlighting the need for intervention.5 Patients with SUD are also at increased risk of hospital readmission.6 Several interventions for patients with alcohol, tobacco, or drug use disorder in general medical settings have been shown to improve retention in treatment and clinical outcomes.7 – 14 Implementation of these interventions in real-world clinical practice, however, remains rare, and rigorous evaluations of hospital-based interventions are lacking.
The goal of this study was to evaluate the impact of addiction consultation during hospitalization on addiction severity and self-reported abstinence at 30 days post-discharge.
METHODS
Study Design
We conducted a prospective, quasi-experimental evaluation of an inpatient addiction consultation service, with 30-day post-discharge outcomes as the primary outcome measures. Because the program was implemented on a subset of medical floors, and not all eligible patients on implementation floors received inpatient consults, we were able to conduct a controlled evaluation of the program.
Study Site
The study was conducted in an urban academic medical center with approximately 48,000 inpatient admissions annually. In November 2014, all inpatients began being screened for substance use by the admitting nurse using the Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) and the National Institute on Drug Abuse (NIDA) single-question screening for drug use.15 , 16 The AUDIT-C, which comprises three questions from the Alcohol Use Disorders Identification Test (AUDIT), has demonstrated validity in identifying heavy drinking and alcohol use disorder. An AUDIT-C score of 8 or higher is 92% specific for alcohol use disorder and 99% specific for heavy drinking and/or alcohol use disorder.15 The single-question drug screen asks “How many times in the past year have you used an illegal drug or used a prescription medication for non-medical reasons?” A response of at least one time is considered positive. This screening question is 100% sensitive and 73.5% specific for the detection of drug use disorder, with test characteristics similar to the longer 10-item Drug Abuse Screening Test (DAST-10).16
Participants
Participants included adults 18 years or older admitted during the study period who had or were at high risk for drug or alcohol use disorder as determined by an AUDIT-C score of 8 or higher, had a positive single-item drug screen, or who were identified by the primary nurse as having a substance use disorder. Exclusion criteria were 1) pregnancy; 2) inability to be interviewed due to acute illness, somnolence, or cognitive impairment; 3) and screening solely for marijuana use.
Recruitment
On 14 study floors, eligible participants were asked by a unit nurse for permission to be approached by a research assistant. The research assistant, available only on weekdays during business hours, completed further screening to confirm eligibility. Participants provided oral informed consent and received a $25 gift card at each follow-up assessment.
Intervention
Twelve of the 14 study floors had access to an addiction consult team (ACT). The remaining two floors did not. The limited access to ACT was due to a phased rollout of this new service. The two floors without access to ACT were floors with a mixed population of medical and surgical or respiratory acute care patients; non-medical patients on these floors were not included in the study. Floor assignment for patients was independent of this study and was based on bed availability at the time of admission. The decision to consult ACT was made by the primary clinical team independently of this study.
ACT is a multidisciplinary team consisting of a psychiatrist, a rotating group of internists with addiction expertise (all of whom had been granted waivers to prescribe buprenorphine, some board-certified in addiction medicine), advanced practice nurses (one full- and one part-time), three clinical social workers, a clinical pharmacist, recovery coach, and resource specialist. ACT provides each patient with a diagnosis and a longitudinal treatment plan, which is initiated in the hospital. The treatment plan includes an assessment of the ideal level of care, appropriateness of pharmacotherapy initiation, psychosocial needs, harm reduction needs, and patient readiness and preferences. Social workers provide bedside motivational enhancement therapy. Pharmacotherapy, when indicated, is initiated during hospitalization. Recovery coaches, who can provide peer support during hospitalization, are available in select outpatient practices and can longitudinally follow patients who receive primary care there. These coaches offer a range of services; examples include attending appointments, providing motivational support, helping with legal or social service needs, and connecting patients to other community supports. The social workers and resource specialist identify community-based treatment resources and make every effort to link patients directly to care following discharge. Patients without a concrete discharge plan are offered care through a low-threshold post-discharge clinic which provides transitional addiction treatment including pharmacotherapy.
Participants in the control group received standard of care for SUD prior to the establishment of ACT. Standard of care included access to a general psychiatry consult liaison team and floor social work. Hospital management of SUD generally included withdrawal treatment and referral to outpatient addiction care. Pharmacotherapy for SUD was rarely initiated in the hospital.
Assessments
Participants were assessed upon enrollment and at 30 and 90 days following discharge. Baseline assessments were conducted in person. Follow-up assessments were completed by phone, in person, or through the mail, depending on participant preference. The research assistant made a maximum of 15 attempts at contact per participant. Participants were asked about substance use and a number of secondary measures, including healthcare utilization.
Substance use severity was assessed using the questions from the Addiction Severity Index (ASI) which make up the alcohol and drug composite scores. The ASI is a standardized instrument for evaluating the severity of problems for patients with SUD.17 The composite score for each problem area of the ASI was derived from sets of items within the ASI, and may be a better indicator of overall problem severity and change in problem status.18 The time period for composite scores is the prior 30 days. The items are combined using a formula to ensure equal weighting of each variable in the composite score. In addition, participants were asked to report the number of days within in the past 30 days that they had used alcohol or drugs.
Self-efficacy was assessed by a question demonstrated to be predictive of relapse.19 Participants were also asked about motivation for abstinence, lifetime and past-30-day history of overdose, treatment engagement, quality of life, and tobacco use and quit attempts. (See online Appendix for full survey).
Main Measures
The primary outcomes of this study were change in ASI composite score for alcohol and drug use and self-reported abstinence at 30 days post-discharge compared to baseline. This combination of self-reported abstinence and ASI composite score has been used effectively in measuring the impact of care interventions.20
Secondary outcomes included change in self-efficacy, motivation for abstinence, self-reported overdose, treatment engagement, mutual help attendance, self-reported health care utilization, and quality of life.
Although the primary endpoint of the study was 30 days, participants were followed and outcomes were collected for up to 90 days.
Analysis
Continuous variables were summarized using the mean with standard deviation, and categorical variables were summarized using frequency with percentage. Separate analyses were conducted for those with 30-day follow-up and those with 90-day follow-up and changes from baseline were compared between the two study groups. For primary outcomes, regression models were used to compare outcomes, adjusting for differences in baseline characteristics. Using an intention-to-treat analysis, all enrolled patients were included and analyzed according to their original treatment groups. In order to include these participants in the analysis, we needed to impute outcomes for participants who were lost to follow-up. We conservatively assumed that participants who were lost to follow-up had no change from baseline severity. For binary secondary outcomes, the differences in change from baseline were compared by testing the interaction between group and time in the logistic regression models. All analyses were conducted using SAS version 9.4 software (SAS Institute Inc., Cary, NC). A two-sided p value of 0.05 or less was considered statistically significant.
The study was designed to enroll 200 intervention patients and 200 control patients in order to detect a mean difference of 0.28 in ASI drug use composite score, assuming a standard deviation of 0.1 with 80% power.20
The Partners Healthcare institutional review board approved the study.
RESULTS
Enrollment and Follow-Up
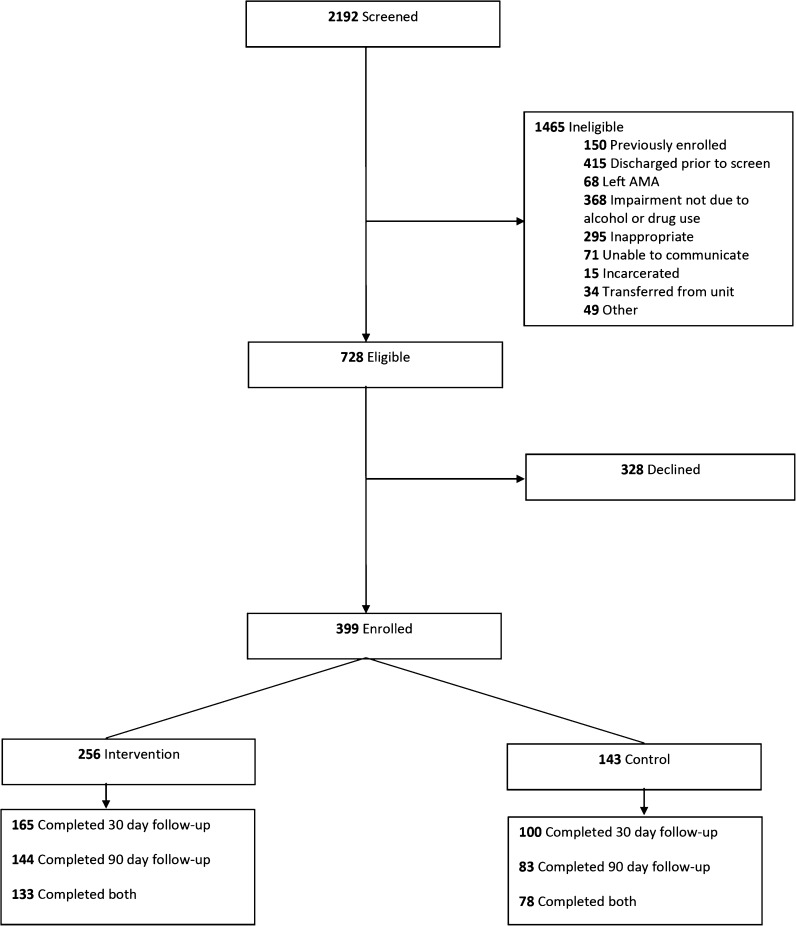
Participants were enrolled from April 1, 2015, until April 1, 2016. During the study period, 2192 participants were available for screening. Of those, 728 were eligible for enrollment; 328 declined and 399 consented to participate (Fig. 1). Of those enrolled, 256 (64%) received the intervention and 143 (36%) did not. Thirty-day follow-up was completed for 165 of 256 (65%) in the intervention group and 100 of 143 (70%) in the control group. Ninety-day follow-up was completed for 144 (56%) in the intervention group and 83 (58%) in the control group. Follow-up rates did not differ significantly between groups (p = 0.27, 0.73 for 30 and 90-day follow-up, respectively). Participants without 30-day follow-up were more likely to be male (78% vs. 69%, p = 0.065), less educated (60% vs. 51% with high school education or less, p = 0.091), unemployed (51% vs. 42%, p = 0.15), and with significantly higher baseline ASI-drug scores (0.16 vs. 0.12, p = 0.012). Participants without 90-day follow-up were more likely to report drugs as the major problem substance (52% vs. 40%, p = 0.049), more likely to be a minority (23% vs. 15%, p = 0.061) and unemployed (50% vs. 41%, p = 0.053), and had higher baseline ASI-drug scores (0.16 vs. 0.11, p < 0.001).
Figure 1.
Participant flow.
Characteristics of Study Participants
Table 1 shows the characteristics for enrolled participants. Among all patients, 218 (55%) identified alcohol as their major problem substance, 143 identified drugs (36%), and 38 identified both (10%). Among those who identified drugs as their major problem, heroin was the primary drug for 52%, cocaine for 15%, multiple drugs for 15%, and other opioids for 9%. The distribution of major problem or primary drug did not differ between intervention and control groups. The groups differed at baseline on several key demographics: those in the intervention group were younger (mean age 46 vs. 51, p = 0.006) and were more likely to be unemployed (53% vs. 31%, p < 0.001) and active smokers (69% vs. 55%, p < 0.001). Mean baseline ASI composite scores were higher in the intervention than in the control group (ASI-alcohol 0.46 vs. 0.31, p < 0.001, ASI-drug 0.15 vs. 0.10, p < 0.001).
Table 1.
Baseline Characteristics of All Enrolled Patients
| All (N = 399) | Intervention (N = 256) | Control (N = 143) | P value | |
|---|---|---|---|---|
| Major substance problem, n (%) | 0.88 | |||
| Alcohol | 218 (55) | 141 (55) | 77 (54) | |
| Drug | 143 (36) | 92 (36) | 51 (36) | |
| Both | 38 (10) | 23 (9) | 15 (10) | |
| Age, mean (SD) | 48 (14) | 46 (13) | 51 (15) | 0.006 |
| Female, n (%) | 110 (28) | 77 (30) | 33 (23) | 0.14 |
| White, n (%) | 324 (81) | 209 (82) | 115 (80) | 0.76 |
| Education, n (%) | 0.88 | |||
| High school or less | 216 (54) | 142 (55) | 74 (52) | |
| More than high school | 91 (23) | 59 (23) | 32 (22) | |
| Unknown | 92 (23) | 55 (21) | 37 (26) | |
| Employment status, n (%) | <0.001 | |||
| Employed/student | 81 (20) | 42 (16) | 39 (27) | |
| Retired | 32 (8) | 11 (4) | 21 (15) | |
| Disabled | 78 (20) | 49 (19) | 29 (20) | |
| Not employed | 179 (45) | 135 (53) | 44 (31) | |
| Unknown | 29 (7) | 19 (7) | 10 (7) | |
| Smoking status, n (%) | 0.002 | |||
| Never | 53 (13) | 31 (12) | 22 (15) | |
| Past | 37 (9) | 14 (5) | 23 (16) | |
| Active | 255 (64) | 177 (69) | 78 (55) | |
| Unknown | 54 (14) | 34 (13) | 20 (14) | |
| Baseline ASI alcohol, mean (SD) | 0.41 (0.33) | 0.46 (0.34) | 0.31 (0.31) | <0.001 |
| Baseline ASI drug, mean (SD) | 0.13 (0.15) | 0.15 (0.16) | 0.10 (0.11) | <0.001 |
Primary Outcomes
Among the 265 patients with 30-day follow-up, a significantly greater reduction in ASI composite scores was found in the intervention group (ASI-alcohol −0.24 vs. −0.08, p < 0.001; ASI-drug −0.05 vs. −0.02, p = 0.003; Tables 2 and 3). Only 10% of participants reported both alcohol and drug use as their major substance problem. The small sample size limited subgroup analyses for this group; however, a greater decrease in ASI composite scores was found in the intervention group (ASI-alcohol −0.25 vs. −0.15, p = 0.49; ASI-drug −0.08 vs. −0.01, p = 0.19.). The intervention group also showed a greater increase in the number of days of abstinence within the past 30 days (12.7 vs. 5.6, p < 0.001). In the adjusted analyses, differences in ASI-alcohol, ASI-drug, and days abstinent all remained statistically significant after controlling for age, gender, employment status, smoking status, and baseline ASI or days abstinent (p = 0.018, 0.018, and 0.02, respectively). In the intention-to-treat analysis, assuming that participants lost to follow-up had no change from baseline, differences between groups remained significant in both bivariate and multivariable analyses. There was a decrease in self-reported past-30-day overdose in both groups.
Table 2.
Substance Use Outcomes
| 30-Day follow-up | 90-Day follow-up | ||||||
|---|---|---|---|---|---|---|---|
| Intervention (N = 165) | Control (N = 100) | P value | Intervention (N = 144) | Control (N = 83) | P value | ||
| ASI alcohol | Baseline | 0.46 (0.33) | 0.28 (0.29) | <0.001 | 0.47 ().32) | 0.30 (0.31) | <0.001 |
| Follow-up | 0.22 (0.22) | 0.20 (0.20) | 0.45 | 0.25 (0.22) | 0.20 (0.22) | 0.12 | |
| Change | −0.24 (0.28) | −0.08 (0.24) | <0.001 | −0.22 (0.29) | −0.10 (0.27) | 0.003 | |
| Adjusted difference* | −0.06 (0.02) | 0.018 | −0.01 (0.03) | 0.79 | |||
| ASI drug | Baseline | 0.13 (0.16) | 0.10 (0.11) | 0.057 | 0.12 (0.15) | 0.09 (0.11) | 0.17 |
| Follow-up | 0.08 (0.11) | 0.08 (0.09) | 0.74 | 0.07 (0.08) | 0.07 (0.08) | 0.89 | |
| Change | −0.05 (0.11) | −0.02 (0.08) | 0.003 | −0.05 (0.11) | −0.02 (0.09) | 0.058 | |
| Adjusted difference* | −0.02 (0.01) | 0.018 | −0.01 (0.01) | 0.12 | |||
| Days abstinent | Baseline | 12.6 (10.6) | 19.1 (11.1) | <0.001 | 13.2 (11.0) | 18.6 (11.2) | <0.001 |
| Follow-up | 25.3 (8.3) | 24.7 (7.9) | 0.57 | 24.3 (9.3) | 24.1 (9.9) | 0.88 | |
| Change | 12.7 (11.7) | 5.6 (10.2) | <0.001 | 11.0 (12.7) | 5.5 (10.5) | <0.001 | |
| Adjusted difference* | 2.59 (1.11) | 0.02 | 1.70 (1.35) | 0.21 | |||
| Overdose | Baseline | 0.23 (0.85) | 0.23 (0.96) | 0.99 | 0.24 (0.89) | 0.17 (0.60) | 0.5 |
| Follow-up | 0.04 (0.30) | 0.06 (0.24) | 0.60 | 0.01 (0.08) | 0.04 (0.33) | 0.43 | |
| Change | −0.19 (0.73) | −0.17 (0.85) | 0.86 | −0.23 (0.90) | −0.13 (0.44) | 0.28 | |
| Adjusted difference* | −0.03 (0.03) | 0.42 | 0.01 (0.01) | 0.48 | |||
*The difference in changes from baseline to follow-up between intervention and control groups from regression models adjusting for age, gender, employment status, smoking status, and baseline value
Table 3.
Days of Abstinence Among Participants at 30 Days, 90 Days, or Both
| Days abstinent | 30-Day follow-up | 90-Day follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||||||||
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | ||
| All | Baseline | 165 | 12.6 | 10.6 | 100 | 19.1 | 11.1 | 144 | 13.2 | 11 | 83 | 18.6 | 11.2 |
| Follow-up | 165 | 25.3 | 8.3 | 100 | 24.7 | 7.91 | 144 | 24.3 | 9.29 | 83 | 24.1 | 9.85 | |
| Change | 165 | 12.7 | 11.7 | 100 | 5.61 | 10.2 | 144 | 11 | 12.7 | 83 | 5.45 | 10.5 | |
| Both | Baseline | 133 | 13 | 10.8 | 78 | 18.6 | 11.1 | 133 | 13 | 10.8 | 78 | 18.6 | 11.1 |
| Follow-up | 133 | 25.7 | 7.87 | 78 | 24.5 | 8.35 | 133 | 24.3 | 9.32 | 78 | 24.2 | 9.71 | |
| Change | 133 | 12.7 | 11.4 | 78 | 5.92 | 10.4 | 133 | 11.2 | 12.6 | 78 | 5.62 | 10.6 | |
| 30-Day only | Baseline | 32 | 10.7 | 10.1 | 22 | 20.9 | 11.2 | ||||||
| Follow-up | 32 | 23.5 | 9.83 | 22 | 25.4 | 6.25 | |||||||
| Change | 32 | 12.8 | 13.3 | 22 | 4.5 | 9.69 | |||||||
| 90-Day only | Baseline | 11 | 15.6 | 13.5 | 5 | 18.4 | 13.2 | ||||||
| Follow-up | 11 | 24.3 | 9.32 | 5 | 21.2 | 12.8 | |||||||
| Change | 11 | 8.73 | 14.4 | 5 | 2.8 | 9.2 | |||||||
Secondary Outcomes
Among the 227 patients with 90-day follow-up, a greater reduction in the mean ASI composite score for alcohol was noted in the intervention group (−0.22 vs. −0.10, p = 0.003; Table 2). The mean change in ASI-drug was also greater in the intervention group, but the difference did not reach significance (−0.05 vs. −0.02, p = 0.058). The change in days of abstinence remained higher in the intervention group (11 vs. 5.5, p < 0.001). There were 16 patients (11 intervention and 5 control) who could not be reached at 30 days but were reached at 90 days. Table 3 shows days of abstinence among all participants and those reached at 30 days, 90 days, or both.
The magnitude of the decrease in self-reported overdose at 90-day follow-up was greater in the intervention group; however, this was not significant (−0.23 vs. −0.13 p = 0.28).
Participants in the intervention group had attended more mutual help meetings at the 30-day (8.1 vs. 4.4, p = 0.004) and 90-day follow-up (9.0 vs. 5.1, p = 0.005). They were also more likely to be engaged in treatment at 30-day (57.9% vs. 41.4%, p = 0.009) and 90-day follow-up (54.6% vs. 40.7%, p = 0.047).
At 30-day follow-up, a significantly greater decrease in self-reported inpatient substance-related hospitalizations was seen in the intervention group (61% vs. 51%, p = 0.001), as well as a significantly greater reduction in substance-related ED visits (66% vs. 53%, p = 0.002; Table 4).
Table 4.
Secondary Outcomes
| 30-Day follow-up | 90-Day follow-up | ||||||
|---|---|---|---|---|---|---|---|
| Intervention (N = 165) | Control (N = 100) | P value | Intervention (N = 144) | Control (N = 83) | P value | ||
| Mutual help attendance | Baseline | 4.5 (9.5) | 2.9 (7.3) | 0.12 | 5.1 (10.2) | 2.9 (7.6) | 0.065 |
| Follow-up | 8.1 (12.3) | 4.4 (8.2) | 0.004 | 9.0 (11.9) | 5.1 (8.7) | 0.005 | |
| Change | 3.6 (12.4) | 1.6 (7.6) | 0.10 | 3.9 (13.4) | 2.2 (8.0) | 0.23 | |
| Treatment engagement (%) | Baseline | 30.5 | 30.3 | 0.97 | 30.5 | 29.6 | 0.89 |
| Follow-up | 57.9 | 41.4 | 0.009 | 54.6 | 40.7 | 0.047 | |
| Change | 27.4 | 11.1 | 0.018 | 24.1 | 11.1 | 0.092 | |
| Hospital admission | Baseline | 1.2 (1.4) | 0.5 (0.9) | <0.001 | 1.1 (1.4) | 0.5 (1.0) | <0.001 |
| Follow-up | 0.4 (1.1) | 0.2 (0.8) | 0.14 | 0.2 (0.8) | 0.1 (0.6) | 0.25 | |
| Change | −0.8 (1.6) | −0.3 (0.9) | 0.001 | −0.9 (1.4) | −0.4 (0.7) | <0.001 | |
| ER use | Baseline | 1.5 (2.1) | 0.7 (1.4) | <0.001 | 1.3 (1.6) | 0.8 (1.5) | 0.017 |
| Follow-up | 0.6 (1.6) | 0.3 (0.9) | 0.10 | 0.3 (1.0) | 0.2 (0.8) | 0.21 | |
| Change | −0.9 (1.6) | −0.3 (1.3) | 0.002 | −0.9 (1.8) | −0.6 (1.4) | 0.084 | |
| Current quality of life | Baseline | 5.0 (3.0) | 5.5 (3.0) | 0.23 | 5.0 (2.9) | 6.0 (2.9) | 0.021 |
| Follow-up | 5.9 (2.9) | 6.2 (2.7) | 0.45 | 6.3 (2.8) | 6.7 (2.9) | 0.29 | |
| Change | 0.9 (3.1) | 0.7 (2.8) | 0.61 | 1.3 (3.0) | 0.7 (2.8) | 0.20 | |
| 30-Day quality of life | Baseline | 3.4 (1.3) | 3.1 (1.5) | 0.13 | 3.3 (1.3) | 3.0 (1.5) | 0.16 |
| Follow-up | 2.5 (1.5) | 2.6 (1.6) | 0.51 | 2.2 (1.3) | 2.8 (1.7) | 0.014 | |
| Change | −0.9 (1.9) | −0.5 (2.0) | 0.11 | −1.1 (1.9) | −0.3 (2.3) | 0.006 | |
| Self-efficacy | Baseline | 6.9 (3.0) | 6.8 (3.7) | 0.65 | 7.0 (2.9) | 7.2 (3.5) | 0.62 |
| Follow-up | 7.2 (2.8) | 7.3 (3.2) | 0.93 | 8.1 (2.6) | 7.4 (3.2) | 0.098 | |
| Change | 0.3 (3.5) | 0.5 (3.6) | 0.61 | 1.1 (3.6) | 0.2 (3.3) | 0.054 | |
| Abstinence motivation | Baseline | 9.2 (2.2) | 8.1 (3.3) | 0.003 | 9.3 (1.7) | 8.5 (3.1) | 0.031 |
| Follow-up | 9.0 (2.4) | 8.3 (3.1) | 0.062 | 9.4 (1.7) | 8.2 (3.1) | 0.001 | |
| Change | −0.2 (2.1) | 0.2 (3.4) | 0.22 | 0.2 (2.0) | −0.2 (2.5) | 0.21 | |
| Medication adherence | Baseline | 1.8 (6.1) | 0.5 (3.1) | 0.023 | 1.9 (6.5) | 0.6 (3.4) | 0.043 |
| Follow-up | 1.0 (4.4) | 0.3 (1.2) | 0.045 | 0.7 (3.1) | 0.2 (0.8) | 0.11 | |
| Change | −0.8 (6.6) | −0.2 (3.3) | 0.34 | −1.3 (7.2) | −0.4 (3.6) | 0.21 | |
DISCUSSION
This prospective, controlled evaluation of an inpatient addiction consultation service for hospitalized medical patients with an SUD found that consultation significantly reduced addiction severity for alcohol and drug use and increased the number of days of abstinence in the first month after hospital discharge. The increase in abstinence days and reduction in alcohol use severity remained significantly greater at 3 months following discharge. The magnitude of improvement with respect to the severity of drug use was also greater at 3 months, although the difference was no longer significant. Addiction consultation also reduced the number of self-reported hospital and ED returns for substance use-related issues.
Our findings build on previous research demonstrating efficacy for hospital-based SUD interventions. An uncontrolled study offering a range of services, including methadone initiation, to non-treatment-seeking hospitalized patients with opioid use disorder showed that 82% of patients engaged in a post-discharge addiction follow-up visit.7 A meta-analysis found that a brief intervention for unhealthy drinkers in a hospital setting reduced alcohol consumption 6 months after discharge.8 A study of hospitalized patients with alcohol use disorder found that bedside assessment with motivational interviewing and facilitated referral to treatment increased treatment participation following discharge.9 Starting pharmacotherapy for alcohol, tobacco, or opioid use disorder prior to discharge is reported to improve treatment retention, reduces substance use, and decreases hospital readmission.10 – 12 Initiation of pharmacotherapy with buprenorphine during an emergency room visit for individuals with opioid use disorder was found to increase treatment engagement and reduce illicit drug use.13 These previous studies have generally examined either pharmacotherapy initiation or behavioral interventions in the hospital. The intervention in this study incorporated elements from all of these prior studies. To our knowledge, no controlled studies have evaluated the impact of comprehensive addiction consultation in the general hospital on substance use outcomes.
Our study suggests that a hospital-based intervention which includes pharmacotherapy initiation, brief behavioral intervention, and direct linkage to outpatient treatment is effective. Hospitalization can offer an opportunity to engage with and initiate treatment for individuals with SUD who are actively experiencing the negative consequences of their disease. The durability of our findings at 3 months for days of abstinence and alcohol use severity is promising, and suggests that such an intervention can have longer-term benefits. This study has important implications for hospital systems, particularly in light of the current opioid epidemic.21
There are limitations to this study. First, assignment to the intervention was not randomized. Consequently, patients in the intervention and control arms differed on a number of variables. We controlled for these differences in the analysis, but in the absence of randomization, unmeasured confounding is possible. We did not examine the impact of psychiatric comorbidity. All patients were general medical patients with SUD; however, it is possible that psychiatric complexity differed between groups. Studies examining the impact of psychiatric comorbidity on substance use outcomes have reported mixed results.22 Co-occurring psychiatric illness has been shown to be more strongly associated with employment problems and suicide risk than with response to treatment or SUD severity. We controlled for baseline addiction severity as measured by ASI composite scores for drug and alcohol, which are the indices within the ASI shown to have the most validity for patients with severe mental illness.23 Second, due to variations in practice across the hospital, the care received by patients in the control arm was heterogeneous. It is possible that some control patients received addiction services, including pharmacotherapy. However, this would only have biased study results toward the null. Third, based on a number of baseline characteristics, including drug use severity, patients who were not reached for follow-up appeared to have been sicker and more marginalized. This is unlikely to have biased our results, because rates of follow-up completion did not differ between study arms. Despite multiple attempts to reach participants, our follow-up rates were relatively low. However, these follow-up rates were similar to those for participants in the control arms of previous studies of hospital-based interventions.12 , 13 In addition, the main outcomes did not change in an intention-to-treat analysis which considered missing participants as not having changed from baseline severity. Fourth, our outcome measures relied on self-reporting and did not use biological confirmation. However, substance use self-reporting has been widely used as a treatment outcome in the literature, even among those with co-occurring psychiatric illness, and a meta-analysis demonstrated good agreement with biological measures for substance use.24 , 25 Lastly, this was a single-site study in an academic medical center that followed patients for up to 3 months. The results may not be generalizable to other care settings and may not be durable.
CONCLUSION
In a non-randomized cohort of medical inpatients, addiction consultation reduced addiction severity for alcohol and drug use and increased the number of days of abstinence during the first month after hospital discharge. Hospitalization appears to be an effective setting in which to initiate addiction treatment. Hospitals have an opportunity to address the current crisis of SUD across the U.S. by offering addiction diagnosis, treatment, and linkage to care.
Electronic supplementary material
(DOCX 14 kb)
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2014 National survey on drug use and health: SAMHSA, 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf Accessed April 24, 2017.
- 2.Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. [PubMed] [Google Scholar]
- 3.Rudd RA, Aleshire N, Zibbell JE, Gladden M. Increases in drug and opioid overdose deaths — United States, 2000–2014. MMWR. 2016;64(50):1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 4.Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27:101–110. doi: 10.1006/pmed.1997.0250. [DOI] [PubMed] [Google Scholar]
- 5.Merrall EL, Bird SM, Hutchinson SJ. A record-linkage study of drug-related death and suicide after hospital discharge among drug-treatment clients in Scotland, 1996-2006. Addiction. 2013;108(2):377–84. doi: 10.1111/j.1360-0443.2012.04066.x. [DOI] [PubMed] [Google Scholar]
- 6.Center for Health Information and Analysis. Behavioral health & readmissions in Massachusetts acute care hospitals. 2016. Available at: http://www.chiamass.gov/assets/docs/r/pubs/16/Behavioral-Health-Readmissions-2016.pdf Accessed April 14, 2017.
- 7.Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803–808. doi: 10.1007/s11606-010-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;8:CD005191. doi: 10.1002/14651858.CD005191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. doi: 10.1186/1940-0640-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365–370. doi: 10.1007/s11606-014-2968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–28. doi: 10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369–76. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–44. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parthasarathy S, Weisner C, Hu TW, Moore C. Association of outpatient alcohol and drug treatment with health care utilization and cost: revisiting the offset hypothesis. J Stud Alcohol. 2001;62(1):89–97. doi: 10.15288/jsa.2001.62.89. [DOI] [PubMed] [Google Scholar]
- 15.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT Alcohol Consumption Questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 16.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155–60. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 18.McGahan PL, Griffith JA, Parente R, McLellan AT. Addiction severity composite scores manual. 1986. Available at: http://www.tresearch.org/wp-content/uploads/2012/09/CompositeManual.pdf Accessed April 14, 2017.
- 19.Hoeppner BB, Kelly JF, Urbanoski KA, Slaymaker V. Comparative utility of a single-item versus multiple-item measure of self-efficacy in predicting relapse among young adults. J Subst Abuse Treat. 2011;41(3):305–312. doi: 10.1016/j.jsat.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massachusetts Department of Public Health. An assessment of opioid-related deaths in Massachusetts (2013–2014). 2016. Available at: http://www.mass.gov/eohhs/docs/dph/stop-addiction/dph-legislative-report-chapter-55-opioid-overdose-study-9-15-2016.pdf Accessed April 14, 2017.
- 22.Ford JD, Gelernter J, DeVoe JS, et al. Association of psychiatric and substance use disorder comorbidity with cocaine dependence severity and treatment utilization in cocaine-dependent individuals. Drug Alcohol Depend. 2009;99(1–3):193–203. doi: 10.1016/j.drugalcdep.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey KB, Cocco KM, Correia CJ. Reliability and validity of the addiction severity index among outpatients with severe mental illness. Psychol Assess. 1997;9(4):422–428. doi: 10.1037/1040-3590.9.4.422. [DOI] [Google Scholar]
- 24.Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances--systematic review and meta-analysis. Addict Behav. 2012;37(3):225–33. doi: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Houck JM, Forcehimes AA, Gutierrez ET, Bogenschutz MP. Test-retest reliability of self-report measures in a dually diagnosed sample. Subst Use Misuse. 2013;48:99–105. doi: 10.3109/10826084.2012.731674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)