Abstract
Red blood cell distribution width (RDW) is a simple, inexpensive, routinely measured and automatically reported blood test parameter, which reflects the degree of anisocytosis of red blood cells in peripheral blood. RDW was found to be associated with and retain clinical significance for assessing disease severity and outcomes in a number of hematological and solid malignancies. Motley of interacting clinical and biochemical factors have an impact on the red cell population biology. Malignancies per se can act as a causative factor, or anisocytosis may develop as a result of chronic inflammation. RDW has also been shown to be affected by nutritional status, which is typically deranged in malignancies. RDW is shown to be a clinically useful marker of disease severity and level of fibrosis in liver cirrhosis of various causes such as hepatitis B, hepatitis C and non-alcoholic fatty liver disease. Whether liver cirrhosis patients with higher RDW are at increased risk of hepatocellular cancer is yet to be determined, but several lines of evidence confirm that RDW has clinical significance in hepatocellular carcinoma (HCC). In this review, we specifically discuss the current literature about the association between RDW and HCC. The available evidences were summarized and the potential underlying mechanisms were analyzed.
Keywords: Red blood cell distribution width (RDW), cancer, hepatocellular carcinoma (HCC), prognosis
Hepatocellular carcinoma (HCC) is the 6th most common form of cancer worldwide, and second leading cause of cancer-related deaths (9% of all cancer deaths; 745,000 people) (https://www.cdc.gov/cancer/international/statistics.htm. Accessed on 03/21/2017). According to American Cancer Society, about 40,710 new cases of HCC and intrahepatic bile duct cancers will be diagnosed in the United States in 2017 and about 28,920 people will die of these cancers (https://cancerstatisticscenter.cancer.org/#/. Accessed on 03/21/2017). The incidence of HCC has more than tripled since 1980 and related death rates have increased by almost 3% per year since 2000. (https://www.cancer.org/cancer/liver-cancer/about/what-is-key-statistics.html Accessed on 03/21/2017). For individual HCC patients, prognostic estimation is crucial because it greatly impact the selection of the most appropriate treatment. During past years, accumulated prognostic factors for HCC have been identified, but these, when used alone or in combination, are not adequate enough for predicting the prognosis of this type of cancer. Therefore, multi-marker approach represents an effective way for HCC management, and it is of great value for exploring innovative prognostic factors.
Red blood cell distribution width (RDW) is a measure of variability of erythrocyte size in peripheral blood (i.e., anisocytosis). It is an automatically generated parameter, which is typically reported as a part of the complete blood count (CBC) (1). In recent past, RDW has gained substantial attention as a prognostic marker of various medical conditions such as sepsis (2), acute myocardial infarction (3), heart failure (4), autoimmune (5) and liver diseases (6). Besides, previous studies indicated that increased RDW is also associated with enhanced all-cause mortality in the general population (7).
Accumulated evidences also indicated that RDW may be a prognostic factors for various malignancies (8), such as gastric (9), lung (10) and ovarian cancers (11). Some studies evaluated the role of RDW as a prognostic marker of poor survival in HCC. However, no review has been published to summarize the available evidences to the best of our knowledge in this regard. In this review, we summarize the published literature and the putative mechanisms involved in this association.
Published studies on role of RDW in HCC
The first report assessing the prognostic role of RDW in HCC was published in 2015 by Smirne et al. (12). The authors retrospectively analyzed a training cohort of 208 patients with HCC and an independently prospectively collected validated cohort of 106 patients with HCC. In both cohorts, median survival time was significantly lower in patients with RDW ≥14.6% at the time of diagnosis. The median survival in training cohort was 1,026 days in low RDW group (RDW ≤14.6%) vs. 282 days in high RDW group (HR =0.43; 95% CI: 0.31–0.60, P<0.0001). The HCC patients were then classified in four quartiles according to their RDW value, and the median survival decreased progressively with each increasing RDW quartile. In the validation cohort, the median survival was 868 days in patients with RDW <14.6% whereas it was 340 days with RDW >14.6%. In the training cohort, the survival rates at 1-, 2- and 3-year were 79%, 57% and 42% in patients with RDW <14.6% compared to 48%, 29% and 18% in patients with RDW >14.6%, respectively. In both the training and validation cohorts, increased RDW was independently associated with higher mortality, with a hazard ratio (HR) of 1.13 and 1.39 per 1% increase, respectively. It was also observed that the Harrell’s C coefficient was 0.709 when using the Barcelona Clinic Liver Cancer (BCLC) staging system as the only independent variable; however, it increased to 0.754 when using both the RDW and BCLC stage. These results suggest that RDW can provide additional prognostic information beyond BCLC stage alone.
Wei et al. (6) carried out a retrospective analysis to evaluate the association between RDW and the clinical characteristic of HCC. They enrolled 110 treatment-naïve HCC patients and compared their RDW with 68 healthy controls, observing that the admission value of RDW was significantly higher in HCC patients. Furthermore, RDW values significantly correlated with liver function tests such as albumin, total bilirubin and prothrombin time (PT). Their study also indicated that RDW increased in parallel with the Child-Pugh stage. However, RDW was not found to be correlated with HCC TNM stage at the time of diagnosis. As albumin, total bilirubin and Child-Pugh stage are well-recognized prognostic factors for HCC, they hypothesized that RDW could be an additional prognostic factor for this type of cancer.
Zhao et al. studied the significance of preoperative RDW in patients undergoing curative radical resection of HCC (13). They retrospectively reviewed the medical records of 106 patients with HCC who received curative radical resection. Patients were categorized in high (>14.5%, n=28) and low RDW (<14.5%, n=78) groups. RDW was found to be associated with many clinical characteristics, including vascular invasion, tumor stage and size. Using Kaplan-Meier curve analysis, patients with higher RDW were found to have significantly lower disease free survival (DFS) and overall survival (OS). In a multivariable Cox regression model, in which common prognostic factors (tumor size, TNM stage, vascular invasion, tumor number) were added as covariables, RDW was independently associated with OS of HCC, with a HR of 1.89 (95% CI: 1.41–2.83) per 1% increase, so underscoring that the risk of death may increase by 89% with each 1% increase in RDW.
In a prospective, multicenter cohort study, Howell et al. investigated the role of RDW in predicting the survival of patients with HCC treated with sorafenib (14). A total number of 442 subjects were enrolled, with OS as the primary study end point and a median follow-up time of 7.1 months. The baseline RDW was found to be significantly associated with OS, with a HR of 1.23 (95% CI: 1.12–1.29). More importantly, using c-statistics, the authors found that the area under receiver operating characteristic (ROC) curve (AUC) was markedly increased from 0.669 to 0.787 after RDW and no treatment-related diarrhea was added to CLIP score (Cancer of Liver Italian Program score) (15) for predicting 12-month survival. Thus, author concluded that addition of RDW and sorafenib side effects (i.e., diarrhea) improves the survival predictability of CLIP score in patients with HCC.
Proposed mechanisms in role of RDW in HCC
Although many studies indicated that increased RDW is associated with poorer outcomes of HCC, the underlying mechanisms of this association remains largely unknown. We, hence, hypothesize that the prognostic value of RDW in HCC can be partially attributed to inflammation and oxidative stress.
It is well-accepted that chronic inflammation is an innate characteristics of HCC (15). Chronically inflamed liver parenchyma due to various viral infections, deposition of substances such as copper and iron and chronic alcoholism, represents a precancerous environment (16). There is increasing evidence that the role of chronic systemic inflammation may be a significant predictor of outcome in various human malignancies, thus including HCC. Multiple studies showed that elevated values of C reactive protein (CRP) predict survival (16,17) and recurrence (18) in HCC. Neutrophil-lymphocyte ratio (NLR), an easily obtainable inflammatory marker, is also proved to be associated with the prognosis of HCC (19). Previous studies indicated that RDW is positively correlated with inflammatory biomarkers, such as CRP and erythrocyte sedimentation rate (ESR) in unselected outpatient (20) and healthy population (21). It can, hence, be hypothesized that RDW may be indicator of inflammation. This is biologically plausible, because chronic inflammation response can suppress erythropoiesis and shorten erythrocyte survival in blood (22).
Oxidative stress has been known to have an important role in development and progression of HCC. Continued oxidative stress triggers to generation of free radical oxidative species, which directly cause lipid peroxidation and DNA damage. Molecular markers of DNA damage and lipid peroxidation such 8-hydroxydeoxyguanosine (8-OHdG), and 4-hydroxynonenal (HNE) have been found elevated in patients with HCC, and were associated with poor clinical outcome (23). Studies are underway to evaluate the role of antioxidant treatment targets in HCC therapy such as the use of metformin, vitamin E and L-carnitine (24,25). Nutritional abnormalities due to direct effect of cancer causing loss of appetite and weight loss can lead to deficiency of various minerals and vitamins such as iron, folate and vitamin B12. It is a well-known fact that RDW is affected by deficiencies of these minerals and vitamins (26). Continued injury to liver parenchyma is also associated with release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α). IL-6 inhibits erythropoietin (EPO) production and downregulates the EPO receptor, thus ultimately impairing efficient erythropoiesis and causing anisocytosis (7) (Figure 1).
Figure 1.
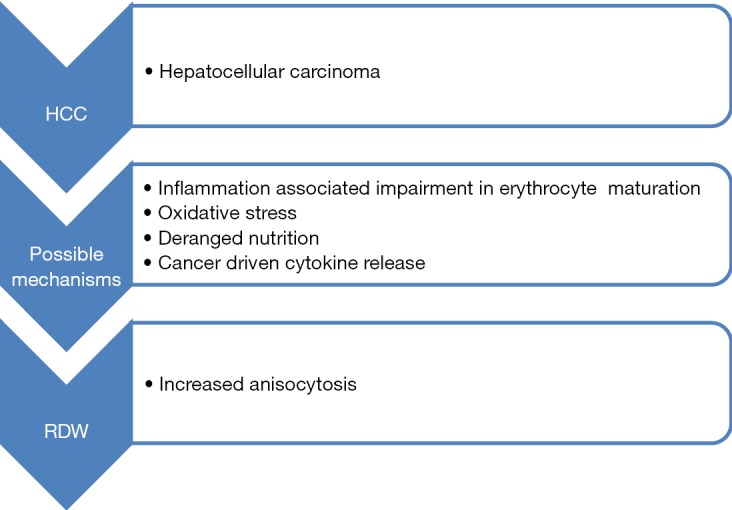
Possible mechanisms of elevated red blood cell distribution width in hepatocellular carcinoma.
Conclusions
Taken together, the available studies point out that RDW may represent an easily obtainable and inexpensive prognostic marker in patients with HCC. The association between RDW and HCC is probably mediated by inflammatory response and oxidative stress. Currently, inflammatory markers have not been included in the prognostic model of HCC, and we, thus, propose that incorporation of RDW into prognostic model of HCC may improve the efficiency of prognostication. Notably, RDW is affected by many factors, such as renal function (27), diabetes (28) and liver function (6,29). Therefore, these factors should be also considered when interpreting the value of RDW in HCC. Although it remains to be established whether liver cirrhosis patients with high RDW are at increased risk of developing HCC, current evidence suggests that RDW may have clinical significance in predicting outcomes after surgery, disease-free survival and OS in HCC.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 2.Jo YH, Kim K, Lee JH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med 2013;31:545-8. 10.1016/j.ajem.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Yang DH, Jang SY, et al. Incremental predictive value of red cell distribution width for 12-month clinical outcome after acute myocardial infarction. Clin Cardiol 2013;36:336-41. 10.1002/clc.22114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YL, Hu ZD, Liu SJ, et al. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta-analysis of cohort studies. PLoS One 2014;9:e104861. 10.1371/journal.pone.0104861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu ZD. Red blood cell distribution width: a promising index for estimating activity of autoimmune disease. J Lab Precis Med 2016;1:4 10.21037/jlpm.2016.10.02 [DOI] [Google Scholar]
- 6.Wei TT, Tang QQ, Qin BD, et al. Elevated red blood cell distribution width is associated with liver function tests in patients with primary hepatocellular carcinoma. Clin Hemorheol Microcirc 2016;64:149-55. 10.3233/CH-162053 [DOI] [PubMed] [Google Scholar]
- 7.Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588-94. 10.1001/archinternmed.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med 2016;4:399. 10.21037/atm.2016.10.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark 2017;18:19-25. 10.3233/CBM-160668 [DOI] [PubMed] [Google Scholar]
- 10.Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. 10.1371/journal.pone.0080240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Hong N, Robertson M, et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep 2017;7:43001. 10.1038/srep43001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smirne C, Grossi G, Pinato DJ, et al. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis 2015;47:488-94. 10.1016/j.dld.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 13.Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark 2016;16:507-12. 10.3233/CBM-160591 [DOI] [PubMed] [Google Scholar]
- 14.Howell J, Pinato DJ, Ramaswami R, et al. Integration of the cancer-related inflammatory response as a stratifying biomarker of survival in hepatocellular carcinoma treated with sorafenib. Oncotarget 2017;8:36161-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber A, Boege Y, Reisinger F, et al. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly 2011;141:w13197. [DOI] [PubMed] [Google Scholar]
- 16.Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology 2013;57:2224-34. 10.1002/hep.26057 [DOI] [PubMed] [Google Scholar]
- 17.Nagaoka S, Yoshida T, Akiyoshi J, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int 2007;27:1091-7. 10.1111/j.1478-3231.2007.01550.x [DOI] [PubMed] [Google Scholar]
- 18.Kornberg A, Witt U, Kornberg J, et al. Postoperative peak serum C-reactive protein is a predictor of outcome following liver transplantation for hepatocellular carcinoma. Biomarkers 2016;21:152-9. 10.3109/1354750X.2015.1118548 [DOI] [PubMed] [Google Scholar]
- 19.Sun XD, Shi XJ, Chen YG, et al. Elevated Preoperative Neutrophil-Lymphocyte Ratio Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients Treated with Liver Transplantation: A Meta-Analysis. Gastroenterol Res Pract 2016;2016:4743808. 10.1155/2016/4743808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [DOI] [PubMed] [Google Scholar]
- 21.Vayá A, Sarnago A, Fuster O, et al. Influence of inflammatory and lipidic parameters on red blood cell distribution width in a healthy population. Clin Hemorheol Microcirc 2015;59:379-85. 10.3233/CH-141862 [DOI] [PubMed] [Google Scholar]
- 22.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am 2014;28:671-81. 10.1016/j.hoc.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki A, Kono H, Gupta M, et al. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol 2007;14:1182-90. 10.1245/s10434-006-9049-1 [DOI] [PubMed] [Google Scholar]
- 24.Li S, Hong M, Tan HY, et al. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid Med Cell Longev 2016;2016:4234061. 10.1155/2016/4234061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Li Z, Ye Y, et al. Oxidative Stress and Liver Cancer: Etiology and Therapeutic Targets. Oxid Med Cell Longev 2016;2016:7891574. 10.1155/2016/7891574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal H, Gupta S, Singla U. Level of red cell distribution width is affected by various factors. Clin Chem Lab Med 2016;54:e387. 10.1515/cclm-2016-0195 [DOI] [PubMed] [Google Scholar]
- 27.Lippi G, Targher G, Montagnana M, et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest 2008;68:745-8. 10.1080/00365510802213550 [DOI] [PubMed] [Google Scholar]
- 28.Magri CJ, Fava S. Red blood cell distribution width and diabetes-associated complications. Diabetes Metab Syndr 2014;8:13-7. 10.1016/j.dsx.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 29.Wang LL, Wei TT, Yin JR, et al. Red blood cell distribution width and mean platelet volume are potential prognostic indices for patients with primary biliary cirrhosis. Clin Chem Lab Med 2017;55:e127-9. 10.1515/cclm-2016-0680 [DOI] [PubMed] [Google Scholar]


