Abstract
The pathogenesis of breast cancer, the most frequent female malignancy, entails both genetic and acquired risk factors. Among the various oncogenetic mutations, those involving the BReast Cancer 1 (BRCA1) and BReast Cancer 2 (BRCA2) genes are associated with an extremely high risk of developing breast cancer, with a penetration approximating 70% in women with a positive family history for this malignancy. This important evidence elicits some pragmatic considerations, such as the clinical effectiveness of screening for the most penetrant BRCA mutations in women with or without a positive familial history, but also raises important issues related to the most appropriate clinical management of these patients. Despite it seems now almost certain that BRCA testing should be offered to women with a positive familial history for breast cancer, the balance between advantages and limitations of a population screening remains largely debated. Whatever conclusion can be reach at this point in time must be accurately weighed against at least four different perspectives, which include the low prevalence of these mutations in the general population, the relatively lower risk of developing breast cancer in women without a familial history for this malignancy, the direct and indirect cost of genetic testing and, last but not least, the many potential psychological and clinical consequences in patients receiving a positive test result. Many of these still unresolved issues will be tentatively discussed in this article.
Keywords: Breast cancer, BRCA, BReast Cancer 1 (BRCA1), BReast Cancer 2 (BRCA2), screening
Breast cancer
Breast cancer is the most frequent female malignancy, with estimated 1,384,155 new cases and approximately 459,000 related deaths around the world (1). According to these figures it can be predicted that one every eight women will develop breast cancer during her lifespan (1). As for many other malignancies, the pathogenesis of breast cancer is clearly multifactorial, thus involving both environmental and genetic risk factors. The former class of risk factors includes different forms of ionizing radiations (e.g., environmental, occupational or diagnostic-therapeutic), synthetic chemicals such as organochlorines (e.g., pesticides, industrial chemicals and dioxins), oral contraceptives (OCs), hormone replacement therapy (HRT), pregnancy and menstrual history. Regarding genetics, dominant gene mutations seem to occur in a minority of breast cancers, and the most common among these are those affecting the BReast Cancer 1 (BRCA1) and BReast Cancer 2 (BRCA2) genes (1) (Figure 1). Both these genes are conventionally known to act as tumor suppressors, since they are actively involved in cell-cycle regulation and double-strand DNA breaks repair by means of homologous recombination (2). In patients with BRCA1 or BRCA2 mutations associated with impaired gene function (e.g., 5' to c.2281, c.2282 to c.4071, c.4072 to 3', c.68_69delAG, c.5266dupC for BRCA1, and 5' to c.3846, c.3847 to c.6275 and c.6276 to 3' for BRCA2, respectively), double strand DNA breaks cannot be efficiently repaired, so that chromosomal rearrangements gradually accumulate during cell division, some of which may engender the development of cell lineages with potency of autonomous cell replication or carcinogenic and metastatic potential (3).
Figure 1.
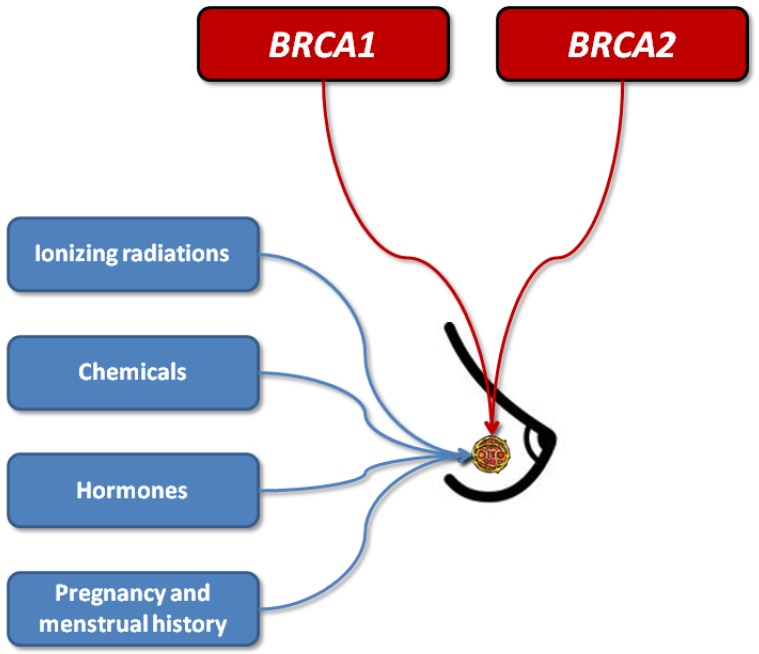
Pathogenesis of breast cancer.
BRCA mutations and breast cancer
It has been convincingly demonstrated that the overall prevalence of BRCA1 or BRCA2 oncogenic mutations may differ widely according to the study populations. In particular, the prevalence is usually found to be very low in the general population (i.e., between 0.2–0.3%), is enhanced to approximately 3% in all breast cancer carriers, further increases to 6% in those with breast cancer development before the age of 40, but can be as high as 20% in women with a positive familial history of breast cancer (4). Despite the overall prevalence in the general population is definitely low, reliable evidence attests that the penetrance (i.e., the risk of developing breast cancer in carriers of BRCA1 or BRCA2 mutations) may be high, especially in women with affected relatives. This concept has been recently emphasized by data published by Kuchenbaecker et al. (5), who carried out a prospective cohort study including 6,036 BRCA1 and 3,820 BRCA2 female carriers, 4,810 of whom with breast or ovarian cancer and 5,046 without. The 5-year prospective follow-up allowed to demonstrate that the overall risk of developing breast cancer was 73% in women with a positive familial history for this malignancy and carrying BRCA1 oncogenic mutations, and as high as 65% in women with positive familial history for breast cancer and bearing BRCA2 oncogenic mutations. Notably, the risk of breast cancer was found to be further increased to 84% in Ashkenazi Jewish carriers of the c.68_69delAG BRCA1 polymorphism, irrespective of the familial history (5).
Population screening?
These important findings elicit some pragmatic considerations about the cost-effectiveness of widespread population screening for identifying subjects carrying oncogenic BRCA mutations. The recent case of the famous actress Angelina Jolie has added more fuel to the fire of the ongoing debate developing between those in favor or against BRCA (population) screening. Briefly, Angelina Jolie decided to undergo preventive double mastectomy because she was found to be carrier of a BRCA1 mutation, but she had previously lost her mother, grandmother and aunt for cancer while bearing the same polymorphism (6). This paradigmatic case has then contributed to generate the so-called “Angelina Jolie effect”, with the number of BRCA tests in the US increasing from 0.71:100,000 to 1.13:100,000 women (i.e., +60%) immediately after the media gave wide and unfiltered airtime to the event. Along with the broad diffusion in the social media, Jolie’s decision also attracted a large number of negative and positive opinions about the clinical efficacy and consequences of BRCA testing in unaffected women or in those with no risk factors (7).
According to the U.S. Preventive Services Task Force, BRCA testing may be offered to women with family members affected by breast, ovarian, fallopian tube or peritoneal cancer, along to those who may have a family history suggestive for the presence of BRCA mutations (4). This recommendation is hence fully confirmed by the data recently published by Kuchenbaecker et al. (5), since the overall risk of developing breast cancer was shown to increase from 53% to 73% in women carrying a BRCA1 oncogenic mutation and having a positive familial history for this type of cancer, and from 39% to 65% in women carrying a BRCA2 oncogenic mutation and having a positive familial history for breast cancer. Yet, a major question remains unanswered. What should be recommended to women without a positive familial history? Inevitably, whatever conclusion can be reached at this point in time must be accurately weighed against at least four different perspectives, which include the low prevalence of these mutations in the general population, the relatively lower risk of developing breast cancer in women without a family history for this malignancy, the direct and indirect cost of genetic testing and, last but not least, the many potential psychological and clinical consequences (Figure 2).
Figure 2.
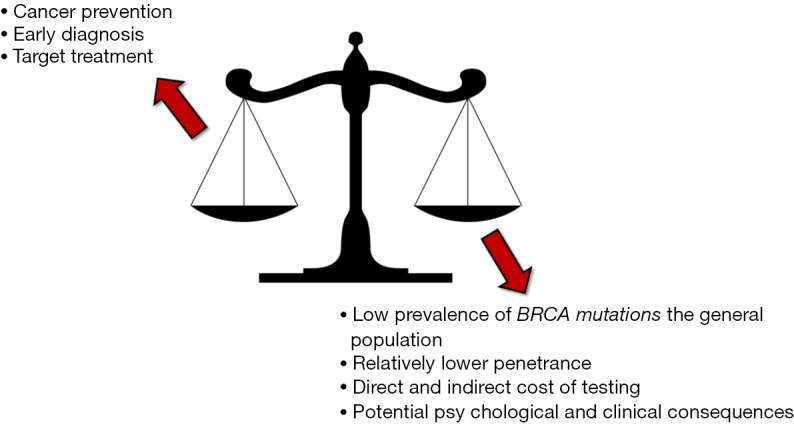
Main advantages and limitations of BRCA population screening.
The first two aspects (i.e., the low prevalence and lower penetrance of BRCA mutations in the general population) have already been argued, but should be further analyzed according to an economic projection. Consistent with a financial and organizational perspective, genetic testing in clinical laboratories is not like screening for cholesterol, glucose or prostate specific antigen (PSA). We may all agree that the cost of measuring cholesterol, regardless of established criteria of appropriates (8), may produce a relatively modest impact on healthcare economics (i.e., the cost of a cholesterol test is approximately 0.045 US$ in the local laboratory). Unlike cholesterol, the cost of BRCA testing currently ranges between 300 and 5,000 US$, depending on the analytical technique and the number or type of mutations being assessed. Therefore, given for granted that none of us would not much complain about performing population screening for hypercholesterolemia, the actual impact of widespread testing for BRCA, even performed once in a lifetime, would be nothing but meaningless. According to the United States Census Bureau, the current US population averages 325.3 million people, 165.8 million of whom are women (9). Therefore, by estimating an optimistic cost of around 1,000 US$ for detecting the most penetrant BRCA mutations, once in a lifetime genetic testing would cost approximately 165,800 million US$ compared to a 325 million US$ (i.e., ~0.20%) expenditure for annual cholesterol screening in all US women in the age range between 18–80 years (Figure 3). This estimation would actually make it unfeasible to even imagine public coverage for this type of genetic testing in a world with limited resources, just now recovering from an unprecedented economic crisis. Yet, someone may then argue that BRCA testing might be ultimately cost-effective, since cancer prevention by “prophylactic” double mastectomy will contribute to lower the overall expenditure for treating breast cancer patients. According to reliable statistics, the cost of treating breast cancer approximated 16.5 billion US$ in the United States in the year 2010 (10). Considering that the prevalence of BRCA mutations is approximately 3% in all breast cancer carriers (4), population screening would theoretically produce a 495 US$ million saving due to prevention of cancer development in carriers of BRCA mutations, but this sum is just 0.30% of the overall predicted expenditure of once in a lifetime BRCA testing (Figure 3).
Figure 3.
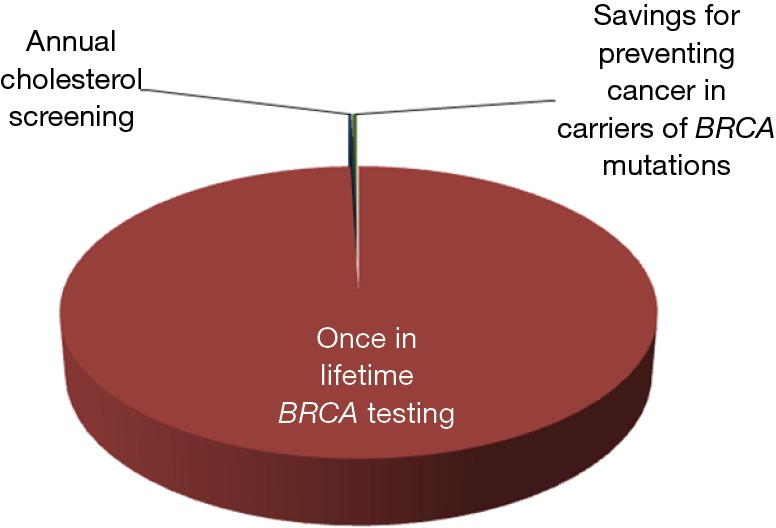
Estimated costs of once in lifetime BRCA testing, expenditure for annual cholesterol screening and potential economic savings for preventing cancer in carriers of BRCA mutations.
Despite we would all agree that each patient can chose to pay out of pocket for a given test, thus including genetic analyses and BRCA “screening” (11), the possible consequences on the healthcare system of a positive test results cannot be discounted. Beside simple surveillance or using chemoprevention, preventive bilateral mastectomy, even with no public health insurance option, may be associated with severe health consequences. Surgery carries a number of immediate risks (e.g., bleeding, infections, lymphedema), but also many long-term complications such as onset of chronic symptoms (i.e., pain, tingling or tightness sensations), fatigue, loss of mobility and the need for cosmetic surgery (12), some of which may ultimately lead the patients to seek publicly funded care, so indirectly raising the economic burden of genetic testing. Indeed, strict surveillance in BRCA-positive women is virtually unavoidable, even though the efficiency of mammography for early cancer detection remains unproven, especially for carriers of BRCA1 oncogenic mutations.
The psychological implication of a positive BRCA test in patients with no familial history for this type of cancer is the last, but not meaningless, issue. As earlier discussed, the penetrance of BRCA mutations is comprised between 39–53% in women with negative familial history (5), so meaning that the probability of developing breast cancer may involve, at worst, one every two women. Some women may hence feel empowered by knowing their BRCA status, but others may suffer this condition as an unsurmountable psychological burden. As for other types of genetic analyses, a positive BRCA testing may disclose unpredictable scenarios, being possibly associated with increased anxiety, distress, persuasion of undergoing “emotionally-driven” but unnecessary preventive surgery, up to possible cases of suicides especially involving psychologically fragile women (13). Then, although prophylactic bilateral mastectomy seems to reduce the future risk of breast cancer by 80-100% in women with highly penetrant BRCA mutations (14), this type of surgery does not have any impact on the future risk of ovarian cancer, which has also been convincingly associated with BRCA status (5). How these women should be managed or treated, especially if they are underage females? Should oophorectomy be considered, despite the risk of developing ovarian cancer in women with negative familial history for this malignancy is only comprised between 16–41% (5)? No definitive answers to these crucial questions have been provided so far, to the best of our knowledge.
Conclusions
Genetic testing, which allows identifying a kaleidoscope of polymorphisms and penetrant mutations with high accuracy and at an affordable cost for many, has completely revolutionized the history of science and medicine (15), thus becoming the ideal bridge connecting laboratory testing and personalized medicine (16). Nevertheless, although a number of extraordinary technological progresses have allowed to increase the quality of in vitro diagnostics and care, some notable threats are also emerging. Widespread genetic testing, without qualified medical advice and guidance, should probably be seen as a risk rather than an opportunity. In particular, the clinical interpretation of genetic cancer testing is already challenging for geneticists and physicians, but it may become awkward or even deceptive for the patients. The screening for BRCA is not an exception to this rule, since the boundaries between over- and under-use are not clearly defined (Figure 4), and the potential risks due to the generation of false-positive results (i.e., unwarranted further testing and unnecessary surgery) have been scarcely explored as well as false-negative findings (i.e., analytical errors, incomplete panels of mutations) (17). Considering that no universal consensus has been reached about the clinical management of patients with oncogenic mutations but no familial history of breast cancer (4,18), being in favor or against a population screening remains a genuine individual opinion. In this still unclear scenario, transparent and patient-oriented genetic counselling becomes imperative. The patient should be clearly informed about the potential clinical advantages and the possible consequences of positive and negative results of BRCA testing, along with the clinical efficacy and the inherent risks of narrow clinical monitoring or management, especially regarding prophylactic mastectomy. Waiting for Godot (i.e., the publication of collective evidence about the clinical efficacy and sustainability of a population BRCA screening), we believe that women at low risk of breast cancer should be allowed to take a medically-driven but autonomous decision about undergoing or declining BRCA testing.
Figure 4.
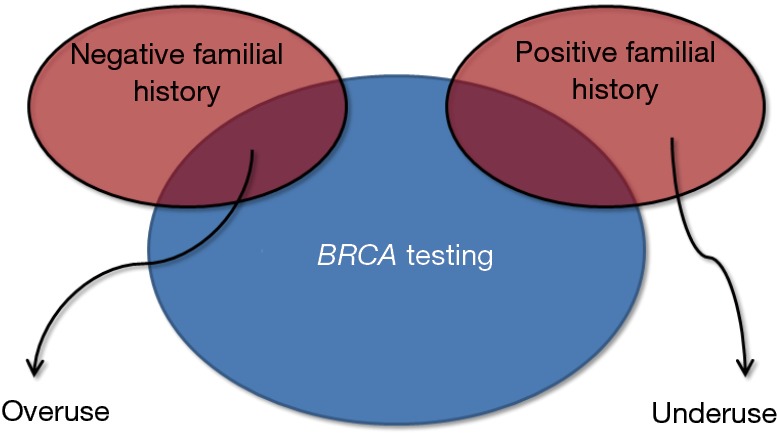
Over- and under-use of BRCA testing.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tao Z, Shi A, Lu C, et al. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys 2015;72:333-8. 10.1007/s12013-014-0459-6 [DOI] [PubMed] [Google Scholar]
- 2.Narod SA, Salmena L. BRCA1 and BRCA2 mutations and breast cancer. Discov Med 2011;12:445-53. [PubMed] [Google Scholar]
- 3.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 2004;4:665-76. 10.1038/nrc1431 [DOI] [PubMed] [Google Scholar]
- 4.Nelson HD, Fu R, Goddard K, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Dec. Report No.: 12-05164-EF-1. [PubMed]
- 5.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 6.McCarthy AM, Armstrong K. The role of testing for BRCA1 and BRCA2 mutations in cancer prevention. JAMA Intern Med 2014;174:1023-4. 10.1001/jamainternmed.2014.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spruill L. Benign mimickers of malignant breast lesions. Semin Diagn Pathol 2016;33:2-12. 10.1053/j.semdp.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Bovo C, Ciaccio M. Inappropriateness in laboratory medicine: an elephant in the room? Ann Transl Med 2017;5:82. 10.21037/atm.2017.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Census Bureau. Available online: https://www.census.gov/popclock/. Last access: 21 June, 2017.
- 10.Costs of Care. The Costs of Breast Cancer in the U.S. Available online: http://costsofcare.org/the-costs-of-breast-cancer-in-the-u-s/. Last access: 21 June, 2017.
- 11.Plebani M, Lippi G. Direct-to-consumer genomewide profiling. N Engl J Med 2011;364:2074-5. 10.1056/NEJMc1103048 [DOI] [PubMed] [Google Scholar]
- 12.Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J 2015;19:48-79. 10.7812/TPP/14-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G, Favaloro EJ, Plebani M. Direct-to-consumer testing: more risks than opportunities. Int J Clin Pract 2011;65:1221-9. 10.1111/j.1742-1241.2011.02774.x [DOI] [PubMed] [Google Scholar]
- 14.Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001;345:159-64. 10.1056/NEJM200107193450301 [DOI] [PubMed] [Google Scholar]
- 15.Katsanis SH, Katsanis N. Molecular genetic testing and the future of clinical genomics. Nat Rev Genet 2013;14:415-26. 10.1038/nrg3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Prec Med 2016;1:7 10.21037/jlpm.2016.12.01 [DOI] [Google Scholar]
- 17.Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 2014;160:255-66. 10.7326/M13-1684 [DOI] [PubMed] [Google Scholar]
- 18.Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 2007;107:159-62. 10.1016/j.ygyno.2007.09.031 [DOI] [PubMed] [Google Scholar]


