Abstract
The present work aims to develop a growth medium to render a wild-type strain of Saccharomyces cerevisiae permeable to the antifungal drug Brefeldin A. In the current study, a synthetic medium containing 0.1% L-proline and supplemented with 3.0 × 10-3% SDS is employed. When Brefeldin A is added to this medium, a wild-type strain shows increased growth sensitivity and a diminished transport of the amino acid L-leucine. Since Brefeldin A exerts its effect on the endoplasmic reticulum and the Golgi apparatus, the medium permits the study of the drug effect on the intracellular traffic of L-leucine permeases.
INTRODUCTION
Before their delivery to the plasma membrane (PM), the different permeases involved in amino acid transport, like most of the membrane proteins, enter the membrane of the endoplasmic reticulum (ER). They then proceed through the protein secretory pathway of the ER, via the Golgi complex (GC) and exocytic vesicles, until they finally reach the PM [1].
A very useful agent for investigating permease transport through the secretory pathway is the antifungal agent, Brefeldin A (BFA), which reversibly blocks the transport of proteins from the ER to the Golgi [2, 3, 4]. This drug can be used to create a temporary block in transport, allowing accumulation of permeases in the ER and depletion of these permeases downstream. In addition, when the BFA block is present, loss of permease molecules from the PM through endocytosis can be studied independent of their replacement via the secretory pathway. Moreover, release of the BFA block would permit the investigation of the dynamics of replacing the permeases in the depleted membrane. Because wild-type yeast has a very low apparent permeability to BFA, previous investigations have used strains bearing the erg6 mutation that blocks the final methylation reaction in ergosterol biosynthesis. The lack of ergosterol in the PM changes the permeability properties of the membrane and renders cells sensitive to several inhibitors, including BFA and the dye, crystal violet (CV) [2]. These changes appear to be at least partly due to decreases in activity of multidrug resistance pumps such as Pdr5p [5].
There are several disadvantages of using the erg6 mutation to obtain BFA sensitivity. The mutation itself causes a marked increase in permeability to sodium and lithium ions [6]. Efficiency of genetic transformation is lowered dramatically, and sexual conjugation is also greatly reduced. Moreover, transport of tryptophan is lowered substantially [7].
We have developed a simple method for obtaining BFA sensitivity without requiring the introduction of erg6. Because the method requires no genetic manipulation, it can be applied to wild-type cells and to strains already bearing various mutations related to secretion, to altered amino acid transport, and to modified permease turnover. The method depends upon the use of an SDS-supplemented synthetic growth medium in which the wild-type strain MMY2 presents increased sensitivity to BFA. At appropriate concentrations, BFA inhibits growth and causes diminished transport of the amino acid L-leucine.
MATERIALS AND METHODS
Strains, media, and growth conditions
Saccharomyces cerevisiae strains MMY2 (MAT a ura3), S288c (MAT α mal gal2), and the strains DBY2057 (MAT a ura3-52) and DBY1885 (MAT a ura3-52 sac1-6), kindly provided by Dr. R. Kölling (Heinrich-Heine-Universität Düsseldorf), were used.
Assays were carried out in media of the following compositions.
MPD (minimal proline medium): 0.17% yeast nitrogen base (Difco, Detroit, Mich, USA), 0.1% L-proline, and 2% dextrose.
MAD(0.2D) (minimal ammonium medium, 0.2% dextrose): 0.17% yeast nitrogen base, 0.3% (NH4)2 SO4, and 0.2% dextrose.
MAD (minimal ammonium medium): 0.17% yeast nitrogen base, 0.3% (NH4)2 SO4, and 2% dextrose.
YPD medium: 1% yeast extract, 1% peptone, and 2% dextrose.
Each medium was supplemented with 20 mg mL-1 uracil. Solid medium contained 2% agar. Following sterilization, for CV resistance assays, the dye (1.7 mg mL-1 ethanolic solution) was added to the medium to a final concentration of 1.0 μg mL-1.
In some experiments, MPD and MAD were supplemented with a sterile SDS solution to a final concentration of 3 × 10-3%. Then, colonies on control plates without SDS represent 100% viable cells. Cells inoculated in liquid medium were grown at 30°C with constant agitation.
Optical Density (OD) of each yeast cell suspension was determined at 570 nm and used to calculate the necessary dilutions needed to reach 350–650 colony-forming units (cfu) on solid YPD, MPD, or MAD plates.
Assays
Crystal violet resistance
For each growth assay, 5-μL spots of yeast suspension were made on the corresponding solid MPD, MAD(0.2D), and MAD media, with or without CV, followed by incubation at 30°C.
Effects of BFA in solid medium
Glass-fiber filters (Schleicher & Schuell, Inc, NH, USA) of 8- mm diameter were coated with 10 μL of 7.7 g L-1 BFA solution (68 : 32 ethanol-DMSO as solvent). After drying at 37°C, filters were deposited on the solid medium surface previously inoculated with 1.0 × 105 cfu of strain MMY2 cell suspension to form a lawn of growth. Halo formation indicating growth inhibition was then recorded.
Effects of BFA in liquid culture
An aliquot of cells from an exponential phase culture in YPD was suspended in MPD with and without 3 × 10-3% SDS, and incubated at 30°C on a rotary shaker, at 5 × 107 cells mL-1 initial concentration. Various concentrations of BFA were tested in MPD-SDS as shown in Results. Samples of cell suspensions were withdrawn at zero, 30, and 60 minutes and plated for single colonies on YPD plates to determine survival.
Determination of BFA effects on L-leucine uptake
Samples containing 108 cells from an exponential phase culture in YPD medium were centrifuged at 4000 rpm using a bench centrifuge. Cell pellets were then washed with sterile distilled water and resuspended in 2.0 mL of MPD-SDS or in the same medium containing BFA. Mixtures were incubated for 10 or 20 minutes at 30°C with agitation, followed by a 5-minute centrifugation at 4000 rpm, and the supernatant fluid containing BFA was removed. The pellet was immediately resuspended in 2.0 mL of fresh MPD without SDS and then incubated for 60 minutes at 30°C with agitation. This incubation allows the derepression of the L-leucine transport systems [8]. Following incubation, a sample was withdrawn for colony counting. The remaining cells were centrifuged, and the pellet was resuspended in water to give a final concentration of 5 × 108 cells mL-1. The suspension was then used to determine L-leucine uptake values at zero and after 5 minutes.
A 100-μL aliquot containing 5 × 107 cells of the above MMY2 suspension, previously incubated in the presence or absence of BFA, was mixed with an equal volume of 0.1 mmol L-1 L-14C leucine (5 mCi mmol-1) in 40 mmol L-1 potassium phthalate, pH 4.5. The mixture was then incubated with shaking for zero or 5 minutes at 30°C. At the end of the incubation, the sample was filtered through glass-fiber filters, washed with three portions of 2.0 mL ice-cold 20 mmol L-1 potassium phthalate, pH 4.5, and the radioactivity of the cells on each filter was determined with a liquid scintillation counter. Values were expressed as the difference between the values at zero and at 5 minutes. Using the colony counts obtained after 48 hours, the uptake of the amino acid was expressed as nmol/108 cfu. For each assay, all measurements were made in duplicate, and the values presented are the average of two experiments (average variation was less than 5%).
RESULTS
Yeast growth inhibition on solid media containing crystal violet
When the erg6 mutation is introduced into a yeast strain, the cells become hypersensitive to multiple inhibitors, including BFA, the dye CV, and cycloheximide [7]. Therefore, the possibility was considered that if a medium could be developed that enhanced CV permeability, it might also increase BFA permeability. To select a medium in which sensitivity to the dye is increased, CV resistance of the wild-type strains MMY2, S288c, DBY2057, and the sac1 mutant DBY1885 on different growth media was determined.
McCusker and Davis [9], while studying the sensitivity of yeast to 5-fluorouracil (5FOA), found that the wild strain S288c is more sensitive when grown in medium containing the amino acid L-proline as the only nitrogen source. Similarly, we previously found that a medium containing L-proline or L-leucine as the sole source of nitrogen also produces a condition in which S288c cells are more sensitive to the antifungal drug fluconazole [10]. On the other hand, cells grown in a medium containing ammonium ion as the only nitrogen source and a low glucose concentration (0.2%) have a decreased cell wall thickness [11]. Based on this information, CV resistance to concentrations previously used [2] was tested on three different growth media, MPD, MAD(0.2D), and MAD. A 5-μL sample of each yeast suspension, 1.0 × 107 cells mL-1, was added to the solid medium surface. Cell growth was evaluated after 24 hours at 30°C in MAD(0.2% and 2% glucose), and after 48 hours in MPD, by observing the respective growth density of the four strains (Figure 1). The greatest resistance to the dye was found in MAD. This resistance decreases when the carbon source concentration is lowered to 0.2%, MAD(0.2D), and is minimal in medium in which L-proline is the only nitrogen source (MPD). Although Hughes et al [12] described an increased sensitivity to BFA for certain sac1 mutants, increased sensitivity to CV of the sac1 mutant DBY1885 was not observed in this experiment. Because use of proline as the sole nitrogen source increased sensitivity to CV, subsequent experiments with BFA were carried out on MMY2 grown in MPD. We have previously demonstrated that in this medium the transport of L-leucine in MMY2 is carried out by three systems, GAP, S1, and S2 [8]. Therefore, to study the effects of BFA on leucine transport, strain MMY2 was chosen for the rest of the study.
Figure 1.
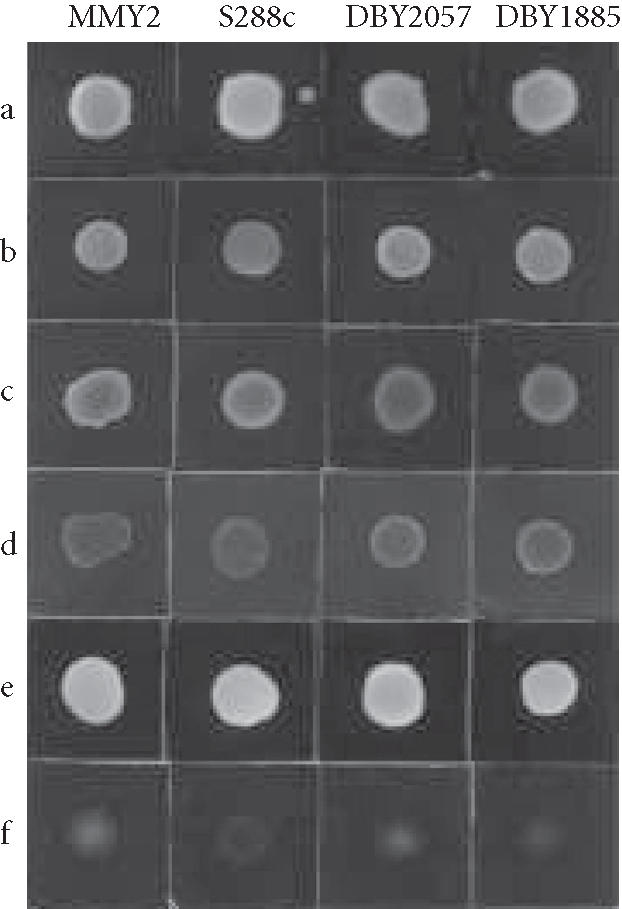
Effect of growth medium on the sensitivity of different strains of S cerevisiae to crystal violet. Aliquots (5 μL) of suspensions of MMY2, S288c, DBY2057, or DBY1885 in distilled water were added to the surface of the solid medium: (a) MAD; (b) MAD + CV; (c) MAD(0.2D); (d) MAD(0.2D)+CV; (e) MPD; and (f) MPD + CV.
Effects of SDS on cellular viability in MPD
In a wild-type strain of the fission yeast Schizosaccharomyces pombe, Nagao et al [13] showed that SDS increases the permeability to BFA. To render S cerevisiae MMY2 cells sensitive to BFA, we found that the addition of low concentrations of SDS to MPD was effective. Initially, the SDS concentration of 6 × 10-3% used for S pombe was tried, but this resulted in nearly 90% viability loss (data not shown). Therefore to select a concentration that did not considerably reduce viability, similar assays were made with a series of SDS dilutions in MAD. The chosen value was 3 × 10-3% SDS (final concentration) which was then tested on both MAD and MPD media. At this concentration, the detergent alone had no detectable effect on viability in medium containing ammonium ion (MAD), and it produced only a 26% decrease in viability in medium containing L-proline (MPD) (data not shown).
Inhibition of yeast growth by BFA
To verify that SDS increases the permeability to BFA in MMY2 cells grown on solid MPD, a test for formation of inhibition halos was performed. For this purpose, 200 μL of a yeast suspension (1.0 × 106 cells mL-1) was distributed homogeneously on plates of solid MPD and on the same medium supplemented with 3.0 × 10-3% SDS. Following this, glass-fiber filters containing 25, 39, and 77 μg of BFA, respectively, were placed on the plate. With the two lower BFA quantities, no inhibition halos were observed. With 77 μg of BFA on MPD-SDS, an inhibition halo of approximately 2 mm was observed, but no halo appeared in the control lacking SDS (Figure 2).
Figure 2.
Effect of BFA on growth of MMY2 on medium containing SDS. MMY2 cells were grown for 4 days at 30°C on MPD (left) or on the same medium supplemented with 3 × 10-3% SDS (right). Each disk contained 77 μg BFA. Two-mm halos are formed on the plate with SDS.
Considering the results obtained on solid medium supplemented with SDS, and estimating the volume of the medium below the halo as 1.0 mL, the concentration of BFA should be close to 77 μg mL-1. To establish whether the BFA-sensitive trait is also observed in liquid medium, cells were incubated with this concentration of drug in MPD-SDS broth, and viability of MMY2 cells was determined by colony counting.
Effects of BFA in liquid culture
The initial concentration of BFA used was 80 μg mL-1. Following an incubation of 60 minutes, viability was found to have decreased 80% (data not shown). Two other concentrations, 40 or 60 μg mL-1, were therefore chosen to obtain greater cell viability, and samples were taken as described in Methods (Figure 3). Addition of SDS and the solvent (ethanol-DMSO) did not alter viability compared with nonsupplemented medium, whereas both lower concentrations of BFA resulted in decreases in viability less than that obtained with 80 μg mL-1. The effect of 40 μg mL-1 and 60 μg mL-1 on cell viability was 42% and 56%, respectively, during the first 30 minutes, while a very small change was observed during the second 30 minutes. Therefore, 40 μg mL-1 was chosen as the BFA concentration in which most of the cells are viable.
Figure 3.
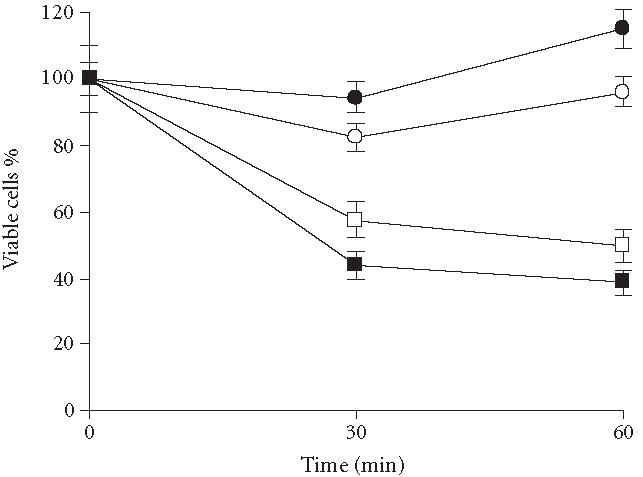
Effect of BFA on the viability of strain MMY2 in broth culture. Cells of strain MMY2 were incubated 60 minutes on MPD containing 40 μg mL-1 or 60 μg mL-1 of BFA. Incubation was performed with and without SDS (3 × 10-3%). Following incubation, a sample was withdrawn to inoculate plates containing YPD medium. After 3 days at 30°C, colony numbers were recorded. (○) MMY2 control; (•) MMY2 + SDS + solvent; (□) MMY2 + SDS + BFA (40 μg mL-1); and (▪) MMY2 + SDS + BFA (60 μg mL-1).
L-leucine transport in MMY2 cells previously incubated with BFA
To determine the effects of BFA on L-leucine transport, a 40 μg mL-1 concentration of BFA was used, and incubation intervals were decreased to 10 and 20 minutes, thereby minimizing any lethal effects of BFA. The results of these experiments are summarized in Figure 4. When cells were incubated for 10 minutes, the decrease in the uptake value was 49% compared to controls, whereas at 20 minutes, the BFA caused a 59% decrease in leucine uptake. Values obtained at zero time (L-leucine binding) in cells incubated with and without BFA showed no significant difference (). In cells with no previous MPD incubation to derepress leucine permeases, an uptake value of 2 nmol/108 cells was obtained.
Figure 4.
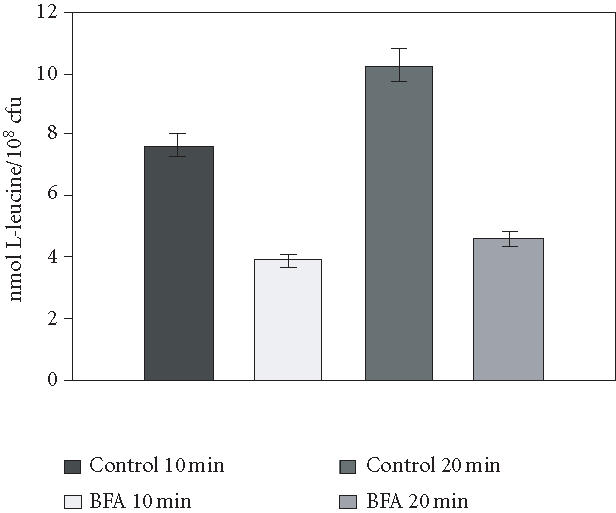
Effects of BFA on L-leucine transport in strain MMY2. MMY2 cells were incubated on MPD broth containing SDS (3 × 10-3%) and BFA (40 μg mL-1) during 10 or 20 minutes. To eliminate BFA, cells were centrifuged and the precipitate resuspended in fresh MPD. After another 60-minute incubation, cells were again centrifuged, and each pellet was resuspended at a concentration of 5 × 108 cells mL-1 in distilled H2O. For amino acid uptake values at zero or 5 minutes, 100 μL of the suspension was mixed with 100 μL-14C-Leucine, 0.1 mmol l-1 (5 mCi mmol-1). As a control for each time, an incubation in MPD with SDS was used. Viability determinations on aliquots of each incubation gave 70% for the 10-minute incubation and 58% for the 20-minute incubation.
Considering that the L-leucine uptake was determined after the BFA treatment, we measured the capacity of the cell to resume growth after the treatment. For this purpose, the doubling time of the cells was established in MPD and the same medium with SDS or SDS-BFA. No significant difference in doubling time among the treatments was found (MPD: 529 minutes, MPD-SDS: 517 minutes, and MPD-SDS-BFA: 506 minutes). Experiments performed in media with sucrose instead of dextrose as the carbon source gave similar values (control: 465 minutes; plus SDS: 491 minutes; and plus SDS-BFA: 463 minutes). These measurements indicate that the treatment affected neither the capacity of MMY2 cells to grow nor their invertase secretion. Neither the amino acid transport value nor cell viability was affected when only 20 μg mL-1 of BFA was used in similar experiments.
DISCUSSION
Susceptibility of yeast to a toxic compound varies with the growth conditions. Therefore, it is possible to alter the apparent toxicity of an inhibitor by modifying the growth medium. In the present work, we observe that a poor nitrogen source like L-proline results in increased sensitivity of cells to toxic compounds, such as CV or SDS.
This result was expected, considering that the same nitrogen source increases the susceptibility to 5FOA and fluconazole [9, 10] and decreases rhodamine 6G efflux [14]. Nevertheless, under our experimental conditions, using unsupplemented L-proline-based medium and the established BFA concentrations for a BFA-susceptible erg6 strain (FKY 212), MMY2 was not sensitive to the drug. This result indicates that the phenotype obtained with MPD is not exactly analogous to the one obtained with an erg6 mutant. Therefore, addition of SDS to the medium was required to produce a significant increase in the sensitivity of MMY2 to BFA.
It may be considered that addition of the SDS detergent, besides having an effect on the susceptibility to BFA, might alter the L-leucine uptake values. However, the SDS concentration used here resulted in less than a 10% decrease in the values of L-leucine transport (data not shown).
In order to extend the utility of the medium to L-leucine transport studies, the amino acid uptake was measured immediately after BFA treatment. We found that BFA plus SDS decreased the measured uptake values by 53% compared to the control medium or the medium with only SDS addition (data not shown). Clearly, the use of MPD-SDS growth medium allows one to obtain BFA-sensitive cells which exhibit a significant alteration in L-leucine transport. In contrast, under similar growth conditions, and without the BFA-SDS addition, an erg6 mutant presents a 50% decrease in leucine uptake values relative to the wild-type strain (data not shown). Moreover, the inconvenience of erg6 strains used in permeases studies has recently been shown by Umebayashi and Nakano who demonstrated that the erg6 mutation causes missorting of the high-affinity tryptophan permease, Tat2p [15].
The effects of BFA on leucine transport indicate that cells of strain MMY2 contain a BFA-sensitive target, probably in the secretion pathway, that becomes blocked, preventing transport of newly synthesized permease molecules from the ER to the PM via the Golgi.
Because conditions were selected that minimized cell death, and L-leucine-specific uptake activity is based only on surviving cells, it is likely that the decreased activity is primarily due to inhibition by BFA of vesicle assembly at the ER membrane [16, 17]. The incomplete inhibition could indicate the existence of separate pathways for transport of different permeases capable of L-leucine uptake.
The growth medium described presents the following advantages. (a) It is a standard synthetic medium requiring only the particular nitrogen source L-proline and addition of the detergent. (b) The concentrations of BFA used to obtain a significant effect on growth are smaller than, or of the same order as, those employed in erg6 strains. (c) The medium would allow the isolation of BFA-resistant mutants starting from wild-type strains. (d) With this method, we have developed a new probe to study the turnover of amino acid permease proteins.
ACKNOWLEDGMENT
This work was supported by Grant PICT99 01-06133 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of Argentina. Vanesa G. Pannunzio is supported by a fellowship from ANPCyT.
References
- 1.Bennett M.K, Scheller R.H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham T.R, Scott P.A, Emr S.D. Brefeldin A reversibly blocks early but not late protein transport steps in the yeast secretory pathway. EMBO J. 1993;12(3):869–877. doi: 10.1002/j.1460-2075.1993.tb05727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, Klausner R.D. Brefeldin A reversibly inhibits secretion in Saccharomyces cerevisiae. J Biol Chem. 1993;268(8):5345–5348. [PubMed] [Google Scholar]
- 4.Vogel J.P, Lee J.N, Kirsch D.R, Rose M.D, Sztul E.S. Brefeldin A causes a defect in secretion in Saccharomyces cerevisiae. J Biol Chem. 1993;268(5):3040–3043. [PubMed] [Google Scholar]
- 5.Kaur R, Bachhawat A.K. The yeast multidrug resistance pump, Pdr5p, confers reduced drug resistance in erg mutants of Saccharomyces cerevisiae. Microbiology. 1999;145(pt 4):809–818. doi: 10.1099/13500872-145-4-809. [DOI] [PubMed] [Google Scholar]
- 6.Welihinda A.A, Beavis A.D, Trumbly R.J. Mutations in LIS1 (ERG6) gene confer increased sodium and lithium uptake in Saccharomyces cerevisiae. Biochim Biophys Acta. 1994;1193(1):107–117. doi: 10.1016/0005-2736(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 7.Gaber R.F, Copple D.M, Kennedy B.K, Vidal M, Bard M. The yeast gene erg6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989;9(8):3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotliar N, Stella C.A, Ramos E.H, Mattoon J.R. L-leucine transport systems in Saccharomyces cerevisiae participation of GAP1, S1 and S2 transport systems. Cell Mol Biol (Noisy-le-grand) 1994;40(6):833–842. [PubMed] [Google Scholar]
- 9.McCusker J.H, Davis R.W. The use of proline as a nitrogen source causes hypersensitivity to, and allows more economical use of 5FOA in Saccharomyces cerevisiae. Yeast. 1991;7(6):607–608. doi: 10.1002/yea.320070608. [DOI] [PubMed] [Google Scholar]
- 10.Stella C.A, Costanzo R, Burgos H.I, Saenz D.A, Venerus R.D. L-proline as a nitrogen source increases the susceptibility of Saccharomyces cerevisiae S288c to fluconazole. Folia Microbiol (Praha) 1998;43(4):403–405. doi: 10.1007/BF02818581. [DOI] [PubMed] [Google Scholar]
- 11.Gaskova D, Brodska B, Herman P, et al. Fluorescent probing of membrane potential in walled cells: diS-C3(3) assay in Saccharomyces cerevisiae. Yeast. 1998;14(13):1189–1197. doi: 10.1002/(sici)1097-0061(19980930)14:13<1189::aid-yea320>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Hughes W.E, Pocklington M.J, Orr E, Paddon C.J. Mutations in the Saccharomyces cerevisiae gene SAC1 cause multiple drug sensitivity. Yeast. 1999;15(11):1111–1124. doi: 10.1002/(SICI)1097-0061(199908)15:11<1111::AID-YEA440>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Nagao K, Taguchi Y, Arioka M, et al. bfr1+, a novel gene of Schizosaccharomyces pombe which confers brefeldin A resistance, is structurally related to the ATP-binding cassette superfamily. J Bacteriol. 1995;177(6):1536–1543. doi: 10.1128/jb.177.6.1536-1543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stella C.A, Burgos H.I, Vicente J.E. In: Research Advances in Antimicrobial Agents & Chemotherapy. Vol. 3. Kerala, India: Global Research Network; 2003. Rhodamine 6G efflux in Saccharomyces cerevisiae with ammonium or L-proline as nitrogen source; pp. 73–77. [Google Scholar]
- 15.Umebayashi K, Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol. 2003;161(6):1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson C.L, Kepes F. BFR1, a multicopy suppressor of brefeldin A-induced lethality, is implicated in secretion and nuclear segregation in Saccharomyces cerevisiae. Genetics. 1994;137(2):423–437. doi: 10.1093/genetics/137.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyroche A, Courbeyrette R, Rambourg A, Jackson C.L. The ARF exchange factors Gea1p and Gea2p regulate Golgi structure and function in yeast. J Cell Sci. 2001;114(pt 12):2241–2253. doi: 10.1242/jcs.114.12.2241. [DOI] [PubMed] [Google Scholar]



