Abstract
Ocean acidification (OA) increasingly threatens marine systems, and is especially harmful to calcifying organisms. One important question is whether OA will alter species interactions. Crustose coralline algae (CCA) provide space and chemical cues for larval settlement. CCA have shown strongly negative responses to OA in previous studies, including disruption of settlement cues to corals. In California, CCA provide cues for seven species of harvested, threatened, and endangered abalone. We exposed four common CCA genera and a crustose calcifying red algae, Peyssonnelia (collectively CCRA) from California to three pCO2 levels ranging from 419–2,013 µatm for four months. We then evaluated abalone (Haliotis rufescens) settlement under ambient conditions among the CCRA and non-algal controls that had been previously exposed to the pCO2 treatments. Abalone settlement and metamorphosis increased from 11% in the absence of CCRA to 45–69% when CCRA were present, with minor variation among CCRA genera. Though all CCRA genera reduced growth during exposure to increased pCO2, abalone settlement was unaffected by prior CCRA exposure to increased pCO2. Thus, we find no impacts of OA exposure history on CCRA provision of settlement cues. Additionally, there appears to be functional redundancy in genera of CCRA providing cues to abalone, which may further buffer OA effects.
Introduction
Predictions of marine ecosystem function under future ocean acidification (OA) suggest that the acidification rate1–3 is likely to overwhelm the capacity of many species to respond4 because OA is creating conditions organisms may not have experienced in their evolutionary history5. Further, the impacts of OA on single species or groups of species may cascade through ecosystems in ways that alter overall species diversity and ecosystem functions6, especially when the target species have critical ecological roles, such as habitat or food for other organisms. Because most ocean acidification studies focus on individual species, rather than species interactions7, understanding how OA alters interactions and ultimately ecosystem functioning remains a major knowledge gap3, 6, 8 and research priority9, 10.
The strongest potential impacts of OA are on marine calcifiers1, 6. Calcified structures occur widely across many phyla and have evolved independently and repeatedly over geologic time4. Calcifying organisms are abundant in marine systems and include many holoplankton, benthic invertebrates, and benthic algae. If calcifiers are unable to adapt to OA-induced seawater changes, there may be major changes throughout marine communities, because numerous non-calcifying organisms rely on calcifiers for food or biogenic habitat1. Crustose coralline algae (CCA) appear to be the calcifiers most vulnerable to OA6 because they deposit high-Mg calcite, the most soluble form of biogenic CaCO3 11. In experiments with even relatively mild increased pCO2 (600–850 µatm), up to 100% reduction in CCA growth or cover have been reported12–14. CCA are not only widespread and abundant (forming 25–70% of the benthos in tropical and temperate reefs15–17), but also play important ecological roles including substrate consolidation, providing food for grazers, and providing space and cues for invertebrate larval settlement15, 18, 19. In addition to CCA, crustose and calcifying but non-coralline red algae from the genus Peyssonnelia are common on the world’s shelves20. Unlike CCA, Peyssonnelia deposit aragonitic calcite. The majority of studies on Peyssonnelia are from the tropics and Mediterranean, or from rhodolith beds, but Peyssonnelia has been found as a common substate in at least parts of the Pacific coast of the USA21.
The role CCA (and potentially other red algal crusts) play in providing cues for invertebrate larval settlement is critical: loss or change in coralline crusts can cause dramatic reduction in recruitment15, 22, thus indirectly altering invertebrate population dynamics. Diverse taxa (including sea urchins, abalones, limpets, scleractinian corals, and octocorals) have chemosensory systems that recognize particular chemical cues from CCA or their associated bacterial biofilms that induce larval settlement19. At least one type of non-coralline red algae induces abalone larval settlement (Hildenbrandia dawsonii)23.Thus, changes in the settlement cues provided by crustose calcifying red algae (CCRA, including both CCA and other calcifying red crusts) may have ecosystem-level consequences, mediated by changes in settlement of invertebrate species19, 24. Previous studies show that OA may reduce CCA coverage, thickness, skeletal strength, or physiological properties. The few studies that have focused on the effects of OA on CCA cues provided to settlers have focused on corals, and acidification of CCA led to reductions in coral settlement of up to 86% due to changes in settlement cues25, 26.
In the temperate rocky reefs of California, CCRA comprise ~30% of benthos (JKO unpublished data) and are critical settlement substrates for seven species of California abalone, including endangered species (the white and black abalone, Haliotis sorenseni and H. cracherodii) and the recreationally fished red abalone (H. rufescens). Despite several studies on the impacts of OA on larval development, growth, and survival of abalone in the California Current27–29 there have been no studies to date that evaluate the potential indirect impacts of OA on abalone larval settlement. During the upwelling season, over a period of several months CCRA are exposed to repeated low pH events, raising the question of how this exposure may affect their properties as a settlement substrate for invertebrate larvae. While abalone larvae may also be affected by exposure to OA, larvae are in their pelagic larval phase for only 5–10 days30 and may not see low pH conditions during this period. Exposure of tropical CCRA to OA has led to changes in both larval recruitment rate and larval preference for algal substrates25.
We grew four genera of common CCRA from California kelp forests under three pCO2 levels ranging from normal to extreme (419–2,013 µatm) to produce living algae differing only in pCO2 history. We used these algae in trials to determine whether abalone have different settlement rates on common CCRA, and whether algal pCO2 exposure history affects interactions between the algae and larval abalone settlers when only the algae are exposed to OA conditions. We hypothesized that elevated pCO2 would greatly diminish abalone settlement through changes in settlement cues, but with differential effects across algal genera. Thus, we also hypothesized that the importance of any OA effects on CCRA for abalone would depend on whether susceptible algae were also preferred by abalone settlers.
Methods
Algal collection and preparation
We collected algae crusts from cobbles at 9 m depth in kelp forests in the Monterey Bay, California, in February 2013. CCRA covered cobbles are the primary abalone settlement habitat31. Fifteen cobbles (1000 to 4000 cm3) were collected at Lovers Point (N36°37′ 32.65, W121°54′ 54.55) and 15 from the Hopkins Marine Reserve (HMR, N36°37′ 10.57, W121°54′ 11.61) and stored in running seawater at the HMR under ambient Monterey Bay temperature (~11.7 °C) and pH (~7.81) until early March 2013. Fluorescent bulbs provided light on a 12 hour cycle at ~15 µmol/m2/s, approximating average light levels measured at mid-day under kelp canopy at 9 m depth at HMR in October 2011 and March 2012 (LI-COR PAR measurement unit with an LI-193 spherical quantum sensor). Thin rock pieces (30–405 mm2) covered with a single algal morphology were cut from the cobbles using a rock saw, maintained in running seawater, and examined under an Olympus SZX-ILLD100 dissecting scope to ensure that no invertebrates were attached or embedded. Each piece was assigned to one of five morphologically distinct groups based on: type of reproductive structure (conceptacle versus nemathecium), size, and distribution; surface growth patterns; growth margins; crust thickness; and hypothallus cell arrangement (for CCA only).
To identify each morphological group, we sequenced 5–10 representatives per group. After extracting total genomic DNA for CCRA32, 33, we amplified partial sequences from two plastid encoded genes (rbcL and psbA) used extensively to distinguish genera and species of CCRA32, 34. Amplification and sequencing protocols were those of 33. Sequences were obtained from an ABI 3100 Genetic Analyzer at the University of North Carolina, Wilmington (see32). Identifications were based on sequencing ~20% of the rbcL gene and matching with sequences from curated type specimens. Reference samples of each sequenced crust have been deposited in the University of North Carolina Herbarium (NCU) herbarium. Four of the five morphological groups were taxonomically consistent to genus, and these were used in the experiments.
We created thirty replicate vials per algal taxon on March 2, 2013 by epoxying 1–3 pieces of alga into clear polystyrene vials (47.75 mm diameter ×102.12 mm height, 147 mL) using Z-spar epoxy compound. The living algal surfaces were flush with the epoxy, ~20 mm above the vial bottom, with space to grow laterally to at least double the initial area. Vials were open at the top and submerged in seawater in an upright position at the Hopkins Marine Station (conditions described above). Crust area was measured from digital photographs (Canon Powershot S100) using Image J (http://rsbweb.nih.gov/ij/). On March 7, 2013 the vials were transported in cooled seawater to the Monterey Bay Aquarium Research Institute (MBARI).
Algal pCO2 Conditioning
Experimental water was prepared at three pCO2 levels: ambient, high and extreme (Table 1a). The ambient treatment (418.5 µatm pCO2; pH 9.2; Ωarag 1.81) represented non-upwelling conditions in central California (pH 7.7–8.1)35. The high pCO2 treatment (1,175.5 µatm; pH 7.52; Ωaragonite 0.77) resembled the IPCC 2007 high-level projections for global oceanic pCO2 (worst-case stabilization level VI, pCO2 > 900)36 and the worst-case representative carbon pathway (RCP 8.5)37. In the Monterey Bay, this “high” level of pCO2 can occur during upwelling events. The extreme treatment (2,012.9 µatm; pH 7.31; Ωaragonite 0.49) exceeded both predictions for the year 210033 and levels found in nearshore waters during strong upwelling38, 39. We report Ωaragonite because the solubility of high-Mg calcite is closer to aragonite than calcite24 (see Table 1a for Ωcalcite).
Table 1.
(a) Water chemistry set points (mean ± 1 SE) in gas-control tanks at MBARI, measured continuously every 60 seconds between March 7 and July 10, 2013. (b) Water chemistry as measured in the bins containing coralline algae and calculations of pCO2 and Ω (from pH and total alkalinity). Salinity was 33.9 ± 0.05 ppm.
| (a) | ||||||
|---|---|---|---|---|---|---|
| Treatment | pH | O2 (µM) | Temperature (°C) | |||
| Ambient | 7.906 ± 0.053 | 247.51 ± 0.44 | 14.77 ± 0.03 | |||
| High | 7.502 ± 0.001 | 247.89 ± 0.14 | 14.20 ± 0.03 | |||
| Extreme | 7.205 ± 0.001 | 252.22 ± 0.15 | 14.25 ± 0.03 | |||
| (b) | ||||||
| Treatment | DIC (µmol/kg) | Alkalinity (µmol/kg) | pH | pCO 2 (µatm) | Ω aragonite | Ω calcite |
| Ambient | 2100.1 | 2256.5 | 7.92 | 418.5 | 1.81 | 2.84 |
| High | 2227.0 | 2246.4 | 7.52 | 1175.5 | 0.77 | 1.20 |
| Extreme | 2291.6 | 2247.6 | 7.31 | 2012.9 | 0.49 | 0.76 |
The water was prepared by passing through a series of partially recirculating tanks. Oxygen and CO2 were stripped by bubbling nitrogen gas through membrane contactors40. The low-O2, low-CO2 water was the source water for 3 gas-controlled tanks where specified pH and water chemistry was maintained, continuously monitored40, and delivered at ~50 l*h−1 to flow-through plastic aquaria (56 × 30 × 25 cm) containing the algae vials.
One plastic flow-through aquaria was used for each pCO2 treatment, in a temperature controlled room with no outside light. Ten vials per algal taxon were assigned haphazardly to each of the three pCO2 levels along with 10 control vials (containing epoxy but no algae) and submerged vertically in the aquaria (top of vials under 15 cm of water) with vial tops open. Aquaria contained 4.2 L of water and water delivery was at 50 l*h−1, so water in aquaria and vials would be replaced every ~5 minutes. Vials were systematically mixed in the aquaria and rotated weekly. The vials were kept in the pCO2 treatments from March 7 to July 10, 2013. Interior and exterior walls of the vials, algal surfaces, and plastic aquaria were gently brushed weekly with a soft toothbrush to remove any accumulated diatoms which can reduce light levels and are normally removed by grazers. Algal surfaces were examined via digital photographs during the first month of the experiment, and under an Olympus dissecting microscope (40x magnification) immediately upon removal from MBARI, and there was no evidence of algal surface damage.
Sensors in the reservoir tank and each gas-controlled tank measured temperature, oxygen and carbon dioxide concentrations, and pH every 60 seconds (Table 1a). Oxygen and temperature were sensed using oxygen optodes (Aanderaa Inc., model 3835) immersed in the reservoir tank and in the three gas-controlled tanks, and pH was measured with Honeywell Durafet pH sensors. Temperature in the aquaria was maintained at a constant average of 14.4 °C (Table 1) because during upwelling events (when surface pH is naturally reduced) temperature remains low38. Two fluorescent bulbs ~0.5 m above each of the aquaria provided 13–15 µMol m−2 s−1 irradiance on a 12-hour cycle, measured at the experiment start and end using the LI-COR instrument. These levels mimicked average mid-day light levels at 9 m depth under kelp canopy at Hopkins Marine Station (measured in October of 2011 and March of 2012).
We also sampled water chemistry in the experimental aquaria three times monthly during the course of the experiment (March–July 2013) to measure salinity, total alkalinity, and dissolved inorganic content (DIC). On each collection date, nine water samples (three for each parameter/bin) were collected in 30 ml Borosilicate Glass serum bottles from beneath the surface of each bin. However, due to malfunction of water testing equipment for alkalinity and DIC, we were not able to measure the water samples until 4 months after the experiment terminated and data was unreliable. Therefore, in April 2014, we recreated the conditions of the experiment at MBARI using the same set points for the gas-controlled tanks, the same bins, and the same mass of coralline in each treatment bin. We let the system equilibrate for one week, then measured pH using a Shimadzu UV-1601 spectrophotometer, and re-sampled the water as described above (but with six replicates/parameter/pCO2 treatment) for salinity, total alkalinity, and DIC. For samples used to measure alkalinity and DIC, 10.9 μl HgCl2 was added to kill living organisms. Each bottle was sealed, stored in a dark refrigerator, and processed two days after collection. Salinity was measured with a YSI 3200 Conductivity instrument with a YSI 3252 cell, total alkalinity using a SI Analytics Titroline 6000 titrator, and DIC using a UIC Inc. Model 5015 CO2 Coulometer with a CM5230 Acidification module. We used the program CO2SYS to calculate pCO2 and Ω using the data from April 2014, with pH from the spectrophotometer readings and total alkalinity measured from collected water. The pCO2 values calculated were almost identical to the measures recorded within the gas-controlled tanks that fed water into experimental bins (Table 1b).
On July 10, vials were transported in cooled seawater to the Hopkins Marine Station for immediate use in larval settlement experiments. Lateral algal crust area was compared in before- and after-treatment photographs taken of individual vials (Canon Powershot S100 using Image J) as an indication of the effects of the pre-conditioning treatments. We do not report vertical growth because crusts were thin (≤0.2 mm), exhibited no visual change in vertical growth across treatments, and surface area is more important for maintenance of larval settlement space.
Abalone settlement
Red abalone larvae from the Cayucos Abalone Farm (Cayucos, CA) were shipped overnight to the Hopkins Marine Station in seawater in a sterile Nalgene container surrounded by ice packs. Larvae were spawned on July 3, 2013, shipped on July 9th, and arrived on July 10, 2013. Because we were interested in how OA might alter the role of calcifying algae in species interactions, we exposed only algae crusts, not the larvae, to the different pCO2 treatments. To test whether OA affected algae cues for settling larvae, 95–110 red abalone larvae (Haliotis rufescens, 7-day old) were added to each of 7 haphazardly selected vials per taxon from each pCO2 treatment on July 10th within hours of removal of algae from pCO2 treatments and immersion in ambient seawater (~14.2 °C and pH ~7.82). Abalone larvae were placed in sterile petri dishes and were inspected under an Olympus 40x dissecting microscope: all were actively swimming with no apparent abnormalities. Vials were filled with 65 mL of seawater (described above) leaving a 1 cm air space in each vial and 95–110 larvae were pipetted into each vial. Vials were closed with watertight polyethylene (LDPE) caps and put into a flowing seawater table to maintain temperature at 14 °C, under lights providing irradiance of ~15 µMol/m2/s on a 12 hour cycle. After 24-hours the number of settled (metamorphosed), swimming (veliger stage), and dead larvae were counted in each vial under a microscope. These counts were repeated 48 hours after abalone insertion.
Statistical Analyses
All analyses were conducted in SYSTAT 13. Prior to conducting the pCO2 treatments, we assessed the surface area of CCRA assigned to the 3 treatments using analysis of variance (ANOVA). We used pCO2 treatments to pre-condition algae for later species interaction trials, which were multiple independent replicates. Data were normally distributed for algal area prior to treatment and no data transformation was applied.
We then tested for differences in growth to judge the effect of pre-conditioning. Because data were skewed, we used a logarithmic (base 10) transformation (plus the constant 20 to make all data positive as a few specimens had slight decline in healthy surface area). We compared the change in algal surface area between treatments and genera using a 2-way ANOVA with percent change in algal surface area (or cover) as the response variable, and pCO2 (3 levels) and algal genera (4 levels) as fixed, independent and orthogonal factors, and with the interaction term pH*genera. We then used a posthoc Tukey’s Honestly Significant Difference test to evaluate pairwise differences in growth between pCO2 levels and between algal genera.
To evaluate differences in abalone settlement across CCRA genera, we first ran an ANCOVA considering only the vials with CCRA (excluding non-CCRA control vials from each pCO2 treatment) so that we could include algal area as a predictor variable. We used the proportion of abalone that settled within 24 hours as the response variable and the predictor variables: algal pCO2 treatment (3 levels), algal genera (4 levels), algal surface area (as a covariate to account for any differences in the amount of algae in each vial), and the interaction between algal genera, pCO2 treatment, and algal area. We then ran an ANOVA with the same response variable and prior algal pCO2 treatment, substrate type (algal genera type or control vials with no algae), and the interaction between substrate type and prior algal pCO2 treatment. In both cases, the number of abalone settled was normally distributed and had homogeneous variances, so we did not use a transformation. Following the above analyses, where results were significantly different, we used a posthoc Ryan-Einot-Gabriel-Welsch (REGW-Q) test to evaluate which pCO2 or genera were significantly different from each other in inducing settlement. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Algal Species Identity
Four of five morphological groups of CCRA collected from cobbles in shallow kelp forests were consistently identified visually to genus (confirmed genetically), and these were used in the experiments. Three groups (each containing 2–3 closely related species) are common genera of CCA in the Northeast Pacific: Lithothamnion spp. (2 species), Lithophyllum spp. (3 species), and Leptophytum spp. (2 species; Fig. 1). The fourth group is a subtidal, lightly calcified aragonitic Peyssonnelia species (order Peyssonneliales) that may be undescribed (Fig. 1). These were the four most common genera on the cobbles collected from sites in the Monterey Bay. In addition, based on preliminary data from samples collected every 2.5 m on 30 m benthic transects in central California (13 transects from 2 sites in the Monterey Bay) and northern California (29 transects at 6 sites in Mendocino and Sonoma Counties), the 3 CCA genera (Liththamnion, Lithophyllum, and Leptophytum) are also common on reef substrates in kelp forests, accounting for 52% of the algal crust from transects in central California and 76% of crusts from transects in northern California. Peyssonnelia sp. was not found on any of the samples identified from transects and may be found only on cobbles rather than on rocky reefs (JKO, unpublished data).
Figure 1.
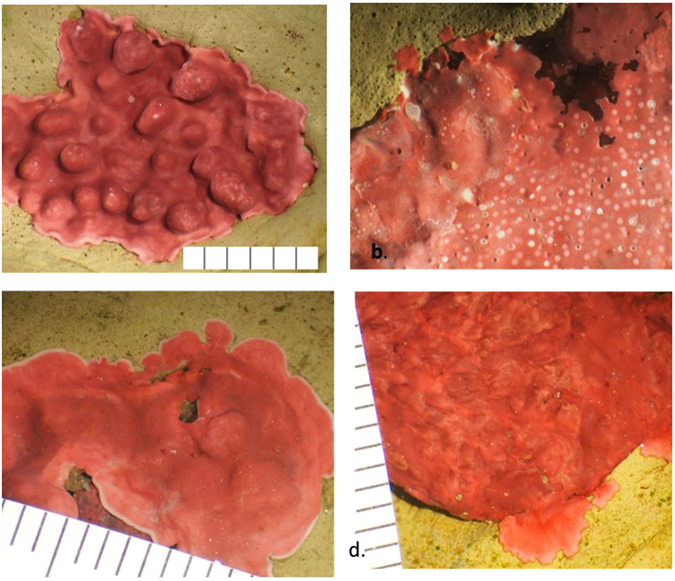
Surface morphology for each morphological group. Scientific names based on DNA sequencing of 5–10 samples/group. (a) Morphological group 1: Five samples of Group 1 were sequenced, with three identified as Lithothamnion phymatodeum and two as L. glaciale. Lithothamnion spp. specimens had raised multiporate conceptacles (~500 µm width) and short non-articulated protuberances that appear as distinct bumps on the surface (~1 × 1 × 1.5 mm). (b) Morphological group 2: Nine samples of this group sequenced as three related, undescribed species of Lithophyllum, some of which have also been found in northern Washington (PG pers obs). Lithophyllum spp. specimens had a very thin crust with numerous, small uniporate conceptacles (~150 µm width) that are flush with or slightly raised above the surface. (c) Morphological group 3: Six samples of this group were sequenced, with three identified as Leptophytum adeyi and three identified as a closely related, but undescribed Leptophytum species. Leptophytum spp. specimens were never found with conceptacles and had flowing growing margins, a very smooth surface, and fast growth in the laboratory compared to the other three groups. (d) Morphological group 4: Five samples were sequenced and all were a single lightly calcified aragonitic species of Peyssonnelia. These had a distinctive darker red coloration and flowing surface patterns. (Scale bars are in mm).
Algal Growth (Response to pre-conditioning period)
Initial mean algal area (per vial) did not differ between pCO2 treatments (2-way ANOVA df = 2,6, F = 1.05, p = 0.35). The pre-conditioning of algae with elevated pCO2 negatively affected algal growth with significantly greater growth (+32.6 mm2) under normal pCO2 conditions compared to the high and extreme pCO2 conditions (+22.3 mm2 and + 17.8 mm2 respectively; Fig. 2, Tables 2a and 3a). Regardless of pCO2 treatment, there was variation in growth among the CCRA genera, with one genus, Leptophytum spp., growing significantly more than the others (Fig. 2, Tables 2a and 3b). All algal tissue appeared healthy at the end of the experiment with little bleaching (<7% of samples and with <2.6% of surface area affected).
Figure 2.
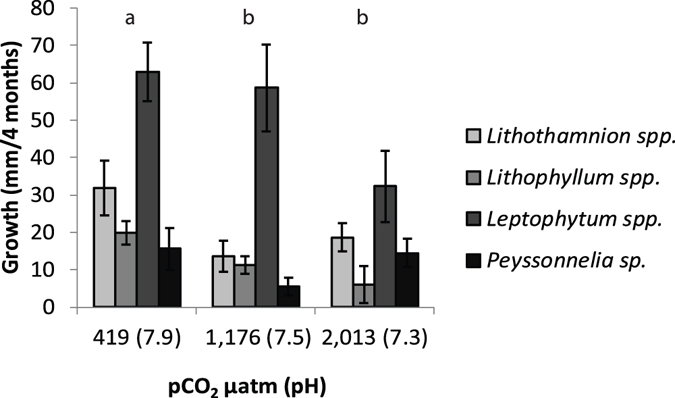
Change in surface area of the CCRA genera after four months in each of the pCO2 treatments, representing ambient conditions (419 µatm and pH of 7.9), high pCO2 (1,176 µatm and 7.5 pH), and extreme pCO2 (2,013 µatm and 7.3 pH). This data is shown to demonstrate that the pCO2 treatments had an effect on the CCRA. Letters above bars denote significant differences in growth considering all four genera.
Table 2.
Statistical Results. (a) Effects of pre-condition pCO2 treatments on algal growth, (b) larval substrate preference (no control vials included) and accounting for algal area in vials, (c) larval settlement preference including control vials, and (d) larval substrate preference (no control vials included).
| Parameter | Statistical Test | Factor | Results |
|---|---|---|---|
| (a) Algal Growth | Two-way ANOVA | pCO2 | df = 2,108, F = 7.93, p = 0.0006 |
| CCRA genera | df = 3,108, F = 20.59, p < 0.0001 | ||
| pCO2*CCRA genera | df = 6,108, F = 1.32, p = 0.25 | ||
| (b) Larval Substrate Preference | ANCOVA | CCRA genera (no control) | df = 3,68, F = 7.60, p = 0.0002 |
| pCO2 | df = 2,68, F = 1.27, p = 0.29 | ||
| CCRA surface area | df = 1,68, F = 0.44, p = 0.51 | ||
| pCO2*genera*area | df = 6,68, F = 0.60, p = 0.73 | ||
| (c) Larval Substrate Preference (no control vials) | Two-way ANOVA | CCRA genera (& control) | df = 4,89, F = 50.91, p < 0.0001 |
| pCO2 | df = 2,89, F = 1.68, p = 0.19 | ||
| pCO2*genera | df = 8,89, F = 0.56, p = 0.81 |
Table 3.
Posthoc Tukey’s Honestly Signficant Difference (HSD) tests for algal growth following pCO2 treatments for the model in Table 2a with algal growth as the response variable and CCRA genera and pCO2 as predictor variables. Growth changes have been back transformed. (a) Growth differences by pCO2 (b) growth differences by CCRA genera.
| (a) | ||
|---|---|---|
| pCO2 Pairwise Comparison | Difference (mm) | p |
| Normal and High | 10.29 | 0.01 |
| High and Extreme | 4.41 | 0.65 |
| Normal and Extreme | 14.70 | 0.008 |
| (b) | ||
| Genera Pairwise Comparison | Difference (mm) | p |
| Leptophytum spp. and Lithothamnion spp. | 29.9 | <0.0001 |
| Leptophytum spp. and Peyssonnelia sp. | 39.43 | <0.0001 |
| Leptophytum spp. and Lithophyllum spp. | 39.9 | <0.0001 |
| Lithothamnion spp. and Lithophyllum spp. | 9.0 | <0.31 |
| Lithothamnion spp. and Peyssonnelia sp. | 9.5 | =0.26 |
| Lithophyllum spp. and Peyssonnelia sp. | 0.57 | =0.99 |
Abalone Settlement
The majority of abalone that settled (94%) did so within 24 hours, so we used the 24-hour numbers in analyses. We found no significant difference in the number of abalone settlers based on variation in CCRA surface area in vials, which ranged from 147 to 405 mm2 (Table 2b). Treatments that had no CCRA (control vials) had only 11% settlement compared to 48–69% in vials with CCRA (Table 2c and Fig. 3). There were significant differences in settlement rates between the four CCRA genera (p < 0.0001, Table 2c and Fig. 3): two genera, Lithothamnion spp. and Leptophytum spp., had higher settlement with 62 and 69% of larvae settling respectively while the other two genera, Lithophyllum spp. and Peyssonnelia sp, each had 48% of larvae settle (REGW-Q Test). To our knowledge, this is the first observation that Peyssonnelia may be an important settlement substrate for abalone. However, there was no change in settlement rates with prior pCO2 treatments of algae (the interaction term between algal genera and prior algal pCO2 treatment was not significant; Table 2c). Thus, while there was no significant difference in settlement associated with prior algal pCO2 treatment, there were strongly significant associations with presence or absence of CCRA as well as between algal genera.
Figure 3.
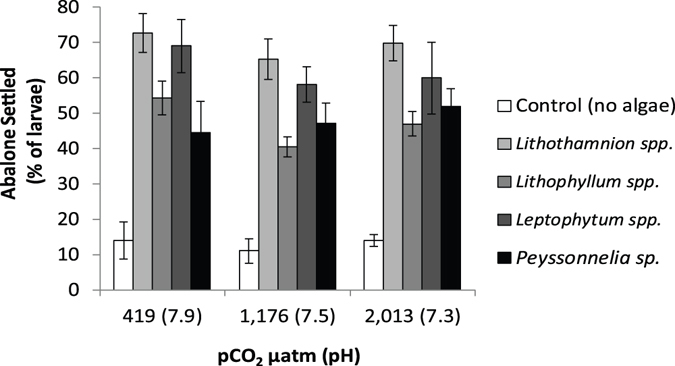
Abalone settlement (as percent of larval settled and attached) on the four CCRA genera and in control vials with no algae, and across the pCO2 treatments representing ambient conditions (419 µatm and pH of 7.9), high pCO2 (1,176 µatm and 7.5 pH), and extreme pCO2 (2,013 µatm and 7.3 pH).
Discussion
One of the major unknowns in OA research is how species will respond to OA in a community context via species interactions8–10. For other environmental impacts like global warming, changes in species interactions have fundamental impacts on communities41. CCRA cover a high proportion of marine benthic substrates, are generally thought to be highly susceptible to OA42, and mediate multiple species interactions43. We tested the response of abalone larval settlement to CCRA that were preconditioned in OA treatments. Contrary to our hypothesis (that pre-conditioning CCRA with OA would disrupt larval settlement), we found that CCRA maintained their ability to induce larval abalone settlement despite prior exposure to strong OA (pCO2 of up to 2,013 µatm) for 4 months, at least when the algae but not the larvae were exposed.
Response of CCRA to OA treatments
CCRA generally show diverse responses to experimentally increased pCO2 (Supplementary Table 1), likely reflecting the high diversity within this group. Prior OA experiments have found strong negative effects such as reduced growth and tissue integrity as well as increased likelihood of dissolution and tissue necrosis, but also sometimes no effect of increased pCO2 on growth10. While our ability to discuss the generality of changes in growth due to OA is constrained by the design of our experiment (e.g. one tank per algal pCO2 treatment), the level of CCRA growth reduction we observed is generally less severe than reported elsewhere. In our experiment, after 4 months, algal growth declined by 48% in the extreme pCO2 treatment (2,013 µatm). In four (of 6) prior laboratory and field studies in the extreme pCO2 range (1,400–2,200 µatm), coralline growth or cover was reduced by 93–100% (Supplementary Table 1). In our high pCO2 treatment (1,176 µatm), CCRA growth reduced by 32%, whereas in prior studies, cover or growth loss in similar pCO2 ranges varied from 22–100%, and most (9 of 13 studies) found >50% loss (Supplementary Table 1). Even in comparatively mild increased pCO2 treatments (600–850 µatm), prior studies have found cover or growth losses ranging from 15–100%, with 8 of 14 studies exhibiting >50% loss (Supplementary Table 1). Further, in our study, the fastest growing CCRA genus (Leptophytum spp.) maintained growth under high pCO2 and only reduced growth under the extreme pCO2 treatment (Fig. 2), indicating the potential for some taxa to resist at least moderate pH changes, which should be tested further.
CCRA genus-specific settlement cues to abalone
Unlike corals, which have high species specificity for CCA15, 19, 44, red abalone larvae responded to cues from three genera of CCA and from the lightly calcified aragonitic crust, Peyssonnelia sp. To our knowledge, this is the first demonstration of Peyssonnelia providing abalone settlement cues. In a study on corals, Peyssonnelia was not found to be a particularly inductive substrate45. Although some CCRA genera in our study induced 1.4 times more settlement than others (Fig. 3), these differences pale when considering that there was >5 times more settlement when CCRA were present compared to when they were absent (10% compared to 48–69% settled). This finding is similar to the results of previous experiments where red abalone settled on multiple genera including the CCRA species Lithothamnium californicum and L. glaciale, Lithophyllum spp., Clathromorphum circumscriptum, and a CCRA non-coralline Hildenbrandia dawsonii, but did not settle when provided with foliose red, green, or brown algae23. There thus appears to be some functional redundancy among CCRA in providing settlement cues to red abalone. The functional redundancy in settlement cues found with red abalone is not necessarily the case for other abalone species (e.g. Haliotis laevigata), which show distinct settlement preferences among CCA species46.
Abalone settlement on CCRA exposed to OA
We found that CCRA maintain settlement cues after prolonged and extreme OA exposure. This finding contrasts with previous studies on coral larvae showing settlement declines ranging between 20–86% on OA-treated CCRA25, 26, and showing settlement reductions at milder algal pCO2 treatments (600–1,300 µatm). The lack of OA-induced changes in larval settlement with elevated pCO2 in our experiment could be due to conditions not being extreme enough, short treatment times, or lack of power, but these scenarios are unlikely. Our OA treatments represented strong scenarios, with the most elevated treatment (2,013 µatm) set to higher pCO2 than global 2100 predictions2, 36. Further, pCO2 treatments had the expected negative effect on growth, with growth slowing significantly at high and extreme pCO2 treatments, especially for the fastest growing genus (Leptophytum spp.) in the extreme pCO2 treatment. The duration of the exposure of the algae to pCO2 treatments in this study was 126 days, well within the range of other similar laboratory studies (mean 138 days, range from 14–420 days; Supplementary Table 1), although one prior study found that CCRA showed a much stronger negative response at 420 days than at 90 days47. In California, upwelling of high pCO2 water lasts hours to days, not months, so our treatments were sufficient to evaluate potential effects of prolonged upwelling followed by the return of lower pCO2 water.
It should be noted that many impacts of OA on chemical communication are due to acid-base disturbances and subsequent impairment of neuronal ion channel function48 or due to changes in protonation of chemical signal compounds49 that lead to disrupted chemical signaling. Both types of effects would only be visible during direct and potentially prolonged exposure of both abalone larvae and CCRA to ocean acidification during settlement assays. However, our study focused on potential changes to CCRA provision of cues, rather than cue reception by larvae.
Our findings indicate that under expected future changes in pH in the California current system, cues to larval settlers like abalone that rely on CCRA may be retained following the cessation of upwelling and high pCO2. We found that several CCRA genera induced settlement (regardless of OA exposure history), suggesting functional redundancy that may provide an additional buffer against the effects of OA on loss of benthic coverage of any one coralline taxon. There is also evidence that the presence of algae, rather than percent cover, is sufficient for settlement50. In this experiment, there was no change in abalone settlement with algal surface area varying between 147 and 405 mm2. Direct contact by the larvae with the inducing algal surface is necessary for induction23, so at least locating CCRA is a requirement for abalone settlement, and this should be influenced by CCRA surface area. Nonetheless, reliance on presence rather than area will further buffer against the effects of slowed CCRA growth under future OA scenarios.
Conclusions
Upwelling regions (like the California Current) have highly variable pCO2 conditions that can result in periodic exposure to pH well below normal38, 39. It has been suggested that for taxa that have evolved under conditions of pCO2 variation, OA effects may be less pronounced due to population acclimatization or adaptation8 than for species evolved under stable pCO2 conditions51. Thus, while California CCRA are susceptible to growth reductions under OA, they may be better able to withstand significant and prolonged changes in pCO2 than corallines from non-upwelling systems, and thus maintain chemical settlement cues. The hypothesis of environmental variability evolutionarily favoring physiological resistance to climate change is supported by studies showing that temperature variation is a key factor enhancing bleaching resistance in calcifying corals. This has been demonstrated in region-wide analyses52 and in field and laboratory experiments showing both acclimation and adaptation53, 54. Highly variable pCO2 may operate similarly, and background environmental variability may be a generally important factor in determining when to expect resistance. For example, in a fjord system with wide pCO2 fluctuations, barnacles showed a strong tolerance to high pCO2 (1,000 µatm)55. Though few species from the California upwelling system have been studied in an OA context56, prior studies have found evidence of OA adaptation in sea urchin (S. purpuratus) gametes56 and larvae57, and in mussel growth56. Abalone (H. rufescens) were found to have distinct differences in biomineralization genes between regions with different upwelling conditions, indicating local population adaptation58. Some calcifying species in California thus appear to tolerate broad pCO2 fluctuations or have sufficient genetic diversity to allow rapid evolution. For CCRA, individuals from a naturally variable tropical environment calcified 42% more than individuals from a uniform environment when experimentally placed under increased pCO2 59. A study on a central California geniculate coralline, Corallina vancouveriensis, also found evidence of local adaptation, and it may be that the ability of CCRA spores to attach rapidly limits dispersal distance, restricts gene flow among populations, and increases the potential for local adaptation60.
Research is just revealing which taxa might be more vulnerable to OA and scaling up to species interactions and ecosystem functions is critical to predict and manage future changes. Species interactions of abalone larval settlers with CCRA in the California upwelling-dominated ecosystems appear to be resistant to the impacts of a four month history of OA exposure, and functional redundancy in settlement inducing CCRA may also buffer future OA impacts to abalone. The ability of CCRA to maintain chemical cues in this system may be due to periodic exposure to OA due to upwelling. If so, areas with variable OA environments like the California Current might represent areas with high resilience to OA in the future, compared with areas where pCO2 is more stable. Regardless, our findings demonstrate maintenance of some level of ecosystem function under OA in the California Current and possibly other upwelling ecosystems.
Electronic supplementary material
Acknowledgements
Support this work came from the Hopkins Marine Station Marine Life Observatory (support for postdoctoral researcher JKO), Monterey Bay Aquarium Research Institute (for lab facilities) and the CDFW Invertebrate Fisheries Project (PCA 25368 to LRB). FM was supported by NSF-CNH grant DEB‐1212124. PWG is grateful to T. Vision, University of North Carolina, Chapel Hill for lab space and equipment and to D. W. Freshwater Farm for processing the DNA samples in the Center for Marine Sciences DNA Analysis Core Facility (UNC, Wilmington). J.O. thanks R. Steneck for guidance and support in classifying coralline algae. We thank the Cayucos Abalone and R. Fields for generously donating abalone larvae. We thank the interns who assisted in data collection: A. Makukhov, M. Morse, A. Blackwell from CSUMB; J. Bien, R. Bohrman, A. Greene, G. Mwakumuna, and J. Seawell from Monterey Peninsula College; K. Schnurle, J. Lo, and M. Mikhail from Stanford; and K. Chumbe from Pacific Grove Middle School. We are particularly grateful for field assistance and coordination from F. Sommer, budget coordination by J. Thompson, advice and logistical assistance from C. Katton, chemical analysis of water samples at Hopkins by C. Patton and B. Compton, and laboratory operation and water chemistry analysis at MBARI by C. Lovera. We are grateful to G. Somero for reviewing and improving the manuscript. This is a contribution of the Bodega Marine Laboratory, UC Davis and the Hopkins Marine Station, Stanford University.
Author Contributions
J.O., F.M., D.P., and S.P. conceived of and designed the experiments. F.M., S.P., and D.P. also contributed to writing various sections of the manuscript. L.R.B. assisted with manuscript preparation as well as large-scale surveys of coralline algae to identify appropriate surveys. P.G. did all genetic identification of coralline algae and contributed to writing the manuscript. J.B. conducted testing of water conditions at MBARI and contributed to writing the manuscript. J.O. conducted experiments, analyzed results, and prepared figures.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
James P. Barry, Paul W. Gabrielson, Laura Rogers-Bennett, Donald C. Potts, Stephen R. Palumbi and Fiorenza Micheli contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05502-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 2009;1:169–92. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 2.Feely RA, et al. Impact of Anthropogenic CO2 on the CaC03 System in the Oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 3.Orr JC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–6. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 4.Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008;65:414–432. doi: 10.1093/icesjms/fsn048. [DOI] [Google Scholar]
- 5.Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Glob. Chang. Biol. 2012;18:1491–1498. doi: 10.1111/j.1365-2486.2012.02656.x. [DOI] [Google Scholar]
- 6.Kroeker K, Micheli F, Gambi M. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Chang. 2012;3:156–159. doi: 10.1038/nclimate1680. [DOI] [Google Scholar]
- 7.Nagelkerken I, Russell BD, Gillanders BM, Connell SD. Ocean acidification alters fish populations indirectly through habitat modification. Nature. 2015;6:89–95. [Google Scholar]
- 8.Hofmann GE, et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One. 2011;6:e28983. doi: 10.1371/journal.pone.0028983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaylord B, et al. Ocean acidification through the lens of ecological theory. Ecology. 2015;96:3–15. doi: 10.1890/14-0802.1. [DOI] [PubMed] [Google Scholar]
- 10.McCoy SJ, Kamenos NA. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 2015;51:6–24. doi: 10.1111/jpy.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamenos NA, et al. Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Glob. Chang. Biol. 2013;19:3621–8. doi: 10.1111/gcb.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 2007;1:114–117. doi: 10.1038/ngeo100. [DOI] [Google Scholar]
- 13.Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Bio. Ecol. 2011;400:278–287. doi: 10.1016/j.jembe.2011.02.011. [DOI] [Google Scholar]
- 14.Russell BD, Thompson J-AI, Falkenberg LJ, Connell SD. Synergistic effects of climate change and local stressors: CO 2 and nutrient-driven change in subtidal rocky habitats. Glob. Chang. Biol. 2009;15:2153–2162. doi: 10.1111/j.1365-2486.2009.01886.x. [DOI] [Google Scholar]
- 15.O’Leary JK, Potts DC, Braga JC, McClanahan TR. Indirect consequences of fishing: reduction of coralline algae suppresses juvenile coral abundance. Coral Reefs. 2012;31:547–559. doi: 10.1007/s00338-012-0872-5. [DOI] [Google Scholar]
- 16.Lacey EA, Fourqurean JW, Collado-Vides L. Increased algal dominance despite presence of Diadema antillarum populations on a Caribbean coral reef. Bull. Mar. Sci. 2013;89:603–620. doi: 10.5343/bms.2012.1015. [DOI] [Google Scholar]
- 17.Connell SD. The monopolization of understorey habitat by subtidal encrusting coralline algae: A test of the combined effects of canopy-mediated light and sedimentation. Ecol. Monogr. 2003;142:1065–1071. [Google Scholar]
- 18.Nelson WA. Calcified macroalgae – critical to coastal ecosystems and vulnerable to change: A review. Mar. Freshw. Res. 2009;60:787–801. doi: 10.1071/MF08335. [DOI] [Google Scholar]
- 19.Morse ANC, Morse DE. Flypapers for coral and other planktonic larvae. Bioscience. 1991;46:254–262. doi: 10.2307/1312832. [DOI] [Google Scholar]
- 20.Basso D. Carbonate production by calcareous red algae and global change. Geodiversitas. 2012;34:13–33. doi: 10.5252/g2012n1a2. [DOI] [Google Scholar]
- 21.Dethier M, Paull K, Woodbury M. Distribution and thickness patterns of subtidal encrusting algae from Washington. Bot. Mar. 1991;34:201–210. doi: 10.1515/botm.1991.34.3.201. [DOI] [Google Scholar]
- 22.Harrington L, Fabricius K, De’ath G, Negri A. Recognition and settlement substrata determine post-settlement survival in corals. Ecology. 2004;85:3428–3437. doi: 10.1890/04-0298. [DOI] [Google Scholar]
- 23.Morse ANC, Morse DE. Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J. Exp. Mar. Bio. Ecol. 1984;75:191–215. doi: 10.1016/0022-0981(84)90166-7. [DOI] [Google Scholar]
- 24.Smith AM, Sutherland JE, Kregting L, Farr TJ, Winter DJ. Phylomineralogy of the coralline red algae: correlation of skeletal mineralogy with molecular phylogeny. Phytochemistry. 2012;81:97–108. doi: 10.1016/j.phytochem.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ. Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett. 2012;15:338–346. doi: 10.1111/j.1461-0248.2012.01743.x. [DOI] [PubMed] [Google Scholar]
- 26.Webster NS, Uthicke S, Botté ES, Flores F, Negri AP. Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob. Chang. Biol. 2013;19:303–315. doi: 10.1111/gcb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TW, Barry JP, Micheli F. The effects of intermittent exposure to low-pH and low-oxygen conditions on survival and growth of juvenile red abalone. Biogeosciences. 2013;10:7255–7262. doi: 10.5194/bg-10-7255-2013. [DOI] [Google Scholar]
- 28.Guo X, Huang M, Pu F, You W, Ke C. Effects of ocean acidification caused by rising CO2 on the early development of three mollusks. Aquat. Biol. 2015;23:147–157. doi: 10.3354/ab00615. [DOI] [Google Scholar]
- 29.Byrne M, et al. Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean. Proc. Biol. Sci. 2011;278:2376–83. doi: 10.1098/rspb.2010.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers-Bennett L, et al. Tracking larval, newly settled, and juvenile red abalone (Haliotis rufescens) recruitment in Northern California. J. Shellfish Res. 2016;35:601–609. doi: 10.2983/035.035.0305. [DOI] [Google Scholar]
- 31.Sheppard SA. Studies on southern Australian abalone (genus Haliotis) XIX: Long-term juvenile mortality dynamics. J. Shellfish Res. 1998;17:813–825. [Google Scholar]
- 32.Gabrielson PW, Miller KA, Martone PT. Morphometric and molecular analyses confirm two distinct species of Calliarthron (Corallinales, Rhodophyta), a genus endemic to the northeast Pacific. Phycologia. 2011;50:298–316. doi: 10.2216/10-42.1. [DOI] [Google Scholar]
- 33.Hughey JR, Silva PC, Hommersand MH, G S, R P. Solving taxonomic and nomenclatural problems in Pacific Gigartinaceae (Rhodophyta) using DNA from type material 1. J. Phycol. 2001;37:1091–1109. doi: 10.1046/j.1529-8817.2001.01048.x. [DOI] [Google Scholar]
- 34.Broom JES, et al. Utility of psbA and nSSU for phylogenetic reconstruction in the Corallinales based on New Zealand taxa. Mol. Phylogenet. Evol. 2008;46:958–73. doi: 10.1016/j.ympev.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf. Science. 2008;320:1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 36.IPCC, 2007: Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team, Pachauri, R. K. & Reisinger, A. (eds). IPCC, Geneva, Switzerland, 104 pp.
- 37.Doney SC, Bopp L, Long M. Historical and future trends in ocean climate and biochemistry. Oceanography. 2014;27:108–119. doi: 10.5670/oceanog.2014.14. [DOI] [Google Scholar]
- 38.Booth JAT, et al. Natural intrusions of hypoxic, low pH water into nearshore marine environments on the California coast. Cont. Shelf Res. 2012;45:108–115. doi: 10.1016/j.csr.2012.06.009. [DOI] [Google Scholar]
- 39.Checkley D, Jr, Barth J. Patterns and processes in the California Current System. Prog. Oceanogr. 2009;83:49–64. doi: 10.1016/j.pocean.2009.07.028. [DOI] [Google Scholar]
- 40.Barry, J. P. et al. A gas controlled aquarium system for ocean acidification studies. In Proc. International Conference OCEANS 2008 and MTS/IEEE Kobe Techno-Ocean ‘08, 774-778 (OCEANS-IEEE 2008).
- 41.Cahill AE, et al. How does climate change cause extinction? Proc. R. Soc. B Biol. Sci. 2012;280:20121890–20121890. doi: 10.1098/rspb.2012.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010;13:1419–34. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 43.McCoy SJ, Pfister CA. Historical comparisons reveal altered competitive interactions in a guild of crustose coralline algae. Ecol. Lett. 2014;17:475–83. doi: 10.1111/ele.12247. [DOI] [PubMed] [Google Scholar]
- 44.Doropoulos C, Diaz-Pulido G. High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar. Ecol. Prog. Ser. 2013;475:93–99. doi: 10.3354/meps10096. [DOI] [Google Scholar]
- 45.Ritson-Williams R, Arnold SN, Paul VJ, Steneck RS. Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs. 2014;33:59–66. doi: 10.1007/s00338-013-1113-2. [DOI] [Google Scholar]
- 46.Daume S, Brand-Gardner S, Woelkerling WJ. Settlement of abalone larvae (Haliotis laevigata Donovan) in response to non-geniculate coralline red algae (Corallinales, Rhodophyta) J. Exp. Mar. Bio. Ecol. 1999;234:125–143. doi: 10.1016/S0022-0981(98)00143-9. [DOI] [Google Scholar]
- 47.Ragazzola F, et al. Phenotypic plasticity of coralline algae in a high CO2 world. Ecol. Evol. 2013;3:3436–46. doi: 10.1002/ece3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson GE, et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012;2:201–204. doi: 10.1038/nclimate1352. [DOI] [Google Scholar]
- 49.Roggatz CC, Lorch M, Hardege JD, Benoit DM. Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Glob. Chang. Biol. 2016;22:3914–3926. doi: 10.1111/gcb.13354. [DOI] [PubMed] [Google Scholar]
- 50.O’Leary J, Potts D, Schoenrock K, McClahanan T. Fish and sea urchin grazing opens settlement space equally but urchins reduce survival of coral recruits. Mar. Ecol. Prog. Ser. 2013;493:165–177. doi: 10.3354/meps10510. [DOI] [Google Scholar]
- 51.Kelly MW, Hofmann GE. Adaptation and the physiology of ocean acidification. Funct. Ecol. 2013;27:980–990. doi: 10.1111/j.1365-2435.2012.02061.x. [DOI] [Google Scholar]
- 52.McClanahan T, Ateweberhan M, Muhundo C, Maina J, Mohammed M. Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecol. Monogr. 2007;77:503–525. doi: 10.1890/06-1182.1. [DOI] [Google Scholar]
- 53.Palumbi S, Barshis D, Traylor-Knowles N, Bay N. Mechanisms of Reef Coral Resistance to Future Climate Change. Science. 2014;344:895–898. doi: 10.1126/science.1251336. [DOI] [PubMed] [Google Scholar]
- 54.Barshis DJ, et al. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA. 2013;110:1387–92. doi: 10.1073/pnas.1210224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pansch C, Nasrolahi A, Appelhans YS, Wahl M. Tolerance of juvenile barnacles (Amphibalanus improvisus) to warming and elevated pCO2. Mar. Biol. 2012;160:2023–2035. doi: 10.1007/s00227-012-2069-4. [DOI] [Google Scholar]
- 56.Hofmann G, et al. Exploring local adaptation and the ocean acidification seascape – studies in the California Current Large Marine Ecosystem. Biogeosciences. 2014;11:1053–1064. doi: 10.5194/bg-11-1053-2014. [DOI] [Google Scholar]
- 57.Pespeni MH, Barney BT, Palumbi SR. Differences in the regulation of growth and biomineralization genes revealed through long-term common-garden acclimation and experimental genomics in the purple sea urchin. Evolution. 2013;67:1901–14. doi: 10.1111/evo.12036. [DOI] [PubMed] [Google Scholar]
- 58.De Wit P, Palumbi SR. Transcriptome-wide polymorphisms of red abalone (Haliotis rufescens) reveal patterns of gene flow and local adaptation. Mol. Ecol. 2013;22:2884–97. doi: 10.1111/mec.12081. [DOI] [PubMed] [Google Scholar]
- 59.Johnson MD, Moriarty VW, Carpenter RC. Acclimatization of the crustose coralline alga Porolithon onkodes to variable pCO2. PLoS One. 2014;9:e87678. doi: 10.1371/journal.pone.0087678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padilla-Gamino JL, Gaitan-Espitia JD, Kelly MW, Hofmann GE. Physiological plasticity and local adaptation to elevated pCO2 in calcareous algae: an ontogenetic and geographic approach. Evol. Appl. 2016;9:1043–1053. doi: 10.1111/eva.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


