Abstract
Influenza virus causes life-threatening infections in pregnant women and their newborns. Immunization during pregnancy is the most effective means of preventing maternal and infant mortality/morbidity; however, influenza vaccination rates of pregnant women remain under 50%. Furthermore, the availability of vaccines in low-resource populations is limited. Skin immunization with microneedle patches (MN) is a novel and safe vaccination platform featuring thermostable vaccine formulations. Cold-chain independence and the potential for self-administration can expand influenza vaccination coverage in developing countries. In this study of pregnant BALB/c mice immunized with subunit H1N1 influenza vaccine, we demonstrate the advantage of skin vaccination over intramuscular delivery of a two-fold higher vaccine dose. MN vaccine induced superior humoral immune responses and conferred protective immunity against a lethal challenge dose of homologous influenza virus. Importantly, MN vaccination of mice at mid-gestation resulted in enhanced and long-lasting passive immunity of the offspring, measured by neutralizing antibody titers and survival rates after virus challenge. We conclude that skin vaccination using MN is a superior immunization approach with the potential to overcome immune tolerance observed in pregnancy, and lower vaccination costs through antigen dose-sparing, which is especially relevant in underserved countries.
Introduction
For nearly a century, the immunotolerant status during pregnancy has been an acknowledged risk factor for severe complications from various infectious agents due to reduced immune responses to antigens. Influenza infections during the second and third trimester of pregnancy cause up to fivefold increases in cardiopulmonary complications compared to a non-pregnant population1. The risk of influenza-associated hospitalization and mortality increases as the pregnancy progresses2. Mortality rates were highest among pregnant women (as high as 45%) during major influenza pandemics (1918, 1957, 1968 and 2009)3. Infection-related complications extend to the fetuses and neonates, with well-known increased risk of miscarriage, stillbirth, neonatal death, preterm birth, and low birth weight neonates1, 3–8.
For safety reasons, influenza vaccines are not licensed for use in infants less than 6 months old. Therefore, the most effective way to protect embryos in utero or newborns postnatally from adverse effects of influenza infection is through vaccination of pregnant women. Efficacy of influenza vaccines in mothers and infants has been evaluated in several human studies with varying outcomes (reviewed in ref. 9). Those studies which are based on laboratory-confirmed cases or clinically diagnosed influenza infection have reported that vaccination during pregnancy reduced the risk of influenza infection by approximately 70% and the risk of preterm birth by 37% compared to non-vaccinated pregnant women10, 11.
Flu vaccine is recommended for administration to unvaccinated pregnant women in the late second or third trimester (after 20 weeks gestation) for two reasons: a) the current subunit or split influenza vaccines induce a fairly short-lived immunity, with antibody titers waning after 6–7 months post-vaccination, so that a late vaccination would successfully protect the mother until labor, and b) since infants are not vaccinated against influenza before 6 months of age, it is desirable to confer a robust passive immunity to them by transplacental transfer of maternal antibodies while in utero or by breast milk during the nursing period of the infant. Influenza vaccines given to pregnant women can be up to 91.5% effective in preventing influenza-related hospitalization of their infant children at 6 months or younger12.
In recent years, the World Health Organization’s (WHO) Expanded Program on Immunization Practices recommended influenza vaccination for all pregnant women regardless of pregnancy trimester, as well as for women of childbearing age13, 14. Despite the more relaxed immunization timelines, only 50% of women in the U.S. were vaccinated either before (15.3%) or during pregnancy (35.0%) in 201515. Major bottlenecks for the implementation of influenza vaccination programs in developing countries include the lack of access to health care services, as well as shortages in trained health care personnel. Other logistical and economic obstacles are vaccine cold chain requirements with increased costs, ineffective immunization campaigns due to lack of information or socioeconomic factors, and needle-phobia16.
We have previously demonstrated in animal models that skin immunization with influenza vaccine using microneedle patches (MN) induces potent and longer-lasting immune responses as compared to conventional vaccination with needle and syringe17, 18. In addition other investigators have reported that IM or intraperitoneal (IP) influenza vaccination of pregnant mice protects them and their fetuses from influenza infection19, 20.
MN are patches containing micron-scale, solid needles made of biocompatible, water-soluble materials encapsulating vaccines, drugs or other compounds of interest21–24. MN can be painlessly applied to the skin by minimally trained personnel or patients themselves25–27. The microneedles dissolve in the skin, leaving no biohazardous sharps waste16. Thermostability of MN vaccines has been shown, including stability of influenza vaccine for at least two years at 25 °C28. By targeting Langerhans cells, dermal dendritic cells and other antigen-presenting cells in the skin, immunological advantages have been shown for skin vaccination using MN with a number of different vaccines21–24, including influenza29–33. Excellent safety, immunogenicity and acceptability of MN for influenza vaccination in a phase I clinical trial was recently reported34.
Based on these findings, we fabricated MN to deliver subunit seasonal influenza vaccine in the skin of pregnant BALB/c mice at mid-gestation. The goal of this study was to determine for the first time whether influenza vaccination with MN could overcome the immune tolerance during pregnancy by harnessing the innate immune cell machinery of the skin and confer robust immunity to mothers and their offspring. We also compared the MN-induced immune responses to those elicited by the conventionally used vaccine delivery method, intramuscular (IM) vaccination or another cutaneous approach; intradermal injection (ID).
Results
MN patches showed successful skin penetration and antigen release
MNs containing influenza vaccine (Fig. 1) were prepared with sulforhodamine in order to visualize the microneedles. The microneedles showed successful penetration and dissolution in mouse skin within 10 min (Fig. 1C and D). On average we observed approximately 10% losses in antigen loading per patch; although we targeted to encapsulate 15 μg of HA, SRID data showed an average of 13.49 ± 1.32 μg HA (average ± standard deviation, n = 4) loading (Table 1). After insertion in the skin, the residual material was 3.86 ± 1.03 μg, indicating a delivery efficiency of 71% (Fig. 1E).
Figure 1.
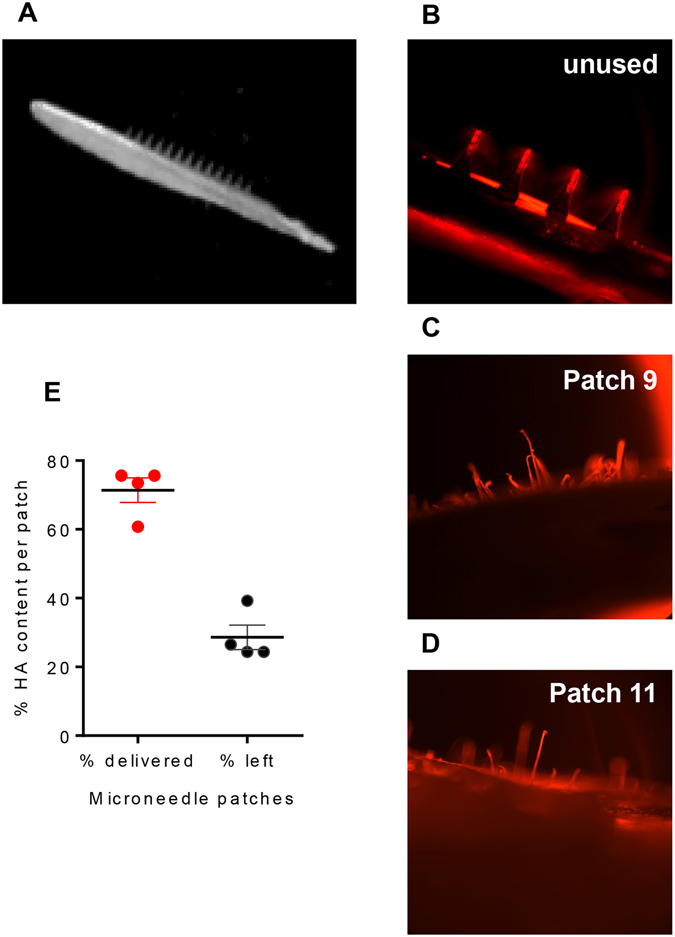
Encapsulation and Delivery of influenza vaccine using dissolving MN patches. (A) Microneedles were loaded with high dose of A/Brisbane/59/07 subunit vaccine (15 μg). The patches were sealed with desiccant and kept @ +4 °C. After being brought to the room temperature and unsealed, MN retained their structure and rigidity. (B) Unused MN patches were imaged with inverted microscopy (20x magnification). (C,D) Used MN patches 10 minutes after insertion into murine skin (20x magnification, inverted microscopy). Some stretched out protrusions can be seen in the used patches possibly containing undelivered vaccine. (E) Percent residual A/Brisbane/59/07 vaccine per patch after insertion into murine skin. The vaccine content after loading and after insertion was determined by SRID. The vaccine was extracted from the patches in the same volume of PBS and run in triplicates. SRID standards ranged from 10 to 70 μg/ml HA. The values were expressed as mean ± SD.
Table 1.
Delivery efficiency of FG patches.
| Patch # | HA in fresh patch, μg | HA in used patch, μg | HA, % remaining |
|---|---|---|---|
| 9 | 15.30 | 3.73 | 24.38 |
| 10 | 13.64 | 5.35 | 39.22 |
| 11 | 12.78 | 3.39* | 27.52 |
| 12 | 12.27 | 2.99* | 24.37 |
| Av ± SD | 13.49 ± 1.32 | 3.86 ± 1.03 | 28.62 |
*Slightly below lowest STDEV.
Vaccination of animals at mid-gestation
Following immunization of pregnant mice, body weights were recorded daily until delivery. Similar to our previous observations made with tetanus vaccine in pregnant mice35, no adverse effects of IM, ID or MN vaccination on the outcome of pregnancy were observed (i.e. body weight fluctuations or preterm labor).
Vaccination of pregnant mice induces lower humoral immune responses than those in non-pregnant mice
We observed that a single dose of influenza subunit vaccine induced 2–5 fold lower serum IgG, IgG1 or IgG2a titers in IM, ID or MN immunized pregnant mice when compared to non-pregnant mice (Fig. 2A–C). The reproducibility of these observations corroborates with reports by other investigators on the role of pregnancy hormones in suppression of the immune response36–38.
Figure 2.
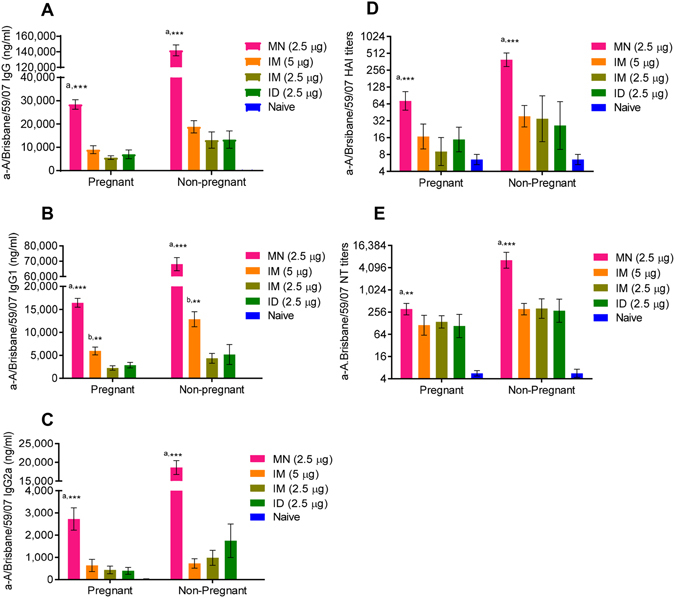
Humoral immune responses in pregnant mice immunized with A/Brisbane/59/07 H1N1 subunit vaccine via intramuscular or transcutaneous routes. Anti-influenza binding antibodies were determined by ELISA in sera collected from mice 28 days after immunization. (A) IgG (B) IgG1 and (C) IgG2a antibody titers. Values are expressed as mean ± SEM, (n = 5–20). (D) †Hemagglutination inhibition (HAI) and (E) neutralizing antibody (NT) titers in sera collected 28 days after immunization. Values are expressed as geometric mean with a ±95% confidence interval (n = 5–20). †Naive: unimmunized mice; IM: vaccine administered intramuscularly; ID: vaccine administered intradermally with syringe and needle; MN: microneedle patches encapsulating the vaccine. †[Pregnant mice: MN (2.5 µg), n = 14; IM (5 μg), n = 8; IM (2.5 μg), n = 5; ID (2.5 μg), n = 5; naïve, n = 5. Non-pregnant mice: MN (2.5 µg), n = 20; IM (5 µg), n = 20; IM (2.5 µg), n = 5; ID (2.5 µg), n = 5; naïve, n = 5]. a[MN vs IM (5 μg), IM (2.5 μg), and ID (2.5 μg)]. b[IM (5 μg) vs IM (2.5 μg), and ID (2.5 μg)]. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Data were analyzed with Mann-Whitney unpaired non-parametric t-test.
MN immunization conferred superior humoral responses compared to ID or IM immunizations in both pregnant and non-pregnant mice
Non-pregnant mice immunized with MN significantly outperformed all other groups in the extent of their immune responses as early as one month post-vaccination (Supplementary Table 1), showing 10-fold higher vaccine-specific IgG serum antibody titers than non-pregnant mice vaccinated IM with the same vaccine dose (p < 0.0001) and 7.5-fold higher vaccine-specific IgG antibody titers than mice immunized IM with a two-fold higher dose of the vaccine (p < 0.0001) (Fig. 2A). The difference between MN and IM vaccinated groups was even more pronounced in IgG2a titers (25-fold higher), and it was 10-fold higher when comparing MN and ID groups (p < 0.001), showing that skin vaccination with either microneedles or intradermal needle improves the Th1 responses (Fig. 2C).
Microneedle immunization was also found to confer superior immune responses in pregnant mice compared to IM immunization. Mice immunized with low dose of subunit vaccine (2.5 µg HA) via MN, had IgG, IgG1, and IgG2a antibody titers about 3–4 fold higher than pregnant mice immunized with 5 µg of HA via the standard IM route (p < 0.001) and 4–7 fold higher than pregnant mice immunized with 2.5 µg of HA via the IM or ID routes (p < 0.001) (Fig. 2A–C) (Supplementary Table 1). Overall, these data provided further evidence on the role of pregnancy in shaping the magnitude and quality of humoral immune responses, although both pregnant and non-pregnant animals showed dose-dependent antibody responses. Most importantly, MN delivery enhanced these responses and showed significant dose-sparing as this route outperformed the standard IM approach using twice-higher vaccine dose.
MN patches induced superior influenza-specific functional antibody titers
HAI and neutralizing antibodies are frequently considered as the most reliable immune correlates in predicting protection against influenza infection. Overall, we observed that the production of influenza-specific HAI and NT titers was dose-dependent in systemic immunization of non-pregnant mice, as seen in the IM vaccinated high (5 μg) and low dose (2.5 μg) groups. The state of pregnancy compromised the production of antibodies by all vaccination route tested; nevertheless, MN immunization with low vaccine dose elicited 4-fold (p < 0.001) and 8-fold (p < 0.0001) higher HAI titers (Fig. 2D) and 3-fold (p < 0.05) and 30-fold (p < 0.0001) higher NT titers than IM immunization with a higher vaccine dose in pregnant and non-pregnant mice respectively, demonstrating significant dose sparing (Fig. 2E) (Supplementary Table 1).
Skin immunization with MNs conferred complete protection against lethal homologous seasonal influenza virus challenge in both pregnant and non-pregnant mice
Thirty days after immunization, which corresponded to about 21 days after delivery, the mothers were challenged with 5xLD50 of homologous influenza virus. All infected animals displayed signs of disease including ruffled hair, hunched posture, and body weight loss; however, pregnant and non-pregnant mice immunized with MNs lost significantly less body weight and demonstrated earlier signs of recovery compared to the groups immunized through traditional routes (Fig. 3A and C). Superior protection of the MN group was also reflected in survival rates. MN-immunized pregnant mice demonstrated 93% survival, significantly higher than the other vaccinated pregnant groups. Mice immunized IM with 5 or 2.5 µg HA of vaccine or ID with 2.5 µg HA showed 25% (p = 0.0015), 20% (p = 0.0015) and 40% (0.0007) survival respectively (Fig. 3B). Interestingly, survival was not dose-dependent in pregnant animals as observed in the non-pregnant cohort. MN vaccination of non-pregnant mice conferred 100% protection whereas IM vaccination with double the vaccine dose (5 µg HA) conferred reduced survival (86%). IM and ID immunized groups that received 2.5 µg HA exhibited a 43% survival rate (Fig. 3D). We observed a significant correlation between HAI titers and protection in the pregnant cohort (R2 = 0.972, p = 0.04), whereas this was not the case in the non-pregnant cohort. The lack of a correlation in the non-pregnant population is indicative of involvement of humoral and cellular immunity in survival. In contrast, pregnant mice rely mainly on humoral responses for protection against a lethal challenge dose of influenza virus.
Figure 3.
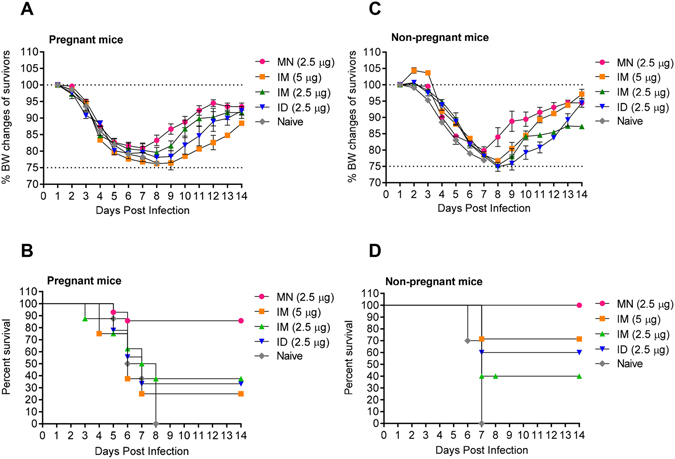
Protective immunity after lethal challenge with homologous virus of mice immunized during pregnancy. Immunized groups were challenged with mouse adapted A/Brisbane/59/07 (H1N1) virus 4 weeks after immunization. (A and C) Body weight changes and (B and D) survival rates after challenge with 5xLD50 of virus were monitored for 14 days. Values are expressed as mean +/− SEM. (n = 5–14). Groups and animal numbers are as described in Fig. 2; *p < 0.05; **p < 0.01.
Offspring born to MN-influenza vaccinated mothers during pregnancy showed superior passive immunity to pups born to IM or ID mice immunized pregnant mice
According to CDC recommendations infants younger than 6 months of age should not receive the influenza vaccine. However, immunized mothers can transfer antibodies to their offspring during gestation through the placenta or post-birth through breast milk39, 40. Influenza-specific antibody levels in mouse pups were correlated with the humoral responses elicited in the mothers. At the end of the weaning period (week 3), the influenza-specific IgG, IG1, and IgG2A antibody titers were at least 2.5 times higher (p < 0.05) in pups born to MN-vaccinated mice compared to mice born to mothers vaccinated with either the same vaccine dose IM or ID or two-fold higher dose administered IM (Fig. 4A,B and C) (Supplementary Table 2). In all groups, influenza-specific antibody titers decreased over time, but the MN offspring had the longest lasting passive immunity. From weeks 4 through 12, IgG and IgG1 antibody titers in the MN offspring ranged from 2 to 10-fold higher than pups born to mothers with the higher IM dose (Fig. 4A and B)
Figure 4.
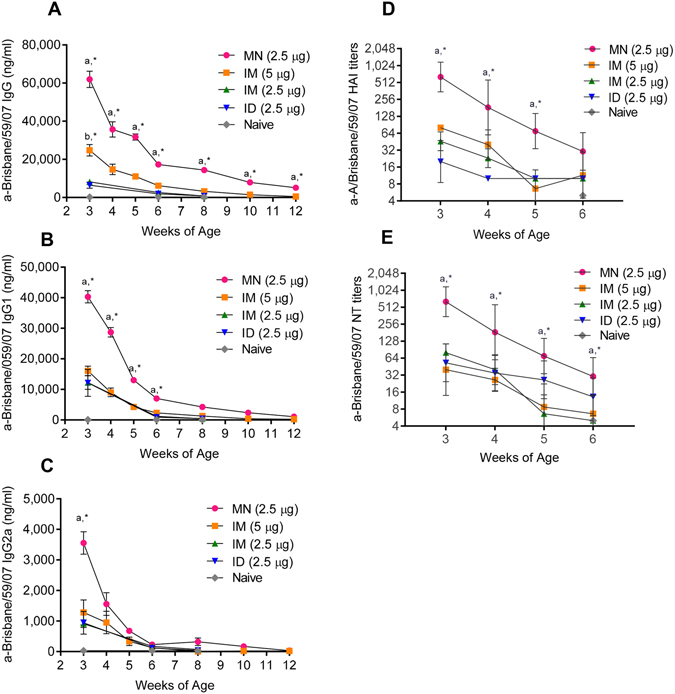
Humoral immune responses in pups born to mothers immunized during pregnancy. Anti-influenza binding antibodies were determined by ELISA in sera collected from pups at weeks 3 to 12 after birth. (A) IgG, (B) IgG1 and (C) IgG2a antibody titers [MN (2.5 ug), n = 10; IM (5 µg), n = 10; IM (2.5 µg), n = 5; ID (2.5 µg), n = 5; Naïve, n = 5]. Values are expressed as mean ±SEM. (D) Hemagglutination inhibition (HAI) and (E) neutralizing antibody (NT) titers in sera collected 3, 4, 5, and 6 weeks after birth (n = 5 per group). HAI and NT values are expressed as geometric mean with ±95% confidence interval. a[MN vs IM (5 μg), IM (2.5 μg), ID (2.5 μg)]. b[IM(5 μg) vs IM (2.5 μg), ID (2.5 μg)]. *p < 0.05. Statistical significance was determined using the Bonferroni-Dunn method, with alpha = 0.05.
The HAI and neutralizing antibody levels were at least 8-fold higher (p < 0.001) in pups from MN-immunized mothers for the first 3 weeks after birth compared to the remaining groups (Fig. 4D and E) (Supplementary Table 2). Six week old offspring from MN vaccinated mothers had HAI and NT titers close to 40 suggestive of protective immunity whereas mice born to mothers immunized IM or ID with a low or high vaccine dose had titers around 10. Interestingly, we noted a dose-dependent response in the production of HAI antibodies in the IM vaccinated mothers’ offspring until week 4 (p = 0.015) (Fig. 4D).
Microneedle immunization of pregnant mice resulted in improved protection of their offspring compared to IM or ID vaccination
The protective efficacy of maternal anti-influenza IgG antibodies transferred to fetuses via the placenta or to the newborns via maternal milk was tested in 6 week-old pups following challenge with 5xLD50 mouse adapted homologous influenza virus (Fig. 5A,B). Although the percent changes of BW over the initial period of 7–8 days (weight loss) were not as prominent for the various offspring groups as in the adult population, the averages at any given time point show that the MN group benefited most from the vaccination (Fig. 5A). It should be mentioned here that the average percentage changes in body weights are affected by the numbers of surviving mice. The highest survival rates were observed in pups from MN-immunized mothers (50% survival) and correlated well with the levels of neutralizing antibodies (Fig. 5B and D). These rates were significantly higher than those observed in pups of pregnant mice immunized intramuscularly with 5 µg or 2.5 µg of HA, which showed survival rates of 10% (p = 0.03) and 0% (p = 0.05) respectively. There was a strong correlation between hemagglutination inhibition antibody titers (HAI) and percent survival (R2 = 0.986, p = 0.01). These data confirm the superiority of MN vaccination, since the passive immunity observed in the offspring of vaccinated mothers was robust and long-lived, with protective antibodies persisting at least up to 6 weeks after birth.
Figure 5.
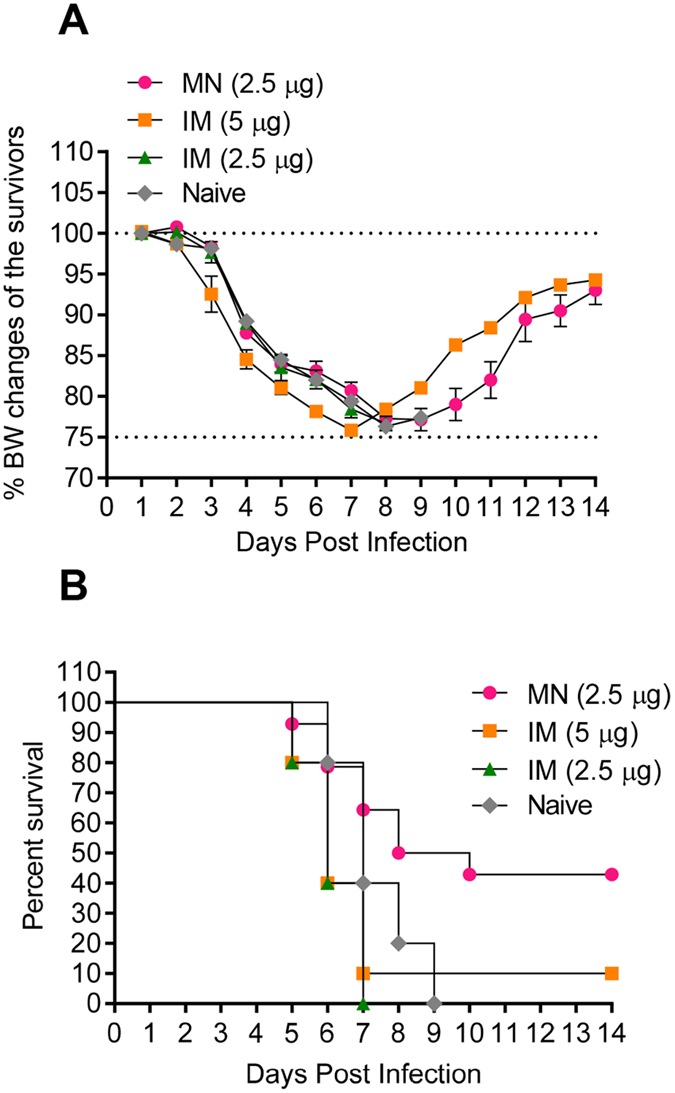
Protective immunity after challenge with A/Brisbane/59/07 virus of offspring born to mothers immunized during pregnancy. Offspring were challenged with 3xLD50 of mouse adapted A/Brisbane/59/07 virus 6 weeks after birth. (A) Body weight changes were monitored for 14 days (B) Survival rates. Values are expressed as mean +/− SEM. Groups and numbers are as described in Fig. 4.
Discussion
During pregnancy, the immune system adapts to tolerate a genetically different fetus as a foreign entity. Dramatic alterations in pro- and anti-inflammatory cytokine profiles and changes in Th1 and Th2 responses exert a suppressive effect on a mother’s immune system pre-disposing her to increased susceptibility to infection and endangering the outcome of pregnancy41. Vaccination of pregnant women has a “two-in-one” benefit of protecting both the mother and infant. Mothers can transfer protective antibodies through placenta or through breast milk to the offspring, providing protection in infants until they are old enough to be immunized39, 40. Several reports showed that influenza vaccination of pregnant mothers is associated with improved pregnancy outcomes11, 42–44 and is up to 63% effective in preventing laboratory confirmed influenza infection in infants45.
In this study we investigated the potential of influenza skin immunization with MNs as a platform to improve host immune responses in pregnancy that could in turn effectively protect newborns by passive transfer through the placenta or breast-feeding milk. We used the BALB/c mouse model that shares the same hemochorial placentation as humans, and although the gestation period in BALB/c mice is short (approximately 20–21 days) we were able to time their pregnancies and immunize at mid-gestation simulating human immunizations, as the second or last trimester is the preferred time for pregnant women to receive influenza vaccine. We did not observe any adverse effects of MN vaccination in pregnant mice. No physical marks were left on the skin at the site of immunization, no behavioral changes were noted, the body weight increase was constant, and premature deliveries were not observed suggesting that the MN application did not affect the pregnant females or their fetuses.
We first compared the magnitude of humoral immune responses by assessing the levels of functional antibody titers, including HAI and neutralizing antibody titers, which are considered correlates of protection, in the sera from MN, IM and ID vaccinated pregnant and non-pregnant mice. Although pregnant vaccinated mice had significantly lower humoral immune responses than their non-pregnant counterparts, we found that delivery of influenza vaccine with MNs could confer superior protective immune responses compared to IM or ID immunization in both cohorts. In addition to the induction of robust immune responses, significant dose sparing was observed when using the MN method of vaccine delivery. Adult non-pregnant and pregnant mice that received a single dose of 2.5 μg HA A/Brisbane/59/07 H1N1 subunit vaccine delivered with MN produced higher anti-influenza antibody titers than mice having received a 2-fold higher dose of the vaccine via the IM route.
Complete protection against lethal infection (5xLD50) from homologous mouse adapted virus was observed in pregnant and non-pregnant animals vaccinated with MN, whereas animals vaccinated via IM or ID administration with 2.5 or 5 μg HA of A/Brisbane/59/07 during gestation were not protected and non-pregnant IM or ID vaccinated animals were partially protected. These findings are similar to those observed in our previous study of tetanus toxoid vaccination using MNs in pregnant mice35.
Previous studies showed that pups born to mice that were vaccinated IP or IM during pregnancy were protected from lethal challenge with influenza virus19, 20. In addition, pups born to non-immunized mice but cross-fostered by immunized mothers were also protected, suggesting that protective immunity can be transferred through breast milk19, 20. In the present study, we demonstrated that pups born to mothers immunized using MNs encapsulating subunit influenza vaccine had higher levels of specific anti-influenza antibodies in sera than mice that were born to mothers immunized with a two-fold higher dose of the same vaccine via the IM route, The superiority of the MN route was more prominent when compared to the ID or IM vaccination route of mothers that received the same vaccine dose. The highest antibody titers were observed in 3 week-old pups that were still housed with the mothers; a gradual decrease was observed with time following separation from mothers. These results are consistent with reports showing that protective immunity decreases with time after separation from mothers19, 20. Pups born to MN immunized mothers maintained significantly higher antibody titers at any tested time and, for up to nine weeks after weaning than pups born to intramuscularly immunized mice. Higher survival rates after lethal challenge with homologous virus three weeks after weaning were observed in pups born to MN immunized mice.
These data demonstrate the benefit of developing a simple-to-administer vaccination method that overcomes the lack of adequate health care in developing countries while achieving protective immunity superior or at least equivalent to conventional immunization. Considering the advantages of the MN technology (thermostability, self-administration, safety, no biohazardous sharps, dose sparing, and robust induction of immune responses at lower doses of vaccine), we believe that a MN vaccination strategy is an attractive option for vaccination of pregnant women, which can aid in achieving the third United Nations Sustainable Development Goal which aims to reduce mortality of children younger than 5 and to reduce maternal mortality46.
Materials and Methods
Cells and virus stocks
Madin-Darby canine kidney (MDCK) cells (CCL 34, ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle’s Medium (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Hyclone, Thermo Scientific, Rockford, IL). Influenza virus stocks (A/Brisbane/59/07 (H1N1)) were propagated in MDCK cells. Mouse-adapted virus was serially passaged in BALB/c mouse lungs, and viral stock titers were determined by plaque assay47. The LD50 was determined using the Reed-Munch formula48. The hemagglutination activity was determined using turkey red blood cells (LAMPIRE, Pipersville, PA)49.
MNs containing influenza vaccine
MNs were manufactured in a two-step micro-molding process described previously35 and stored with a desiccant in individual sealed pouches until use. The bending and brittleness of the microneedles was visually evaluated under the microscope before and after insertion of MN into pig skin explants (not shown). The antigen dose was adjusted to 2.5 μg per patch and mounted onto a 1 cm2 paper disk for application.
Delivery efficiency of MNs
To determine antigen delivery efficiency, MNs were prepared according to the protocol described by Vassilieva50. Freshly prepared MNs encapsulating A/Brisbane/59/2007 monovalent vaccine were cut in halves. One half was manually applied to mouse skin, firmly held in place for 1 minute and left on skin for an additional 9 minutes while the other, unused half was kept as a control. The hemagglutinin (HA) content was measured in both used and unused halves with SRID assay.
Single Radial Immunodiffusion (SRID) assay
Antigen content in each MN was quantified in triplicate via SRID as described previously50.
Animals
Eight-week-old male and female BALB/c mice were purchased from Harlan Laboratories (Dublin, VA). All mice were bred and housed in the Emory University Whitehead Animal Facility. Immunizations were performed in a Biosafety Level 1 facility, and influenza challenge studies were conducted in a Biosafety Level 2 facility at Emory University (Atlanta, GA). All animal breeding, vaccination, and infection experiments were performed in accordance with relevant guidelines and regulations and approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University.
Breeding protocol
Breeding cages were set up with three females in proestrus or estrus and one male for 3 days51, 52. The timing of pregnancies was determined by the presence of copulation plugs and body weight increases in female mice after mating35.
Immunization and sample collection
Female pregnant and non-pregnant mice were immunized cutaneously with MN, intramuscularly (IM) or intradermally (ID) at mid-gestation (days 11–13) as outlined in Fig. 6. For MN immunization, mouse skin was exposed by trimming hair and 1 minute application of depilatory cream (Nair, Church and Dwight, Ewing, NJ) on the caudal area on the dorsal side. MNs were manually inserted with constant pressure into the skin for 1 minute and left in place for 10 minutes to allow microneedle dissolution and delivery of 2.5 µg HA antigen. For IM immunizations, 5 or 2.5 µg HA antigen was diluted in PBS and injected into the biceps femoris in the hind leg of the mice. Blood was collected submandibularly at 4 weeks post-immunization and serum was stored at −20 °C until analysis. The pups were weaned 3 weeks after birth (day 21) and bled at weeks 3, 4, 5, 6, 8, 10 and 12 after birth (Fig. 6).
Figure 6.
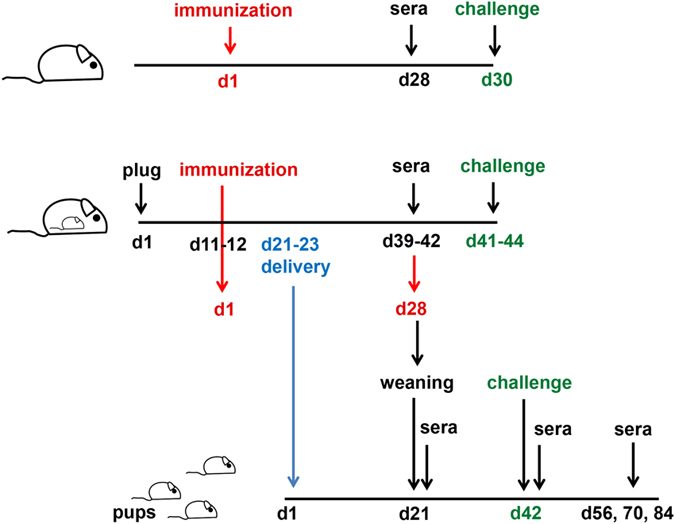
Schematic presentation of animal vaccination and challenge. Pregnant BALB/c mice were immunized at mid gestation with A/Brisbane/59/07 H1N1 vaccine via the intramuscular (IM), intradermal (IM), or cutaneous route using fish gelatin microneedles encapsulating the vaccine (MN). Non-pregnant female mice were also immunized with the same vaccine via the same routes. On day 28 after immunization mice were bled and then challenged with homologous virus. The pups were weaned three weeks after birth and bled on weeks 3, 4, 5, 6, 8, 10, and 12. A group of 6 week old pups was challenged with A/Brisbane/59/07 H1N1 virus 6 weeks after birth.
Humoral immune responses
Influenza-specific antibody serum concentrations were quantified via ELISA in Nunc 96-well Maxisorb plates (Rochester, NY) coated with 100 ng total protein of seasonal A/Br/59/07 vaccine per well53. Hemagglutination inhibition (HAI) titers were determined using turkey red blood cells (LAMPIRE, Pipersville, PA) WHO protocol49, 54. Sera from vaccinated mice was heat inactivated and neutralizing potency was determined in a microneutralization assay using 100 TCID50/well of H1N1 A/Brisbane/59/07 virus17.
Challenge studies of adult and young mice
Adult mice and their offspring were lightly anesthetized under 2 ml isoflurane in a 500-ml enclosed beaker and infected intranasally with 30 μl 5xLD50 of mouse adapted A/Brisbane/59/07 virus at 6 weeks after birth. The LD50 was calculated separately for adult and young mice. The animals were monitored for 14 days for body weight changes, fever, hunched posture, and mortality. Weight loss exceeding 25% of the starting body weight was used as the experimental end point, at which mice were euthanized according to relevant IACUC guidelines and regulations.
Statistics
Statistical significance between experimental groups was calculated via Mann-Whitney two-tailed unpaired non-parametric t-tests with alpha = 0.05. Antibody assays (ELISA, HAI, microneutralization) were duplicated except where noted otherwise. HAI and NT titers were analyzed as log2 titers. For survival curves, statistics were calculated using a Log-rank (Mantel-Cox) test. Non-linear regression analyses were performed to determine the IC50 (95% confidence interval). A p value ≤ 0.05 was considered significant. Pearson correlation coefficients were applied to correlate humoral responses to survival.
Data Availability
All data generated or analyzed during this study are included in this published article.
Electronic supplementary material
Acknowledgements
We thank Dahnide Taylor-Williams and Donna Bondy for their valuable technical and administrative support. We thank Novartis for the monovalent subunit vaccine. The work was supported by United States Agency for International Development (AID-OAA-F-13–00083 award).
Author Contributions
E.S.E. and J.P.P. designed and executed the animal experiments, processed data, prepared figures and wrote part of the manuscript. H.K. fabricated the fish gelatin microneedle patches. H.K. and D.M. developed the fish gelatin microneedle patches. E.V.V. designed and executed all the vaccine stability studies before and after formulation, E.Q.L. contributed in pregnant mouse studies and advanced the project to new areas. M.R.P. and R.W.C. provided their scientific advice during the study and edited the manuscript. I.S. designed the study, assisted in data processing, analysis and statistics; in figure preparation and in manuscript writing. All authors reviewed the manuscript.
Competing Interests
Mark Prausnitz is an inventor of patents that have been licensed to companies developing microneedle-based products, a paid advisor to companies developing microneedle-based products, and a founder/shareholder of companies developing microneedle-based products (Micron Biomedical). The terms of this arrangement have been reviewed and approved by Georgia Tech and Emory University in accordance with their conflict of interest policies.
Footnotes
E. Stein Esser and Joanna A. Pulit-Penaloza contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05940-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cantu J, Tita AT. Management of influenza in pregnancy. American journal of perinatology. 2013;30:99–104. doi: 10.1055/s-0032-1331033. [DOI] [PubMed] [Google Scholar]
- 2.Dodds L, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2007;176:463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. American journal of obstetrics and gynecology. 2012;207:S3–8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson AM, Yu S, Annicelli CH, Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell host & microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrekoussis T, et al. The role of stress in female reproduction and pregnancy: an update. Annals of the New York Academy of Sciences. 2010;1205:69–75. doi: 10.1111/j.1749-6632.2010.05686.x. [DOI] [PubMed] [Google Scholar]
- 6.Pierce M, et al. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. Bmj. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease, C. & Prevention. Maternal and infant outcomes among severely ill pregnant and postpartum women with 2009 pandemic influenza A (H1N1)–United States, April 2009–August 2010. MMWR. Morbidity and mortality weekly report60, 1193–1196 (2011). [PubMed]
- 8.Michaan N, et al. Maternal and neonatal outcome of pregnant women infected with H1N1 influenza virus (swine flu) The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:130–132. doi: 10.3109/14767058.2011.562569. [DOI] [PubMed] [Google Scholar]
- 9.Manske, J. M. Efficacy and Effectiveness of Maternal Influenza Vaccination During Pregnancy: A Review of the Evidence. Maternal and child health journal (2013). [DOI] [PubMed]
- 10.Haberg SE, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. The New England journal of medicine. 2013;368:333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards JL, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:1216–1222. doi: 10.1093/cid/cit045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51:1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisa, A. et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). United States, 2013–14 Influenza Season. in MMWR Recommendation Report, Vol. 62, 1–43 (CDC, Atlanta, GA, 2013). [PubMed]
- 14.Committee on Obstetric P, et al. Committee opinion no. 608: influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648–651. doi: 10.1097/01.AOG.0000453599.11566.11. [DOI] [PubMed] [Google Scholar]
- 15.Helen Ding, C. L. B. et al. Influenza Vaccination Coverage Among Pregnant Women — United States, 2014–15 Influenza Season. In MMWR Recommendation Report, Vol. 64, 1000–1005 (CDC, Atlanta, GA, 2015). [DOI] [PubMed]
- 16.Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. Journal of controlled release: official journal of the Controlled Release Society. 2016;240:135–141. doi: 10.1016/j.jconrel.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutsonanos DG, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific reports. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsonanos DG, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. The Journal of infectious diseases. 2011;204:582–591. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang SD, et al. Protection of pregnant mice, fetuses and neonates from lethality of H5N1 influenza viruses by maternal vaccination. Vaccine. 2010;28:2957–2964. doi: 10.1016/j.vaccine.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Mbawuike IN, Six HR, Cate TR, Couch RB. Vaccination with inactivated influenza A virus during pregnancy protects neonatal mice against lethal challenge by influenza A viruses representing three subtypes. J Virol. 1990;64:1370–1374. doi: 10.1128/jvi.64.3.1370-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn HL, Kearney MC, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert opinion on drug delivery. 2014;11:1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 22.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Advanced drug delivery reviews. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. Journal of controlled release: official journal of the Controlled Release Society. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Human vaccines & immunotherapeutics. 2016;12:2975–2983. doi: 10.1080/21645515.2016.1171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. The Clinical journal of pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haq MI, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomedical microdevices. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 27.Norman JJ, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. Journal of pharmaceutical sciences. 2015;104:740–749. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skountzou I, Compans RW. Skin immunization with influenza vaccines. Current topics in microbiology and immunology. 2015;386:343–369. doi: 10.1007/82_2014_407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando GJ, et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PloS one. 2010;5:e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nature medicine. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kommareddy S, et al. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. Journal of pharmaceutical sciences. 2012;101:1021–1027. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 33.Hirobe S, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–58. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Rouphael, N. G. et al. The safety, immunogenecity and acceptability of inactivated influenza vaccine delivered by microneedle patch: a randomized, partially blind, placebo-controlled phase 1 trial. The Lancet in press (2017). [DOI] [PMC free article] [PubMed]
- 35.Esser ES, et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. Journal of controlled release: official journal of the Controlled Release Society. 2016;236:47–56. doi: 10.1016/j.jconrel.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS immunology and medical microbiology. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 37.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerging infectious diseases. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HM, Kang YM, Song BM, Kim HS, Seo SH. The 2009 pandemic H1N1 influenza virus is more pathogenic in pregnant mice than seasonal H1N1 influenza virus. Viral immunology. 2012;25:402–410. doi: 10.1089/vim.2012.0007. [DOI] [PubMed] [Google Scholar]
- 39.Englund JA, et al. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. The Journal of infectious diseases. 1993;168:647–656. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 40.Linder N, Ohel G. In utero vaccination. Clinics in perinatology. 1994;21:663–674. [PubMed] [Google Scholar]
- 41.Memoli MJ, Harvey H, Morens DM, Taubenberger JK. Influenza in pregnancy. Influenza and other respiratory viruses. 2013;7:1033–1039. doi: 10.1111/irv.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinhoff MC, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2012;184:645–653. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fell DB, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. American journal of public health. 2012;102:e33–40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omer SB, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS medicine. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaman K, et al. Effectiveness of maternal influenza immunization in mothers and infants. The New England journal of medicine. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 46.Acheampong, M., Ejiofor, C. & Salinas-Miranda, A. An Analysis of Determinants of Under-5 Mortality across Countries: Defining Priorities to Achieve Targets in Sustainable Developmental Goals. Maternal and child health journal (2017). [DOI] [PubMed]
- 47.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/JVI.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muench, L. J. R. A. H. A simple method of estimating fifty percent endpoints. Journal of Hygiene29 (1938).
- 49.WHO Manual on Animal Influenza Diagnosis and Surveillance. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf.
- 50.Vassilieva, E. V. et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug delivery and translational research (2015). [DOI] [PMC free article] [PubMed]
- 51.Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biology of reproduction. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 52.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PloS one. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koutsonanos DG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS one. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO/CDS/CSR/NCS. WHO Manual of Animal Influenza Diagnosis and Surveillance. Department of Communicable Disease Surveillance and Response (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


