Abstract
The aim of this study is to evaluate the efficacy and safety of sugammadex for reversing neuromuscular blockade in pediatric patients. MEDLINE and other three Databases were searched. Randomized clinical trials were included if they compared sugammadex with neostigmine or placebo in pediatric patients undergoing surgery involving the use of rocuronium or vecuronium. The primary outcome was the time interval from administration of reversal agents to train-of-four ratio (TOFr, T4/T1) > 0.9. Incidences of any drug-related adverse events were secondary outcomes. Trial inclusion, data extraction, and risk of bias assessment were performed independently. Mean difference and relative risk were used as summary statistics with random effects models. Statistical heterogeneity was assessed by the I2 statistic. Funnel plot was used to detect publication bias. Ten studies with 580 participants were included. Although considerable heterogeneity (I2 = 98.5%) was detected in primary outcome, the results suggested that, compared with placebo or neostigmine, sugammadex can reverse rocuronium-induced neuromuscular blockade more rapidly with lower incidence of bradycardia. No significant differences were found in the incidences of other adverse events. Compared with neostigmine or placebo, sugammadex may reverse rocuronium-induced neuromuscular blockade in pediatric patients rapidly and safely.
Introduction
Neuromuscular blocking agents (NMBA) are frequently used to facilitate endotracheal intubation and mechanical ventilation and to provide good quality surgical conditions. However, postoperative residual neuromuscular blockade may increase the risk of postoperative pulmonary diseases and respiratory complications, such as pulmonary atelectasis, decreased oxygen saturation and upper airway obstruction, which may result in reintubation in the ICU and prolong the patient’s length of stay1, 2.
To accelerate the recovery time from neuromuscular blockade and to prevent postoperative residual neuromuscular blockade3, 4, acetylcholinesterase inhibitors, the only reversal agents before sugammadex, are often administered. However, these antagonist are usually associated with bradycardia, bronchospasm and other undesirable muscarinic side effects2. Anticholinergic drugs that are used to relieve muscarinic side effects may only be effective when used in high doses, which comes with the possibility of other unacceptable side effects2. Additionally, there is an association between acetylcholinesterase inhibitors and residual blockade in both pediatric and adult patients5–9.
Sugammadex is a selective relaxant binding agent with a modified gamma cyclodextrin, and it is specifically designed to grab and encapsulate the aminosteroidal NMBAs such as rocuronium or vecuronium10. Sugammadex forms a complex with these NMBAs separates NMBAs from nicotinic receptors at the neuromuscular junction therefore resulting in the reversal of the neuromuscular blockade11. Several clinical studies have shown that sugammadex is a safe, effective agent for the rapid reversal of aminosteroidal neuromuscular blockade for any depth of muscle relaxation12–21. Since the pharmacokinetic and pharmacodynamic profiles of neuromuscular blockade are affected by different age, they may be not the same between pediatric and adult patients22.
To examine whether sugammadex can be used to reverse rocuronium or vecuronium in pediatric patients, we reviewed randomized controlled trials (RCTs) that compared the efficacy and safety of sugammadex with neostigmine or placebo in pediatric patients undergoing general anesthesia.
Results
Study selection
We initially identified 166 studies by searching the MEDLINE, EMBASE, CENTRAL, and Web of Science databases, and three additional citations were identified through a Google Scholar search. Seventy-four citations were excluded as duplicates. Next, we scanned the abstracts of the remaining 95 citations and found that 55 did not meet our inclusion criteria. Then, we retrieved the full texts of the remaining 40 citations and excluded 30 studies. The reasons for exclusion are as follows: 13 studies were conducted using adult patients or volunteers; one study was a duplicate of another article; five studies were not RCTs; nine articles were reviews; and two studies compared different doses of sugammadex. Ten studies23–32 that fulfilled the criteria of our study were included. Two review authors (G.L. and R.W.) were in complete agreement regarding the inclusion of selected studies. The study selection process is shown in Fig. 1.
Figure 1.
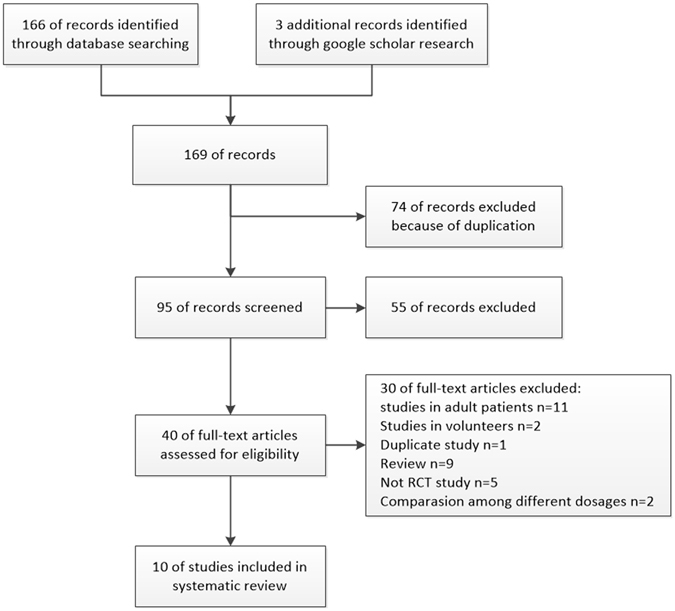
Study selection flow diagram.
To obtain complete data and details to evaluate risk of bias in studies, we contacted the authors of the included studies by email. Only El sayed M replied and gave us additional details that were unreported in article.
Study characteristics
A total of 580 eligible participants were included in the systematic review. Nine studies24–32 were conducted on pediatric patients over the age of 2 years, and in the study conducted by Plaud23, 8 infants met the eligibility criteria. Sugammadex and neostigmine were compared in nine studies24–32, and placebo was used as a control in the study conducted by Plaud23. Aside from one study24 that used 0.45 mg/kg of rocuronium, nine studies23, 25–32 used 0.6 mg/kg of rocuronium as the neuromuscular blockade agent before orotracheal intubation, and additional rocuronium was administered in seven studies24, 27–32. Reversal agents (sugammadex, neostigmine or placebo) were administered upon reappearance of T2 or T3 in eight studies23, 24, 27–32, while the effects of sugammadex on the reversal of deep rocuronium-induced neuromuscular blockade, with a post tetanic count (PTC) < 2–3, were examined in two studies25, 26. However, in one study26 reversal agents were administered at different times between the sugammadex group (PTC < 2–3) and the control group (PTC > 2–3). Inhaled anesthesia was used in six studies24, 27–31 and TIVA was used in one study23, while two studies25, 26, 32 did not report the maintenance of the anesthesia technique. All studies reported the time from the reversal of the neuromuscular blockade to train-of-four ratio (TOFr) > 0.9 as the primary outcome. Table 1 shows the characteristics of all of the included studies.
Table 1.
Characteristics of included studies.
| First author | year | study country | Numbers of patients | Age of patients | ASA PS | Type of surgery | Dose of NMBA | Additional NMBA | Intervention | Control | Time of reverse | Maintenance of anesthesia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plaud B23 | 2009 | Six European centers | 63 | 28 days –17 year (2 infants, 4 children and 5 adolescents in CG/6 infants, 18 children and 23 adolescents in SG)* | 1–2 | Surgery in a supine position | 0.6 mg/kg Roc | None | 0.5 mg/kg Sug 1 mg/kg Sug 2 mg/kg Sug 4 mg/kg Sug | placebo | Reappearance of T2 three times | Opioid + Propofol |
| Veiga RG24 | 2011 | Spain | 24 | 2–9 years | UR | UR | 0.45 mg/kg Roc | 0.15 mg/kg Roc as needed | 2.0 mg/kg Sug | 5 mcg/kg Neo + 2.5 mcg/kg Atp** | Reappearance of T2 three times | 70% N2O |
| Alvarez-Gomez JA25 | 2012 | Multicenter | 96 | 2–11 years | UR | UR | 0.6 mg/kg Roc | UR | 4.0 mg/kg Sug | 50 mcg/kg Neo + 25 mcg/kg Atp | PTC < 2 | UR |
| Gaona D26 | 2012 | Multicenter | 30 | 2–11 years | UR | UR | 0.6 mg/kg Roc | UR | 4 mg/kg Sug | 50 mcg/kg Neo + 25 mcg/kg Atp | PTC < 2–3 (sug group) PTC > 2–3 (control group) | UR |
| Kara T27 | 2014 | Turkey | 80 | 2–12 years (5.07 ± 3.24 years in CG/6.48 ± 2.81 years in SG) | 1 | Lower abdominal or urogenital procedures | 0.6 mg/kg Roc | 0.2 mg/kg Roc as needed | 2 mg/kg Sug | 30 mcg/kg Neo + 10 mcg/kg Atp | Reappearance of T2 | 50% N2O + 2% Sev |
| Ozgun C28 | 2014 | Turkey | 60 | 2–12 years (8.0 ± 2.8 years in CG/7.3 ± 2.2 years in SG) | 1–2 | Ear nose and throat surgery | 0.6 mg/kg Roc | 0.1–0.2 mg/kg Roc as needed | 2.0 mg/kg Sug | 60 mcg/kg Neo + 20 mcg/kg Atp | Reappearance of T2 | 50% N2O + Sev (1.3–1.5MAC) |
| Ghoneim AA29 | 2015 | Egypt | 40 | 7–18years | 1–3 | Posterior fossa tumor excision | 0.6 mg/kg Roc | 0.4 mg/kg/h Roc | 4 mg/kg Sug | 40 mcg/kg Neo + 20 mcg/kg Atp | Reappearance of T2 | 0.5 μg/kg/h Fentanyl + Sev 1MAC |
| El sayed M30 | 2016 | Egypt | 70 | 2–10 years (5.42 ± 2.23 years in CG/5.64 ± 2.41 years in SG) | 1 | Tonsillectomy (outpatient) | 0.6 mg/kg Roc | 0.2 mg/kg Roc as needed | 2.0 mg/kg Sug | 50 mcg/kg Neo + 10 mcg/kg Atp | Reappearance of T2 | Isoflurane |
| Güzelce D31 | 2016 | Turkey | 37 | 2–16 years (7.02 ± 4.46 years in CG/6.37 ± 4.08 years in SG) | 1 | lower urinary tract surgery and inguinal hernia | 0.6 mg/kg Roc | 0.15 mg/kg Roc as needed | 2.0 mg/kg Sug | 50 mcg/kg Neo + 20 mcg/kg Atp | Reappearance of T2 | 2% Sev |
| Mohamad Zaini RH32 | 2016 | Malasia | 80 | 2–12 years | UR | UR | 0.6 mg/kg Roc | 0.2 mg/kg Roc as needed | 2.0 mg/kg Sug | 50 mcg/kg Neo + 20 mcg/kg Atp | Reappearance of T2 or T3 | UR |
ASA PS: American Society of Anesthesiologists’ physical status. NMBA: neuromuscular blockade agents. UR: unreported. Roc: Rocuronium. Sug: sugammadex. Neo: neostigmine. Atp: atropine. PTC: post-titanic count. Sev: Sevoflurane. MAC: minimum alveolar concentration. CG: control group. SG: sugammadex group. *In Plaud’s study, Infant, child, and adolescent were defined as 28 days to 23 months, 2–11 years and 12–17 years, respectively. **The dose of neostigmine and atropine was suspected for that is far less than recommended.
Risk of bias within studies
The Cochrane Collaboration’s risk of bias tool was used to assess the validity and quality of the included Five studies24–26, 28, 32 were not explicit about how they generated random sequences. Six studies24–27, 29, 32 were unclear about their allocation concealment. Four studies24, 25, 29, 31 did not report whether the patients and the assessors were blinded during outcome assessments. In the study conducted by El sayed30, the assessors were not blinded to the allocation of groups, which may have affected the results of the study; therefore, we evaluated the study as ‘high risk’. Four studies24, 26–28 did not provide sufficient detail on whether there was attrition or exclusion. When we assessing risk of selective reporting in one study24, there was insufficient information about whether the study was considered ‘low risk’ or ‘high risk’. Since the primary outcome reports in two studies25, 28 were contradicted by the tables and the article text using incorrect units, we were not confident in the results and marked the study as “other risk of bias” under the high risk category. In one study26 reversal agents were administered at different times between the sugammadex group (PTC < 2–3) and the control group (PTC > 2–3), which we believe may have influenced the primary outcome. We believe that one trial23 had a low risk of bias because all of the study criteria were assessed as low risk, whereas three trials25, 26, 28, 30, 31 had high risks, and the risks of other studies24, 27, 29, 32 were unclear. Assessments of risk of bias are summarized in Fig. 2.
Figure 2.
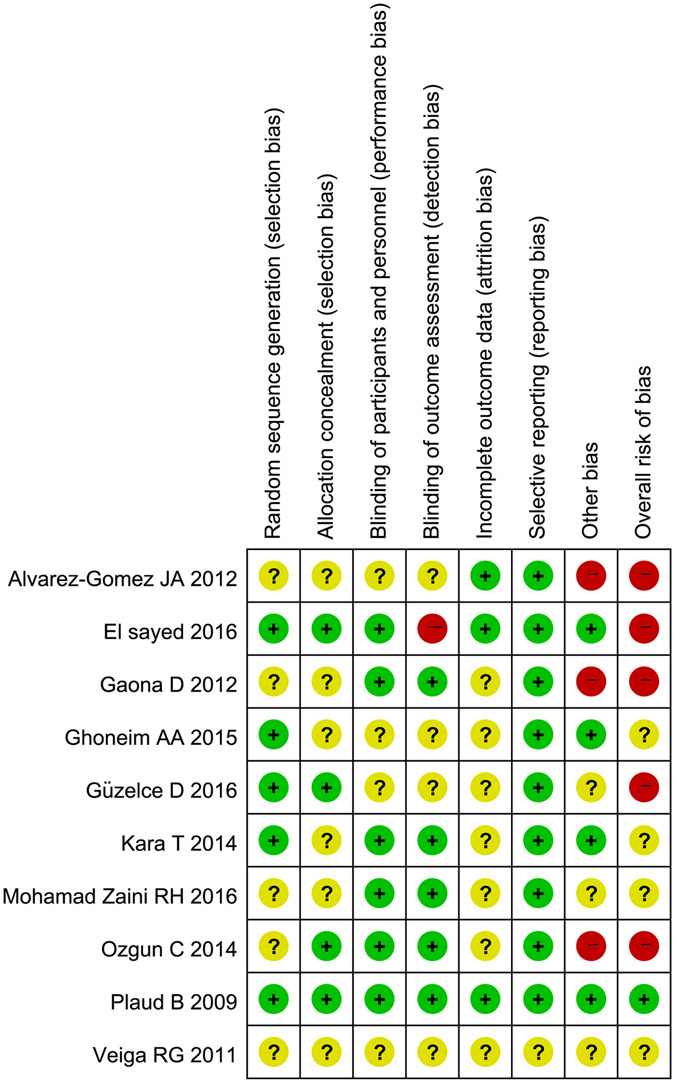
Summary of the risk of bias of the included studies.
Primary outcome
Primary outcome was reported in all of the studies, totaling 575 participants (protocol violations were reported for four participants, and the primary outcome of one participant was missing in one study23). All ten studies showed significant differences between the sugammadex group and the control group. Figure 3 shows that sugammadex was significantly more effective than the control in reducing the time from administration of reversal agents to TOFr > 0.9 in pediatric patients (WMD = −8.51, 95% CI: −11.32 to −5.71), but considerable heterogeneity was detected (I2 = 98.3%). Heterogeneity was not resolved with sensitivity analyses and subgroup analyses.
Figure 3.
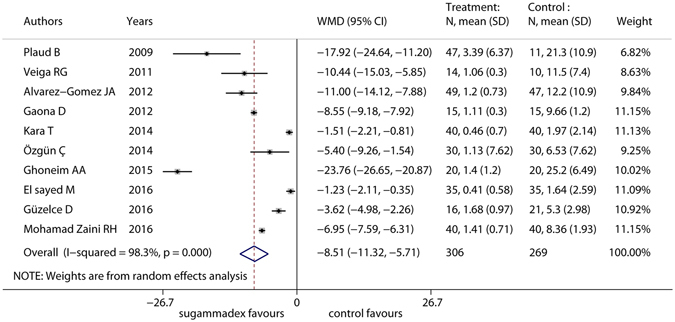
Forest plot of primary outcome of included RCTs. Sugammadex was significantly more effective than control in reducing recovery the reversal of neuromuscular blockade to TOFr > 0.9 in pediatric patients (WMD = −8.51, 95% CI: −11.32 to −5.71), but considerable heterogeneity was detected (I2 = 98.3%).
Secondary outcome
Compared with neostigmine, sugammadex was able to reduce the incidence of bradycardia (RR = 0.08; 95% CI: 0.01 to 0.42), whereas no significant differences were found for the incidence of other adverse events (AEs) between the two groups, such as nausea and vomiting (RR = 0.57; 95%CI: 0.32 to 1.03), diarrhea (RR = 0.75; 95%CI: 0.03 to 17.37), and bronchospasm (RR = 0.73; 95% CI: 0.05 to 10.78). These results are shown in Table 2.
Table 2.
The summary of reported secondary outcome (adverse events) in the included studies.
| Adverse events | Number of studies | Incidence of adverse events/total number of patients | RR [95% CI] | I2 | P-value | References | |
|---|---|---|---|---|---|---|---|
| Sugammadex | Control | ||||||
| Nausea and vomiting | 8* | 27/281 | 25/245 | 0.57[0.32, 1.03] | 9% | P = 0.355 | 24, 25, 27–32 |
| Bradycardia | 5* | 0/190 | 15/149 | 0.08[0.01, 0.42] | 0% | P = 0.823 | 23, 25–27, 30 |
| Tachycardia | 1 | 0/40 | 0/40 | / | / | / | 27 |
| Hypotension | 2 | 0/75 | 0/75 | / | / | / | 27, 30 |
| QTc prolongations | 2 | 0/91 | 0/52 | / | / | / | 23, 27 |
| Constipation | 1 | 2/51 | 0/12 | 1.25[0.06, 24.48] | / | / | 23 |
| Diarrhea | 1 | 1/51 | 0/12 | 0.75[0.03, 17.37] | / | / | 23 |
| Viral gastroenteritis | 1 | 1/51 | 0/12 | 0.75[0.03, 17.37] | / | / | 23 |
| Nasopharyngitis | 1 | 1/51 | 0/12 | 0.75[0.03, 17.37] | / | / | 23 |
| Pharyngitis | 1 | 1/51 | 0/12 | 0.75[0.03, 17.37] | / | / | 23 |
| Rhinitis | 1 | 0/51 | 1/12 | 0.08[0.00, 1.93] | / | / | 23 |
| Bronchospasm | 3* | 1/119 | 2/117 | 0.73[0.05, 10.78] | 34.6% | P = 0.216 | 25, 27, 28 |
| Hypoglycemia | 1 | 1/51 | 0/12 | 0.75[0.03, 17.37] | / | / | 23 |
| Pyrexia | 2* | 0/91 | 1/52 | 0.08[0.00, 1.93] | / | / | 23, 27 |
| Pain | 1 | 2/51 | 0/12 | 1.25[0.06, 24.48] | / | / | 23 |
| Procedural pain | 1 | 17/51 | 4/12 | 1.00[0.41, 2.43] | / | / | 23 |
| Diplopia | 2 | 0/56 | 0/61 | / | / | / | 27, 31 |
| Hyper salivation | 1 | 0/40 | 0/40 | / | / | / | 27 |
| Dysgeusia | 1 | 0/40 | 0/40 | / | / | / | 27 |
| Rash | 3 | 0/91 | 0/96 | / | / | / | 27, 30, 31 |
| Postoperative anemia | 1 | 2/51 | 0/12 | 1.25[0.06, 24.48] | / | / | 23 |
*Several studies were excluded when relative risk was calculated because the incidences of two groups were both zero.
Publication bias
With ten studies included, a funnel plot was used to assess publication bias. Figure 4 shows that the funnel was not entirely symmetrical.
Figure 4.
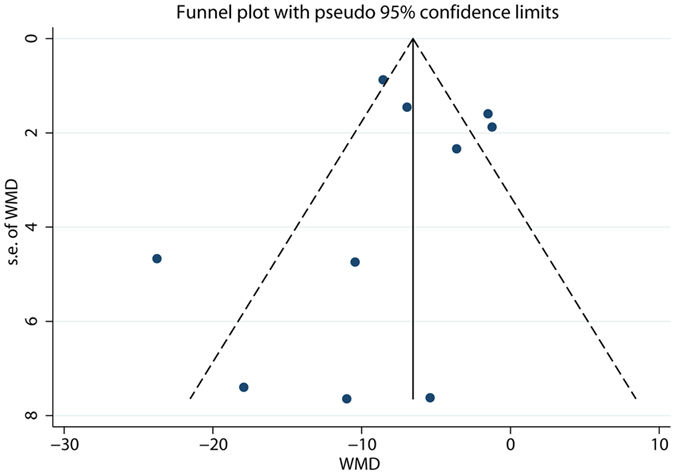
Funnel plot of primary outcome of included RCTs.
Sensitivity analysis and subgroup analysis
Two separate sensitivity analyses were performed (Table 3) as follows: (1) excluding studies with an unclear or high risk of bias and (2) a comparison of weighted mean difference and standard mean difference.
Table 3.
Subgroup analysis and sensitivity analysis.
| Subgroups | Number of studies | Number of patients | Weighted Mean difference (or Standardized mean difference) [95% CI] | I2 |
|---|---|---|---|---|
| Risk of bias | ||||
| Low risk | 1 | 58 | −17.92 [−24.64, −11.20] | / |
| High or unclear risk | 9 | 517 | −7.82 [−10.70, −4.94] | 98.4% |
| Intervention effect measure scale | ||||
| Weighted mean difference | 10 | 575 | −8.51 [−11.32, −5.71] | 98.3% |
| Standardized mean difference | 10 | 575 | −2.56 [−3.46, −1.66] | 94.2% |
| controls | ||||
| neostigmine | 9 | 517 | −7.82 [−10.70, −4.94] | 98.4% |
| placebo | 1 | 58 | −17.92 [−24.64, −11.20] | / |
| Dose of sugammadex | ||||
| 4 mg/kg | 4 | 188 | −15.66 [−23.61, −7.70] | 97.3% |
| 2 mg/kg | 7 | 373 | −5.81 [−8.50, −3.12] | 97.0% |
| 1 mg/kg | 1 | 24 | −18.57[−25.15, −11.99] | / |
| 0.5 mg/kg | 1 | 23 | −13.53[−22.71, −4.35] | / |
| Time of reverse | ||||
| T2 reappeared | 8 | 449 | −8.24 [−11.53, −4.95] | 98.1% |
| PTC < 2–3 | 2 | 126 | −9.28 [−11.47, −7.08] | 56.0% |
According to the protocol, subgroup analyses should be performed according to control (neostigmine or placebo), patient age (infant, child or adolescent), and neuromuscular blocker (rocuronium or vecuronium). However, because of an insufficient amount of data, subgroup analyses were performed according to (1) controls (neostigmine or placebo), (2) sugammadex dose (post-hoc analysis), and (3) time of reverse (post-hoc analysis). The results are listed in Table 3. The results showed that the I2 values were higher than 50% in each subgroup analysis and were higher than 90% in most situations.
Quality of the evidence
Evidence quality of nausea and vomiting was assessed as moderate. Other outcomes were assessed as having a very low or low level of evidence quality, and the results are listed in the Supplementary Table.
Discussion
Our study suggests that, compared with neostigmine or a placebo, sugammadex may reverse rocuronium-induced neuromuscular blockade rapidly in pediatric patients. The included studies demonstrated that sugammadex was well tolerated in the majority of pediatric patients
In this meta-analysis, the authors included all RCTs using sugammadex in pediatrics that met the inclusion criteria, regardless of the publication stage (full-text published or conference abstracts). The inclusion of conference abstracts can have advantages as well as problems. The Cochrane Handbook for Systematic Reviews33 recommends that grey literature (for example, conference abstracts) should be included because a systematic review of data from only published reports may present a misleading picture of an intervention’s efficacy. According to an updated Cochrane methodology review, the exclusion of grey literature may exaggerate the estimates of the intervention’s efficacy by 15% to 38%, depending on the type of grey literature34. Conversely, all of the included conference abstracts24–26, 32 adequately reported the primary and secondary outcomes, which are the time from administration of reversal agents to TOFr > 0.9 and the incidence of adverse events. However, there was inadequate detail on the methodological quality and the adverse events in abstract-only studies. To address this problem, all authors of these studies were contacted and asked for further information about their studies. Unfortunately, only one author replied to our inquiry. Due to the limited details and amount of author feedback, several items from the included studies had an unclear risk of bias.
Regarding primary outcomes, there are some issues that need to be considered before generalizing the results. First, all of the included studies used acceleromyography (AMG) to define the recovery of neuromuscular blockade as TOFr 0.9. However, several methods of quantitative monitoring have been used in clinical studies35. Anesthesiologists should pay attention to the differences between AMG and other methods, such as mechanomyograph (MMG) and electromyography (EMG)35. Second, the reported SDs in the different studies ranged widely, which cannot be explained by the design of the trials. For example, studies conducted by Alvarez-Gomez JA et al.25 and Gaona D et al.26 both examined deep blockades (PTC < 2–3) with rocuronium and compared 4 mg/kg sugammadex to neostigmine + atropine in 2 to 11 year-old patients. However, the SDs in the control groups (10.9 in the study conducted by Alvarez-Gomez JA25 compared to 1.2 in the study conducted by Gaona D26) were quite different. In these studies, unstable tracing during AMG and patient movement may partially account for the unreliable measurements.
The included studies used sugammadex to reverse in moderate (reappearance of T2 or T3) or deep (PTC < 2 or 2–3) of neuromuscular blockade, but RCTs conducted on pediatric patients with shallow (TOFr 0.25 or 0.5) or very deep (just a few minutes after high dosage of neuromuscular blockade agents) neuromuscular blockades were not identified. First, RCTs that compared the use of sugammadex with the use of neostigmine or placebo to reverse shallow neuromuscular blockades (TOFr 0.25 or 0.5) in pediatric patients were not available. Second, eight23, 24, 27–32 of the ten included studies compared sugammadex and neostigmine or placebo in moderate depth neuromuscular blockades (reappearance of T2 or T3). A possible cause for this high ratio is that neostigmine cannot reverse deep neuromuscular blockade and is only recommended for use in moderate depth neuromuscular blockade. Third, the included studies25, 26 also suggested that sugammadex safely and rapidly reversed deep (PTC < 2 or PTC < 2–3) neuromuscular paralysis following rocuronium. Finally, situations including “cannot intubate, cannot ventilate (CICV)” or failed intubation during “rapid sequence induction” are another potential application of sugammadex. Woloszczuk-Gebicka B et al.36 reported a case that CICV happened in a nine-month-old infant, and 8 mg/kg sugammadex was administered after 0.1 mg/kg vecuronium. It was reported that spontaneous breathing returned 25 s after sugammadex administration.
Research has shown that sugammadex is well tolerated in pediatric patients. The incidence of bradycardia in the sugammadex group is lower than that in the placebo or neostigmine group. Additionally, sugammadex, compared with placebo or neostigmine, did not increase the incidence of other AEs in pediatric patients. Unlike neostigmine, sugammadex does not have an anticholinesterase effect and does not require atropine or glycopyrronium; thus, it provides greater cardiovascular stability than neostigmine. On the other side, in experimental research, one study37 showed that clinically relevant sugammadex concentrations may cause apoptotic or neuron death in primary cultures. In an intact brain barrier this will be unlikely, however, if the brain barrier is affected by systemic infection, intracranial bleeding or head trauma, sugammadex may have negative effects on neuronal cells.
In this systematic review, a study conducted by Plaud B23 using eight infant patients provided limited data on sugammadex used in infants. However, several case reports38, 39 and a cohort study40 provided more data on infant patients. Two case reports38, 39 described the use of sugammadex in three neonates following abdominal surgery. In these reports, sugammadex rapidly reversed neuromuscular blockade without any adverse events. Alonso A et al.40 conducted a cohort study with 23 neonates (eight patients were one-day-old). Changes in vital signs were not observed after the administration of sugammadex.
The funnel plot was not perfectly symmetrical. The funnel plot suggested the possible presence of a potential publication bias, a language bias, inflated estimates by a flawed methodological design in smaller studies and/or a lack of publication of small trials with opposite results.
There are some limitations of this study, and the first limitation is its high heterogeneity. The reason for high heterogeneity in the primary outcome data may be related to the fact that volatile anesthetics, such as sevoflurane or isoflurane, can enhance the effect of neuromuscular blockade agents, thus affecting the reversal of rocuronium-induced neuromuscular paralysis. Six studies24, 27–31 used various concentrations of sevoflurane, isoflurane or N2O during operation, whereas three studies25, 26, 32 did not report whether volatile anesthetics were used during operation. Another limitation of this meta-analysis is that most of the included studies24–32 were determined to have unclear or high risk of bias, and the qualities of the primary outcome and most secondary outcomes were assessed as having low risk or very low risk.
In conclusion, compared with neostigmine or placebo, sugammadex may reverse rocuronium-induced neuromuscular blockade rapidly and safely in pediatric patients. Further studies should be conducted to help confirm the efficacy and safety of sugammadex in this special population.
Methods
This study protocol was registered in PROSPERO 2015 and register ID is CRD42015032448. The results of this study were reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Eligibility criteria
Randomized clinical trials were included if they compared sugammadex with either neostigmine or a placebo in pediatric patients who were undergoing surgery involving the use of rocuronium or vecuronium. There were no language or publication date restrictions. Studies comparing reversal with sugammadex at different doses or with placebo were included. However, studies comparing sugammadex and sugammadex combined with neostigmine were excluded. The primary outcome of our study is the time from administration of the reversal agents to TOFr > 0.9. Incidences of any drug-related AEs were analyzed as secondary outcomes.
Search strategy
We searched the MEDLINE (PubMed), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of ScienceTM databases for research that was published prior to Jan 20, 2017 without any language limitations. We also checked the reference lists for reviews and additional studies. In addition, we searched Google Scholar for potentially useful studies. Furthermore, using the System for Information on Grey Literature in Europe (SIGLE) database, grey literature was searched to identify potential RCTs that we could use. The search terms that we used included sugammadex, org 25969, bridion and its Registry Number. Details of our MEDLINE (PUBMED) search strategy are provided in the Supplementary Text that can be found online.
Trial inclusion
Eligibility assessment was performed independently by 2 reviewers (G.L. and R.W.), in an unblended, standardized manner. Disagreements between reviewers were resolved through discussion.
Data extraction
The following data were extracted from every study: first author name; year of publication; study country; sample size; range of participant age and their American Society of Anesthesiologists (ASA) physical status; type of surgery; dose of neuromuscular blockade agents administered; dose of sugammadex and placebo or neostigmine administered; and the occasion for sugammadex and control agents being administered. We also extracted the time from administration of the reversal agents to TOFr > 0.9 and incidences of any drug-related AEs. Two reviewers (R.W. and G.L.) independently extracted all of the data mentioned above. Disagreements were resolved through discussion. To obtain complete outcome data and further details from the studies, we contacted the authors of the included studies via email.
Risk of bias assessment
Using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions33, the two review authors independently assessed the risk of bias for the included trials. If all of the study criteria were assessed as adequate, the study was considered to have a low risk of bias. If one or more of the criteria in a trial were assessed as inadequate, then we considered the study to have a high risk of bias. The other trials were assessed as having an unclear risk of bias.
Statistical analyses
Data on the primary outcome are continuous, and the mean difference is used as a summary statistic with random effects models. The secondary outcomes, drug-related AEs, are binary, and the relative risk is used as a summary statistic. We analyzed the data using the function Metan, Metabias in STATA software version 13 (College Station, TX) with a random-effect model.
Heterogeneity assessment
Statistical heterogeneity was assessed with the I2 statistic, which estimates the percentage of total variance that derives from heterogeneity instead of variance from chance alone. If I2 is greater than or equal to 50% and is statistically significant, then we considered this to be evidence of substantial levels of heterogeneity, although we acknowledged that values of I2 ranging from 30% to 60% might also indicate moderate heterogeneity. When we found substantial levels of heterogeneity, we explored the reasons for the heterogeneity, and a sensitivity analysis was performed to analyze statistical heterogeneity. The standardized mean difference was used as a summary statistic instead of mean difference. Additionally, studies that were considered to have a low risk of bias were analyzed separately from the others.
Publication bias test
To assess publication bias, if at least 10 studies were included, then a funnel plot was used. If not enough studies were included, then an egger test was used to detect publication bias.
Publication bias and sensitivity analysis
If enough studies were included, subgroups analyses were performed according to control (neostigmine or placebo), patient age (infant, child or adolescent), and neuromuscular blocker (rocuronium or vecuronium).
Quality of evidence
We used the principles of the GRADE system41, where appropriate to assess the quality of the primary and secondary outcomes. A summary of findings (SOF) table was constructed to present this assessment using the GRADEpro (available on gradepro.org). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers the study risk of bias (methodological quality), the directness of the evidence, the heterogeneity of the data, the precision of effect estimates and the risk of publication bias.
Electronic supplementary material
Acknowledgements
This work was supported by Beijing 215 high level healthcare talent plan - academic leader 008-0027 and Beijing municipal administration of hospitals’ ascent plan, Code: DFL20150802. G.L. also express his thanks to Qulian Guo (Department of Anesthesiology, Xiangya Hospital of Central South University) and Jixiu Xue (Department of Anesthesiology, XuanWu Hospital Capital Medical University) for giving him assistance in his life.
Author Contributions
G.L. and R.W. conceived and conducted the study. Y.Y., J.X. and T.W. gave the study important advice and help. L.F., G.L. and R.W. analysed the results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Guangyu Liu and Rui Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06159-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth. Analg. 2010;111:120–128. doi: 10.1213/ANE.0b013e3181e33bd9. [DOI] [PubMed] [Google Scholar]
- 2.Bevan DR, Donati F, Kopman AF. Reversal of neuromuscular blockade. Anesthesiology. 1992;77:785–805. doi: 10.1097/00000542-199210000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Baillard C, et al. Postoperative residual neuromuscular block: a survey of management. Br. J. Anaesth. 2005;95:622–626. doi: 10.1093/bja/aei240. [DOI] [PubMed] [Google Scholar]
- 4.Baillard C, et al. Residual curarization in the recovery room after vecuronium. Br. J. Anaesth. 2000;84:394–395. doi: 10.1093/oxfordjournals.bja.a013445. [DOI] [PubMed] [Google Scholar]
- 5.Baxter MR, Bevan JC, Samuel J, Donati F, Bevan DR. Postoperative neuromuscular function in pediatric day-care patients. Anesth. Analg. 1991;72:504–508. doi: 10.1213/00000539-199104000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Booij LHDJ, de Boer HD, van Egmond J. Reversal agents for nondepolarizing neuromuscular blockade: Reasons for and development of a new concept. Semin. Anesth. Perio. M. 2002;21:92–98. doi: 10.1053/sane.2002.34114. [DOI] [Google Scholar]
- 7.Magorian T, Lynam D, Caldwell J, Miller R. Can early administration of neostigmine, in single or repeated doses, alter the course of neuromuscular recovery from a vecuronium-induced neuromuscular blockade? Anesthesiology. 1990;73:410–414. doi: 10.1097/00000542-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Meretoja OA, Gebert R. Postoperative neuromuscular block following atracurium or alcuronium in children. Can. J. Anaesth. 1990;37:743–746. doi: 10.1007/BF03006532. [DOI] [PubMed] [Google Scholar]
- 9.Murphy GS, et al. Residual paralysis at the time of tracheal extubation. Anesth. Analg. 2005;100:1840–1845. doi: 10.1213/01.ANE.0000151159.55655.CB. [DOI] [PubMed] [Google Scholar]
- 10.Adam JM, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J. Med. Chem. 2002;45:1806–1816. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- 11.Zhang MQ. Drug-specific cyclodextrins: the future of rapid neuromuscular block reversal. Drug. Future. 2003;28:347–354. doi: 10.1358/dof.2003.028.04.742715. [DOI] [Google Scholar]
- 12.Sorgenfrei IF, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104:667–674. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Suy K, et al. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106:283–288. doi: 10.1097/00000542-200702000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Vanacker BF, et al. Reversal of rocuronium-induced neuromuscular block with the novel drug sugammadex is equally effective under maintenance anesthesia with propofol or sevoflurane. Anesth. Analg. 2007;104:563–568. doi: 10.1213/01.ane.0000231829.29177.8e. [DOI] [PubMed] [Google Scholar]
- 15.Shields M, et al. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br. J. Anaesth. 2006;96:36–43. doi: 10.1093/bja/aei314. [DOI] [PubMed] [Google Scholar]
- 16.Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth. Analg. 2007;104:555–562. doi: 10.1213/01.ane.0000260135.46070.c3. [DOI] [PubMed] [Google Scholar]
- 17.de Boer HD, et al. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107:239–244. doi: 10.1097/01.anes.0000270722.95764.37. [DOI] [PubMed] [Google Scholar]
- 18.Sparr HJ, et al. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106:935–943. doi: 10.1097/01.anes.0000265152.78943.74. [DOI] [PubMed] [Google Scholar]
- 19.Schaller SJ, Fink H, Ulm K, Blobner M. Sugammadex and Neostigmine Dose-finding Study for Reversal of Shallow Residual Neuromuscular Block. Anesthesiology. 2010;113:1054–1060. doi: 10.1097/ALN.0b013e3181f4182a. [DOI] [PubMed] [Google Scholar]
- 20.Pongracz A, Szatmari S, Nemes R, Fulesdi B, Tassonyi E. Reversal of neuromuscular blockade with sugammadex at the reappearance of four twitches to train-of-four stimulation. Anesthesiology. 2013;119:36–42. doi: 10.1097/ALN.0b013e318297ce95. [DOI] [PubMed] [Google Scholar]
- 21.Kaufhold N, et al. Sugammadex and neostigmine dose-finding study for reversal of residual neuromuscular block at a train-of-four ratio of 0.2 (SUNDRO20) Br. J. Anaesth. 2016;116:233–240. doi: 10.1093/bja/aev437. [DOI] [PubMed] [Google Scholar]
- 22.Fisher DM. Neuromuscular blocking agents in paediatric anaesthesia. Br. J. Anaesth. 1999;83:58–64. doi: 10.1093/bja/83.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Plaud B, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110:284–294. doi: 10.1097/ALN.0b013e318194caaa. [DOI] [PubMed] [Google Scholar]
- 24.Veiga RG, et al. Sugammadex reversal efficacy and security vs neostigmine in the rocuronium‐induced neuromuscular blockade in paediatric patients. Eur. J. Anaesthesiol. 2011;28:153. doi: 10.1097/00003643-201106001-00490. [DOI] [Google Scholar]
- 25.Alvarez-Gomez, J. A. et al. Efficacy and Safety of the Reversal With Sugammadex From Deep Rocuronium-Induced Neuromuscular Blockade in Children, http://www.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2012&index=16&absnum=2981 (2012).
- 26.Gaona D, Carceles MD, Veiga G, Tedesco M, Motta P. Efficacy and safety of the reversal with sugammadex in deep neuromuscular blockade induced by rocuronium in pediatrics. Br. J. Anaesth. 2012;108:308–309. doi: 10.1093/bja/aer372. [DOI] [PubMed] [Google Scholar]
- 27.Kara T, et al. Sugammadex versus neostigmine in pediatric patients: a prospective randomized study. Rev. Bras. Anestesiol. 2014;64:400–405. doi: 10.1016/j.bjan.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Ozgun C, Cakan T, Baltaci B, Basar H. Comparison of reversal and adverse effects of sugammadex and combination of - Anticholinergic-Anticholinesterase agents in pediatric patients. J. Res. Med. Sci. 2014;19:762–768. [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoneim AA, El Beltagy MA. Comparative study between sugammadex and neostigmine in neurosurgical anesthesia in pediatric patients. Saudi J. Anaesth. 2015;9:247–252. doi: 10.4103/1658-354X.154696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El sayed M, Hassan S. Does sugammadex facilitate recovery after outpatient tonsillectomy in children? Egyt. J. Anesth. 2016;32:447–450. [Google Scholar]
- 31.Güzelce D, Kendigelen P, Tütüncü AÇ, Kaya G, Altıntaş F. Comparison of sugammadex and neostigmine in terms of time to extubation in pediatrics. Med. Bull. Haseki. 2016;54:207–211. doi: 10.4274/haseki.3091. [DOI] [Google Scholar]
- 32.Mohamad Zaini RH, Penny Tevaraj JM, Wan Hassan WN, Iberahim MI. & Wan Muhd Shukeri, W. F. Comparison between the efficacy of neostigmine versus sugammadex for reversal of rocuronium induced neuromuscular blockade in paediatric patients. Anesth. Analg. 2016;123:329. doi: 10.1213/01.ane.0000492650.20862.e4. [DOI] [Google Scholar]
- 33.Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0., http://handbook.cochrane.org (2011).
- 34.Hopewell, S., McDonald, S., Clarke, M. & Egger, M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst. Rev. MR000010, doi:10.1002/14651858.MR000010.pub3 (2007). [DOI] [PMC free article] [PubMed]
- 35.Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth. Analg. 2010;111:129–140. doi: 10.1213/ANE.0b013e3181e8b54b. [DOI] [PubMed] [Google Scholar]
- 36.Woloszczuk-Gebicka B, Zawadzka-Glos L, Lenarczyk J, Sitkowska BD, Rzewnicka I. Two cases of the “cannot ventilate, cannot intubate” scenario in children in view of recent recommendations. Anaesthesiol. Intensive Ther. 2014;46:88–91. doi: 10.5603/AIT.2014.0017. [DOI] [PubMed] [Google Scholar]
- 37.Palanca JM, et al. Sugammadex, a neuromuscular blockade reversal agent, causes neuronal apoptosis in primary cultures. Int. J. Med. Sci. 2013;10:1278–1285. doi: 10.7150/ijms.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matinyan NV, Saltanov AI, Mareeva AA. Sugammadex use experience in pediatric oncology. Anesteziol. Reanimatol. 2013;8:34–37. [PubMed] [Google Scholar]
- 39.Martin DP, Crawford J, Uffman J, Michler R, Tobias JD. Sugammadex and fast-track anesthesia for pediatric cardiac surgery in a developing country. Anaesth. Pain Intensive Care. 2016;20:S17–S22. [Google Scholar]
- 40.Alonso A, De Boer HD, Booij L. Reversal of rocuronium-induced neuromuscular block by sugammadex in neonates. Eur. J. Anaesthesiol. 2014;31:163. doi: 10.1097/00003643-201406001-00459. [DOI] [Google Scholar]
- 41.Guyatt GH, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


