Abstract
The effects of prematurity on hippocampal development through early childhood are largely unknown. The aims of this study were to (1) compare the shape of the very preterm (VPT) hippocampus to that of full‐term (FT) children at 7 years of age, and determine if hippocampal shape is associated with memory and learning impairment in VPT children, (2) compare change in shape and volume of the hippocampi from term‐equivalent to 7 years of age between VPT and FT children, and determine if development of the hippocampi over time predicts memory and learning impairment in VPT children. T 1 and T 2 magnetic resonance images were acquired at both term equivalent and 7 years of age in 125 VPT and 25 FT children. Hippocampi were manually segmented and shape was characterized by boundary point distribution models at both time‐points. Memory and learning outcomes were measured at 7 years of age. The VPT group demonstrated less hippocampal infolding than the FT group at 7 years. Hippocampal growth between infancy and 7 years was less in the VPT compared with the FT group, but the change in shape was similar between groups. There was little evidence that the measures of hippocampal development were related to memory and learning impairments in the VPT group. This study suggests that the developmental trajectory of the human hippocampus is altered in VPT children, but this does not predict memory and learning impairment. Further research is required to elucidate the mechanisms for memory and learning difficulties in VPT children. Hum Brain Mapp 35:4129–4139, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: very preterm children, hippocampal size and shape, development, magnetic resonance imaging, neurodevelopmental outcome
INTRODUCTION
The human hippocampal formation undergoes rapid growth and morphological development in the perinatal months, including cytoarchitectural differentiation and infolding of the dentate gyrus and cornu ammonis regions into the medial temporal lobe [Arnold and Trojanowski, 1996; Kier et al., 1997; Okada et al., 2003; Seress, 2001]. One may expect this medial temporal lobe structure to be particularly susceptible to the effects of very preterm birth (VPT; <32 completed weeks of gestational age [GA]) given the degree of hippocampal development in the perinatal period. Infection, hypoxic‐ischemia, lung disease, and stress [Gadian et al., 2000a; Isaacs et al., 2000; Khwaja and Volpe, 2008] are among the complications associated with VPT birth that have been associated with hippocampal pathology [Rees et al., 1999; Volpe, 2000]. Previous studies have reported reduced hippocampal volume in VPT infants [Thompson et al., 2008] and adolescents [Gimenez et al., 2008; Nosarti et al., 2002] and altered hippocampal shape in VPT infants [Thompson et al., 2013] compared with their full‐term (FT) peers. Although cross‐sectional evidence demonstrates compromised hippocampal development as a result of prematurity, there is currently no longitudinal information on hippocampal development in VPT children.
Several cross‐sectional studies have described typical hippocampal growth through childhood. In a post‐mortem study, hippocampal volume increases were reported between 1 and 2 years of age [Kretschmann et al., 1986]. Volumetric magnetic resonance imaging (MRI) studies have demonstrated maximum hippocampal growth to occur between 1 and 2 months of age, with continued rapid growth up to 2 years of age [Knickmeyer et al., 2008; Utsunomiya et al., 1999] and slowed growth up to 14 years [Utsunomiya et al., 1999]. Uematsu et al. reported nonlinear age‐related hippocampal volume changes between 1 month and 25 years, again with most growth occurring during the first few years of life and peaking at 9–11 years of age [Uematsu et al., 2012]. The structural development of the hippocampus may parallel functional development.
Functionally, the hippocampal formation is vitally involved with memory and learning [Cabeza and Nyberg, 2000b; Nadel et al., 2000]. Memory involves the initial registration of information into a temporary storage system, referred to as immediate memory, and the capacity to process this information while in temporary storage is referred to as working memory [Baddeley and Hitch, 1974]. Processes such as articulatory rehearsal [Baddeley, 1996] and attentional refreshment [Barrouillet and Camos, 2001] are used to transfer this material into long‐term memory, where it is stored for later retrieval. VPT children often show memory and learning impairments [Isaacs et al., 2000, 2003; Omizzolo et al., 2013a; Rose et al., 2005; Woodward et al., 2005], and reduced hippocampal volume has been associated with compromised working memory in VPT children at 2 years of age [Beauchamp et al., 2008] and poorer everyday memory in VPT adolescents [Isaacs et al., 2000, 2003]. Left hippocampal volume reduction has also been correlated with reduced verbal recognition memory and learning in preterm teenagers [Gimenez et al., 2004]. While we have previously reported that hippocampal shape in VPT infants is not associated with memory and learning performance at 7 years of age [Thompson et al., 2013], it remains unknown whether hippocampal shape at 7 years, or the change in shape or volume between infancy and 7 years of age relates to memory and learning.
This study aims to: (1) compare the shape of the hippocampus between VPT and full‐term (FT) children at 7 years of age, and determine if hippocampal shape of VPT children is associated with memory and learning impairment at age 7 years, and (2) compare change in (a) shape and (b) volume of the hippocampi from term‐equivalent to 7 years of age between VPT and FT children, and determine if change in the shape or volume of the hippocampus are associated with memory and learning impairment at 7 years in VPT children.
MATERIALS AND METHODS
Participants
Participants were prospectively recruited at birth from the Royal Women's Hospital in Melbourne, Australia from July 2001 to December 2003 as part of the Victorian Infant Brain Studies cohort. The VPT group consisted of 227 infants with either a GA of <30 weeks or a birth weight of <1,250 g. Subjects with congenital abnormalities that would impair neurological function were excluded. A concurrent control group was also recruited from the Royal Women's Hospital, and consisted of 46 FT (37–42 weeks' GA) and normal birth weight (≥2,500 g) infants. All infants underwent an MRI brain scan at term equivalent age (40 weeks ± 2 weeks). A total of 184 VPT and 32 FT infant scans (79% of the original sample) were suitable for hippocampal analysis. Of the remaining subjects, some were unable to be scanned within the term equivalent age range (38–42 weeks' GA; n = 14), and some were excluded due to imaging artefact (n = 43).
At 7 years corrected age, all participants underwent a brain MRI and neuropsychological assessment, with follow‐up rates (from the original sample) of 88% for the VPT group (n = 198) and 93% for the FT group (n = 43). Of these, 145 VPT and 34 FT children had MRI and outcome data at 7 years; the remainder were unable to be scanned (n = 45), or scans were excluded due to image artefacts (n = 17). A total of 125 VPT and 25 FT children had MR images able to be analyzed at both time points (term‐equivalent and 7 years), which is the sample used in the current study.
This research complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki), and the Human Research Ethics Committee of the Royal Women's Hospital and the Royal Children's Hospital granted approval for the study. Written informed consent was obtained from parents.
Imaging
Infant scanning (GA range 38–42 weeks) took place at the Royal Children's Hospital, Melbourne. T 2 / proton density weighted MR images (1.7–3.0 mm coronal slices; repetition time 4,000 ms; echo time 60/160 ms; flip angle 90°; field of view 22 × 16 cm2; matrix 256 × 192, interpolated 512 × 512) were acquired with a 1.5 Tesla General Electric MRI scanner.
MRI scanning was repeated at 7 years of age at the Royal Children's Hospital, Melbourne. Prior to the scan, children underwent a mock MRI scanning session to familiarize each child with the scanning environment and procedure. Scans were conducted without sedation, and T 1 weighted (0.85 mm sagittal slices, flip angle 9°, repetition time 1,900 ms, echo time 2.27 ms, field of view 210 × 210 mm, matrix 256 × 256) structural images were obtained using a 3Tesla Trio Siemens MRI machine (Siemens, Erlangen, Germany).
Hippocampal Segmentation
Hippocampal segmentation was conducted on MRI scans at both term equivalent age and at 7 years of age. Hippocampal formations in the term equivalent data were manually outlined by a single operator (D.K.T) in the coronal view on the combined raw T 2‐ and proton density–weighted image volumes (obtained by volume addition) to increase contrast for optimal visualization of hippocampal boundaries [Thompson et al., 2012, 2008]. Infant hippocampal segmentation was performed with the 3Dslicer 2.5 software (http://slicer.org/), with reference to anatomical atlases [Duvernoy, 1988; Mai et al., 1997]. The tracing scheme has been previously described in detail [Thompson et al., 2012, 2008]. Hippocampal volumes were delineated a second time on 15 randomly chosen images for intra‐rater reliability analyses. Intra‐class correlation coefficients were 0.97 for the right and 0.96 for the left hippocampus.
At 7 years, the hippocampi were manually delineated by operator C.O. The structure was manually outlined in the coronal view of the T 1 scan using ITK‐SNAP 2.2.0. Again, anatomical boundaries generally followed those proposed by Watson et al. and Pruessner et al. [Pruessner et al., 2000; Watson et al., 1992], with reference to an anatomical atlas [Woolsey et al., 2008]. While tracing occurred in the coronal view, reference was made to the sagittal and axial views in order to provide more reliable identification of structural boundaries. The dentate gyrus, four cornu ammonis regions, alveus and the fimbria were included within the hippocampal formation measurement. Boundary definition has been previously described in detail [Omizzolo et al., 2013b]. Repeat segmentations to assess intra‐rater reliability were conducted on 10 subjects. Intra‐class correlation coefficients were 0.97 for the right and 0.96 for the left hippocampal formations.
Because of the longitudinal nature of this study, all 7 year hippocampal segmentations were reviewed and edited by D.K.T to match boundary definitions used at term equivalent. This required deleting several slices of the hippocampal tail in 7‐year segmentations when they extended posterior to the point where the crus of the fornix fused with the pulvinar nucleus. This was necessary because this section of the tail was not included in infant segmentations, as it was not able to be consistently delineated. Inter‐rater reliability was carried out between operators D.K.T and C.O on 10 subjects, and intra‐class correlation coefficients were 0.96 for the right and 0.97 for the left hippocampus.
Hippocampal Shape Analysis
Morphological analysis of the delineated neonatal hippocampal formations was conducted using the spherical harmonics‐point distribution model (SPHARM‐PDM) [Shi et al., 2007; Styner et al., 2003]. This model characterizes both global and local shape [Gerig et al., 2001; Styner et al., 2006]. This technique has been previously described in detail [Thompson et al., 2013]. In brief, both infant and 7 year hippocampal masks were resampled to isotropic resolution and minimally smoothed. Boundaries were converted to triangular surface meshes [Lorensen and Cline, 1987] which were deformed to spheres [Brechbuehler et al., 1995], thereby creating spherical parameterizations. Smoothed PDM shape representations of segmentation boundaries were subsequently registered by Procrustes alignment [Styner et al., 2006].
For the longitudinal morphometric analysis, the 7 year hippocampal surfaces were aligned to the infant surfaces by Procrustes alignment, including a scaling component in order for the 7‐year‐old hippocampi to match the infant hippocampi. Vectors and magnitudes of the difference between infant and 7 year surfaces were created by subtracting the infant meshes from the 7 year hippocampal meshes.
Neonatal Brain Abnormality Score
At term‐equivalent age, data were collected on brain abnormality. Cerebral abnormality was scored by a neonatal neurologist on infant T 1 and T 2 structural scans using a previously described system [Kidokoro et al., 2013]. Presence and severity of white matter abnormality (cystic lesions, signal abnormality, myelination delay, callosal thinning, lateral ventricular volume, white matter volume), cortical grey matter abnormality (extracerebral space, signal abnormality, gyral maturation), deep grey matter abnormality (signal abnormality, deep grey matter volume), and cerebellar abnormality (signal abnormality, cerebellar volume) was rated and combined to give an overall abnormality score from 0 to 17.
Neuropsychological Assessment
At 7 years of age, corrected for prematurity, tests from widely used memory and learning test batteries were administered. Full‐scale intelligence quotient (IQ) was estimated using the four‐subset version of the Wechsler abbreviated scale of intelligence (WASI) [Wechsler, 1999].
Three subsets from the working memory test battery for children (WMTB‐C) [Pickering and Gathercole, 2001] were administered to assess immediate and working memory: (1) Forward digit recall which assesses verbal immediate memory; (2) Backward digit recall which assesses verbal working memory; and (3) Block recall which assesses spatial immediate memory. A total score for each subtest reflects the number of trials completed correctly.
The California verbal learning test (CVLT)—Children's Version [Delis et al., 1994] was used to assess verbal memory and learning, and involves the presentation of a list of 15 words over five trials. Variables of interest included verbal learning (total number of words recalled over five trials) and long delay recall. The dot locations test from the children's memory scale (CMS) [Cohen, 1997] was used to assess visual‐spatial memory and learning, and involves learning the spatial location of dots over three trials. Variables of interest were visual‐spatial learning (total number of correct locations recalled over three trials) and long delay recall.
Due to the restricted age range of the children (6.6–8.1 years), raw rather than standardized data were used for analyses. Scores were reported as “missing” when children were too impaired to complete tasks, refused to participate in tasks, or there was a problem with the testing equipment (e.g., missing components of a task). Impairment was defined as performance <1 standard deviation (SD) below the FT group mean for all memory and learning tasks.
Statistical Analyses
For group‐wise morphometric comparisons at 7 years of age, group‐specific PDMs were generated. For each vertex, the signed distance from the mean surface for each PDM was evaluated for evidence of differences (defined as P < 0.05, Bonferroni corrected), indicating areas of local expansion or contraction. Univariate Hotelling T 2 statistical testing was used on the corresponding boundary points across all subjects, with permutation testing to correct for multiple comparisons. Intracranial volume was included in the analyses as a scaling factor, to correct for the effect of head size. The analysis was repeated including neonatal brain abnormality score as a covariate. The associations between hippocampal shape at 7 years and memory and learning outcomes were assessed by comparing hippocampal shape at 7 years between VPT children with and without memory and learning impairments using univariate Hotelling T 2 tests. This analysis was repeated covarying for neonatal brain abnormality score using multivariate analysis of covariance (ANCOVA).
To determine longitudinal morphometric change in the hippocampus between term‐equivalent and 7 years of age, t tests were used to determine whether the signed distances were different from zero for each vertex separately for the VPT and FT groups. To compare the morphological change over time between VPT and FT children, ANCOVA was carried out on the morphometric change from term to age 7 years. The association between change in hippocampal shape and memory and learning outcomes was assessed by comparing the change in hippocampus shape from term to 7 years between impaired and non‐impaired VPT children using ANCOVA. This analysis was repeated covarying for neonatal brain abnormality score using multivariate ANCOVA. All morphometric analyses were corrected for multiple comparisons using Bonferroni correction.
The change in hippocampal volume from birth to 7 years in the VPT and FT groups was compared using Stata 12. A single mixed regression model was fitted to the left and right hippocampi measurements at term and 7 years, including time as a covariate and using a random effect to allow for the correlations between observations within an individual. Group differences (VPT vs. FT) in change in hippocampal volume were assessed by allowing the effect of time to vary by group (group‐by‐time interaction), and allowing the effect of time and group to vary by hemisphere (three‐way interaction). Secondary analyses adjusted for change in intracranial volume and neonatal brain abnormality score. Finally, the association between change in hippocampal volume and memory and learning impairment at age 7 years in the VPT group was assessed using separate logistic regression models for each hippocampal volume (left and right) and outcome combination. Secondary analyses adjusted for change in intracranial volume and neonatal brain abnormality score.
RESULTS
Sample Characteristics
Characteristics of the VPT and FT cohorts are described in Table 1. As expected GA at birth (P < 0.001) and birth weight (P < 0.001) were lower in the VPT subjects, while brain abnormality score (P < 0.001), and the incidence of multiple births (P < 0.001), bronchopulmonary dysplasia (P = 0.002), and antenatal corticosteroids (P < 0.001) were higher in VPT subjects compared with FT subjects. The VPT sample had lower IQ (P < 0.001) and higher rates of memory and learning impairment compared with the FT group at 7 years of age, with strong evidence of group differences for the verbal working memory (P = 0.002) and immediate spatial memory (P = 0.004) domains.
Table 1.
Perinatal and 7 year characteristics of the very preterm and full‐term cohorts
| Very preterm (n = 125) | Full‐term (n = 25) | |
|---|---|---|
| Perinatal characteristics | ||
| GA (weeks), M (SD) | 27.5 (1.9) | 38.7 (1.3) |
| Birth weight (g), M (SD) | 968 (223) | 3255 (517) |
| Small for gestational age, n (%) | 13 (10) | 1 (4) |
| Singleton, n (%) | 68 (54) | 24 (96) |
| Intracranial volume (cc), M (SD) | 447.3 (72.7) | 456.3 (54.6) |
| Neonatal brain abnormality score, M (SD) | 5.3 (3.1) | 1.5 (1.3) |
| Male sex, n (%) | 62 (50) | 14 (56) |
| Antenatal corticosteroids, n (%) | 108 (86) | 0 (0) |
| Postnatal corticosteroidsa, n (%) | 7 (6) | 0 (0) |
| Bronchopulmonary dysplasia, n (%) | 37 (30) | 0 (0) |
| Cystic periventricular leukomalacia, n (%) | 5 (4) | 0 (0) |
| Intraventricular haemorhage grades 3/4, n (%) | 5 (4) | 0 (0) |
| 7 year characteristics | ||
| Age at assessment (years), M (SD) | 7.5 (0.2) | 7.6 (0.2) |
| Intracranial volume (cc), M (SD) | 1332.6 (119.7) | 1435.3 (125.7) |
| Full scale IQ, M (SD) | 98.1 (13.4) | 108.9 (11.9) |
| Impaired verbal immediate memorya, n (%) | 29 (23) | 2 (8) |
| Impaired verbal working memoryb, n (%) | 60 (48) | 4 (16) |
| Impaired spatial immediate memoryc, n (%) | 52 (42) | 3 (12) |
| Impaired verbal learning, n (%) | 24 (19) | 5 (20) |
| Impaired verbal long delay recalla, n (%) | 32 (26) | 3 (12) |
| Impaired spatial learninga, n (%) | 50 (40) | 6 (24) |
| Impaired spatial long delay recalld, n (%) | 31 (25) | 3 (12) |
GA = gestational age, M = mean, SD = standard deviation. Impairment defined as <1 SD from mean of full‐term children.
Missing one subject.
Missing nine subjects.
Missing three subjects.
Missing two subjects.
Hippocampal Shape at 7 years of Age
At 7 years of age VPT children's hippocampi were more outwardly displaced (greater expansion) in the anterior–posterior direction and less inwardly displaced (more contracted) along the medial border (Fig. 1a) than FT children. There were several scattered regions where there was strong statistical evidence for group‐wise shape differences, particularly for the right side, which mainly corresponded to areas of expansion in the VPT children (Fig. 1b). The pattern of shape differentiation was explained by greater infolding, or “curling up” of the FT hippocampus, while the VPT hippocampus remained straighter (Fig. 1c). Most regions where there was statistical evidence of expansion in the VPT hippocampus remained after adjusting for neonatal brain abnormality score (Fig. 1d).
Figure 1.
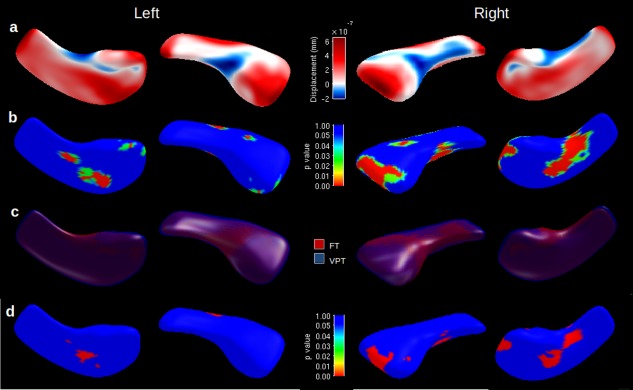
Shape differences for very preterm (VPT) vs. full‐term (FT) children at 7 years, displayed for the right and left hippocampi. (a) Displacement map with areas of positive expansion (red) or negative contraction (blue) of the VPT hippocampal surface from the mean surface, overlaid on mean of all hippocampi. (b) Statistical P value map of shape difference after permutation testing overlaid on mean of all hippocampi. (c) Mean overlay of VPT (blue) and FT (red) hippocampi. (d) Bonferroni corrected statistical maps of shape differences for VPT vs. FT children's hippocampi after correcting for brain abnormality score. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
There was little evidence of an association between hippocampal shape and impairment on memory and learning measures in VPT 7‐year olds (data not shown).
Longitudinal Hippocampal Shape
Between infancy and 7 years of age, the morphological change in the hippocampus was similar for VPT and FT children. In general, both VPT and FT children's hippocampi “curled up” more by 7 years of age. There were several areas of expansion from infancy to 7 years into the inner medial border of the hippocampus, and a corresponding contraction along the lateral border in both groups (Fig. 2a). There was evidence of lateral contraction between time points in all major zones, as well as zones of medial expansion in the VPT population (Fig. 2b). The overlay of the 7 year hippocampus onto the infant hippocampus further demonstrated hippocampal infolding between time‐points for both groups (Fig. 2c). There were some regions where the medial expansion and lateral contraction between time‐points appeared greater in VPT children (Fig. 2d). There were only small portions of the hippocampi where there was statistical evidence that the longitudinal shape change differed between VPT and FT children. These regions mainly corresponded to greater medial expansion and lateral contraction of the VPT hippocampal surface, particularly for the right side (Fig. 2e), and remained after adjusting for neonatal brain abnormality score (data not shown).
Figure 2.
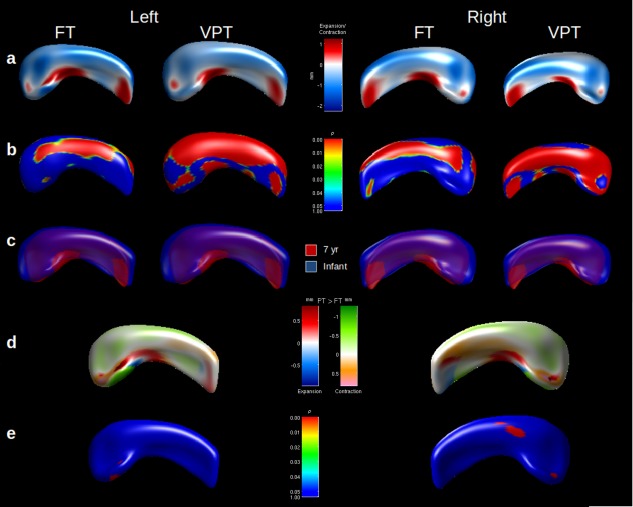
Shape differences between infancy and 7 years for full‐term (FT) and very preterm (VPT) children. (a) Displacement map with areas of expansion (red) and contraction (blue), overlaid on mean of all hippocampi. (b) Statistical P value map of shape difference between time‐points, after Bonferroni correction. (c) Mean overlay of infant (blue) and 7 year (red) hippocampi, where the 7 year surface is scaled to match the infant surface. (d) Displacement map with areas of expansion (red) or contraction (green) greater in VPT children, or expansion (blue) or contraction (orange) greater in FT children, between term and 7 years. (e) Statistical P value map demonstrating the evidence for differences in longitudinal shape change between FT and VPT children, after Bonferroni correction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
There was little evidence that change in hippocampal shape between infancy and 7 years in VPT children was associated with impairment on memory and learning measures (data not shown).
Longitudinal Hippocampal Volume
The average change in hippocampal volume from the neonatal period to 7 years of age was smaller for VPT children than for FT children (Table 2, interaction: −0.22, 95% CI −0.29 to −0.14, P < 0.001). This difference remained following adjustment for change in intracranial volume and neonatal brain abnormality score (interaction: −0.14, 95% CI −0.21 to −0.07, P < 0.001). Overall the change in volume was greater for the right hippocampus compared with the left hippocampus (main effect P = 0.001), however there was little evidence that the effect of group varied by hemisphere (interaction P = 0.82).
Table 2.
Left and right hippocampal volume (cc) at term and 7 years, and change in volume from term to 7 years for very preterm and full‐term childrena
| Hippocampal volume, | Very preterm | Full‐term | ||
|---|---|---|---|---|
| mean (95% CI) | Right | Left | Right | Left |
| Neonatal | 1.14 (1.10) | 1.12 (1.08, 1.17) | 1.21 (1.11, 1.31) | 1.18 (1.08, 1.28) |
| 7‐year | 2.85 (2.80, 2.89) | 2.74 (2.70, 2.77) | 3.15 (3.05, 3.25) | 3.00 (2.90, 3.10) |
| Change | 1.71 (1.67, 1.75) | 1.62 (1.58, 1.66) | 1.97 (1.88, 2.06) | 1.82 (1.73, 1.91) |
Results from a single model including measurements from the left and right hemisphere at both time points, allowing the effect of time and hemisphere to be different in the two groups, and allowing for a three way interaction between group, hemisphere and time.
In the VPT group, there was little evidence for a relationship between change in hippocampal volumes between infancy and 7 years and memory and learning impairment, on both unadjusted analyses (Fig. 3) or analyses adjusted for intracranial volume and neonatal brain abnormality (data not shown).
Figure 3.
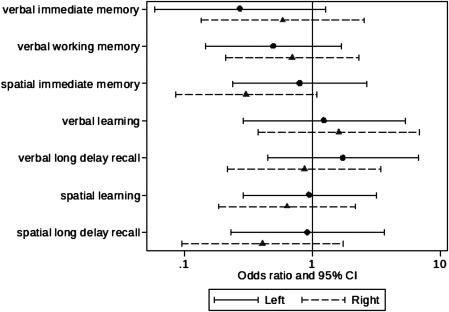
Association between change in hippocampal volume and memory and learning impairment at age 7 years. Results are the odds ratios and 95% confidence intervals (CI) for an impairment per unit difference in the change in hippocampal volume from separate unadjusted logistic regression models.
DISCUSSION
Despite VPT hippocampi showing more immature shape at 7 years than FT hippocampi (less infolded), the morphological change over time was similar between groups, indicating that shape differences present in infancy [Thompson et al., 2013] persisted but the trajectory was relatively typical thereafter in VPT children. Volumetrically, FT children's hippocampi grew more between infancy and 7 years of age than VPT children, with more growth in the right than the left hippocampus for both groups. There was little evidence that hippocampal shape at age 7 or either volumetric or morphological changes from term to 7 years were associated with memory and learning impairments in the VPT group at 7 years.
At 7 years, VPT hippocampi were less infolded than those of FT children. We have previously shown that this cohort of VPT subjects displayed straighter hippocampi than FT infants at term‐equivalent age [Thompson et al., 2013]. This suggests that the alterations in hippocampal shape that occurred in the perinatal period persist into childhood, and therefore hippocampal development did not catch up. Altered hippocampal shape in the VPT child may result from exposures and injury shortly after birth, a period in which the hippocampus undergoes rapid synaptic, dendritic, and oligodendroglial development [Insausti et al., 2010], and is actively establishing cortical‐hippocampal connections [Hevner and Kinney, 1996]. The VPT infant hippocampus is vulnerable to a range of complications following birth which may alter the normal progression of these developmental processes such as neuronal or white matter damage [Ramenghi et al., 2007], which may result in alterations of hippocampal shape [Qiu et al., 2010].
Shape changes in the hippocampus from term to 7 years were similar for the VPT and FT children with both showing more infolding at 7 years. Considering shape alterations are present at term equivalent age in VPT infants [Thompson et al., 2013], the period within the neonatal intensive care unit is likely the period of greatest vulnerability for the hippocampus. It should be noted that this study is the first to show that further hippocampal infolding occurs during childhood, even in healthy FT populations. Previously, hippocampal inversion was thought to be complete by around 25 weeks of gestation [Bajic et al., 2010].
Hippocampal growth is compromised in the neonatal period following VPT birth [Thompson et al., 2008]. Findings from the current study showed that hippocampal growth between term‐equivalent and 7 years of age was slower in VPT children, even after taking into account change in overall brain size. This indicates that the hippocampus continues to be specifically vulnerable in VPT children well after infancy, and into childhood. A major hippocampal growth spurt occurs between infancy and 2 years of age [Utsunomiya et al., 1999], which may be altered in those born VPT. This may explain why, in the same cohort, we found a 3.4% lower hippocampal volume in VPT compared with FT subjects in infancy [Thompson et al., 2008], which had increased to a 6.3% difference by 7 years of age [Omizzolo et al., 2013b]. Furthermore, others have reported even larger differences of around 15% between VPT adolescents and FT controls [Gimenez et al., 2004; Nosarti et al., 2002]. Together these results suggest that the VPT hippocampal volumes do not catch up with those of term‐born peers, and indeed the gap may widen. Altered hippocampal growth in VPT children is likely due to the susceptibility of the hippocampus to complications associated with VPT birth [Gadian et al., 2000b; Khwaja and Volpe, 2008], in particular white matter injury and the subsequent associated neurological sequelae [Volpe, 2009]. However, the slower growth of the hippocampi in VPT children appears to be independent of brain injury, as VPT children still had a slower hippocampal growth rate after adjusting for the neonatal brain abnormality score. Disturbingly, the full impact of prematurity on hippocampal growth may not be apparent until around 9–11 years of age, when hippocampal growth peaks [Uematsu et al., 2012]. On the other hand, our results suggest that there may be a window of time early in development where it may be possible to intervene in order to improve hippocampal growth in VPT populations.
Though hippocampal asymmetry develops in utero [Thompson et al., 2009], we confirmed that asymmetrical development of the hippocampi continues into childhood. The greater growth of the right compared with the left hippocampus during childhood seen in this study is consistent with previous research reporting rightward asymmetry in children [Giedd et al., 1996; Pfluger et al., 1999; Utsunomiya et al., 1999] and adults [Uematsu et al., 2012; Watson et al., 1992]. Given there was little evidence of group by hemisphere interactions, it would appear that VPT children showed similar growth in each hemisphere during childhood as FT children, despite the fact that they have altered hippocampal asymmetry in infancy [Thompson et al., 2009].
Within VPT children, there was little evidence that hippocampal shape at age 7 years, or hippocampal growth from term to 7 years (volumetric or morphological change) were associated with memory and learning impairment at 7 years of age. We conducted additional analyses where we related hippocampal measurements to continuous memory and learning scores, rather than dichotomous impairment variables, which also failed to find evidence of any such associations (data not shown). We were surprised that our hippocampal growth measures were not related to functional impairments in these domains, especially considering we have shown positive associations between infant hippocampal volume and memory functioning at 7 years [Thompson et al., 2013]. However the current results are consistent with our previous findings that hippocampal volume at 7 years is not related to memory and learning performance [Omizzolo et al., 2013b]. In contrast, previous research in populations with memory and learning difficulties has demonstrated an association between hippocampal volume and performance on the CVLT [Riggins et al., 2012; Willoughby et al., 2008]. It could be that the measures of memory and learning employed in this study were not sensitive to subtle volumetric and morphological changes in the VPT hippocampus. An alternative explanation is that memory and learning impairment in the VPT group reflects pathology in other components of the neural memory network, which includes but is not limited to prefrontal and parietal regions [Cabeza and Nyberg, 2000a], as well as the thalamus and basal ganglia [Omizzolo et al., 2013a]. It is commonly accepted that memory and learning functions continue to mature well after 7 years of age [Gathercole, 1998; Gathercole et al., 2004], and given that the hippocampus continues to mature into adolescence [Insausti et al., 2010], the full effects of impaired hippocampal growth and development on memory and learning function may not be apparent until later in development. Although no previous study has examined the relationship between longitudinal hippocampal development and memory and learning in VPT cohorts, a few studies have linked reduced hippocampal volume to everyday memory impairment [Isaacs et al., 2000, 2003] and verbal learning and recognition memory impairment [Gimenez et al., 2004] during adolescence.
Despite the fact that the current study assessed multiple components of memory and learning in both visual and verbal modalities, it is possible that altered hippocampal development in this VPT group would have been related to performance on other memory and learning measures such as those assessing paired associative learning and everyday memory functioning. Furthermore, the hippocampus may be involved in other cognitive and behavioral processes altered in VPT children, such as affective regulation and both full‐scale [Lodygensky et al., 2005] and performance IQ [Isaacs et al., 2004], which were not explored in the current study. Another limitation to this study is that manual segmentation is prone to human error, and there are many different protocols for hippocampal segmentation reported in the literature. The current study employed a common method of hippocampal segmentation based on the protocol put forward by Watson et al. [1992], and both intra‐ and inter‐rater reliability were high. Furthermore, the morphological analysis assumes perfect registration of all hippocampi into a common reference space. As this is not always possible, the accuracy of our results may be affected by alignment error. All alignments were qualitatively assessed for accuracy to minimize this risk.
In conclusion, hippocampal shape is less mature in VPT children at 7 years of age than in FT children, which corresponds with similar morphological differences observed at term equivalent age. The findings from the current study provide unique insight into the developmental trajectory of the human hippocampus from the neonatal period to 7 years of age in children born VPT and at term. These findings are the first to show that the hippocampus undergoes further infolding between infancy and 7 years of age in both VPT and FT children. We also show that the VPT hippocampus does not undergo typical volumetric development throughout childhood, with delayed growth between infancy and 7 years. The relationship between delayed hippocampal growth as a result of VPT birth and cognitive deficits still remains unclear. Further research is required to examine this association later in development, ideally during adolescence. Future studies may wish to examine whether early intervention can close the gap between VPT and FT hippocampal development, which will likely improve later cognitive functioning.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Marc Seal, Richard Beare, and Jian Chen for helpful discussions on data analysis and interpretation. They thank Leona Pascoe, Natalie Reidy, and Shannon Scratch for performing the memory and learning tests. They acknowledge the support of the Victorian Infant Brain Studies and Developmental Imaging teams at the Murdoch Childrens Research Institute and Royal Children's Hospital, University of Melbourne, Victoria. They also acknowledge the support of the Victorian Government's Operational Infrastructure Support Program. They also thank the families and children who participated in this study, as well as Michael Kean and the radiographers at the Royal Children's Hospital for acquiring the MRI scans. The authors have no conflicts of interest to declare.
REFERENCES
- Arnold SE, Trojanowski JQ (1996): Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J Comp Neurol 367:274–292. [DOI] [PubMed] [Google Scholar]
- Baddeley A (1996): The fractionation of working memory. Proc Natl Acad Sci USA 93:13468–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ (1974): Working memory In: Bower GH, editor. The Psychology of Learning and Motivation. New York: Academic Press; pp 47–89. [Google Scholar]
- Bajic D, Ewald U, Raininko R (2010): Hippocampal development at gestation weeks 23 to 36. An ultrasound study on preterm neonates. Neuroradiology 52:489–494. [DOI] [PubMed] [Google Scholar]
- Barrouillet P, Camos V (2001): Developmental increase in working memory span: Resource sharing or temporal decay? J Mem Lang 45:1–20. [Google Scholar]
- Brechbuehler C, Gerig G, Kubler O (1995): Parametrization of closed surfaces for 3‐D shape description. Comp Vis Image Understanding 61:154–170. [Google Scholar]
- Cabeza R, Nyberg L (2000a): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000b): Neural bases of learning and memory: Functional neuroimaging evidence. Curr Opin Neurol 13:415–421. [DOI] [PubMed] [Google Scholar]
- Cohen M (1997): Children's Memory Scale. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA (1994): California Verbal Learning Test—Children's Version. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Duvernoy HM (1988): The Human Hippocampus. An Atlas of Applied Anatomy. Munchen: J.F Bergmann Verlag. 166 p. [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha‐Khadem F (2000a): Developmental amnesia associated with early hypoxic‐ischaemic injury. Brain 123 (Part 3):499–507. [DOI] [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha‐Khadem F (2000b): Developmental amnesia associated with early hypoxic–ischaemic injury. Brain 123:499–507. [DOI] [PubMed] [Google Scholar]
- Gathercole SE (1998): The development of memory. J Child Psychol Psychiatry 39:3–27. [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H (2004): The structure of working memory from 4 to 15 years of age. Dev Psychol 40:177–190. [DOI] [PubMed] [Google Scholar]
- Gerig G, Styner M, Jones D, Weinberger D, Lieberman J (2001): Shape analysis of brain ventricles using SPHARM. Kauai, HI: Proc IEEE Workshop on Math Methods Biomed Image Anal. pp 171–178. [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. (1996): Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex 6:551–560. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado‐Pineda P, Bargallo N, Segarra D, Botet F (2004): Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage 23:869–877. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Soria‐Pastor S, Junque C, Caldu X, Narberhaus A, Botet F, Bargallo N, Falcon C, Mercader JM (2008): Proton magnetic resonance spectroscopy reveals medial temporal metabolic abnormalities in adolescents with history of preterm birth. Pediatr Res 64:572–577. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Kinney HC (1996): Reciprocal entorhinal‐hippocampal connections established by human fetal midgestation. J Comp Neurol 372:384–394. [DOI] [PubMed] [Google Scholar]
- Insausti R, Cebada‐Sanchez S, Marcos P (2010): Postnatal Development of the Human Hippocampal Formation. Heidelberg: Springer. [PubMed] [Google Scholar]
- Isaacs EB, Edmonds CJ, Chong WK, Lucas A, Morley R, Gadian DG (2004): Brain morphometry and IQ measurements in preterm children. Brain 127 (Part 12):2595–2607. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha‐Khadem F, Gadian DG (2000): Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res 47:713–720. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Vargha‐Khadem F, Watkins KE, Lucas A, Mishkin M, Gadian DG (2003): Developmental amnesia and its relationship to degree of hippocampal atrophy. Proc Natl Acad Sci USA 100:13060–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ (2008): Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93:F153–F161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro H, Neil JJ, Inder TE (2013): New MR Imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol 34:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Kim JH, Fulbright RK, Bronen RA (1997): Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. Am J Neuroradiol 18:525–532. [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH (2008): A structural MRI study of human brain development from birth to 2 years. J Neurosci 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F (1986): Growth of the hippocampal formation in man. Bibl Anat 28:27–52. [PubMed] [Google Scholar]
- Lodygensky GA, Rademaker K, Zimine S, Gex‐Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS (2005): Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116:1–7. [DOI] [PubMed] [Google Scholar]
- Lorensen WE, Cline HE (1987): Marching cubes: A high resolution 3D surface construction algorithm. Comp Graph 21:163–169. [Google Scholar]
- Mai JK, Assheuer J, Paxinos G (1997): Atlas of the Human Brain. San Diego: Academic Press. [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M (2000): Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus 10:352–368. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Al‐Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM (2002): Adolescents who were born very preterm have decreased brain volumes. Brain 125 (Part 7):1616–1623. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kato T, Iwai K, Iwasaki N, Ohto T, Matsui A (2003): Evaluation of hippocampal infolding using magnetic resonance imaging. Neuroreport 14:1405–1409. [DOI] [PubMed] [Google Scholar]
- Omizzolo C, Scratch SE, Stargatt R, Kidokoro H, Thompson DK, Lee KJ, Cheong J, Neil J, Inder TE, Doyle LW, et al. (2013a): Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory. DOI: 10.1080/09658211.2013.809765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omizzolo C, Thompson DK, Scratch SE, Stargatt R, Lee KJ, Cheong J, Roberts G, Doyle LW, Anderson PJ (2013b): Hippocampal volume and memory and learning outcomes at 7 years in children born very preterm. J Int Neuropsychol Soc 19:1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, Scheck R, Hahn K (1999): Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia 40:414–423. [DOI] [PubMed] [Google Scholar]
- Pickering S, Gathercole S (2001): Working Memory Test Battery for Children—Manual. London: The Psychological Corporation. [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC (2000): Volumetry of hippocampus and amygdala with high‐resolution MRI and three‐dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex 10:433–442. [DOI] [PubMed] [Google Scholar]
- Qiu A, Tuan TA, Woon PS, Abdul‐Rahman MF, Graham S, Sim K (2010): Hippocampal‐cortical structural connectivity disruptions in schizophrenia: An integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage 52:1181–1189. [DOI] [PubMed] [Google Scholar]
- Ramenghi LA, Fumagalli M, Righini A, Bassi L, Groppo M, Parazzini C, Bianchini E, Triulzi F, Mosca F (2007): Magnetic resonance imaging assessment of brain maturation in preterm neonates with punctate white matter lesions. Neuroradiology 49:161–167. [DOI] [PubMed] [Google Scholar]
- Rees S, Breen S, Loeliger M, McCrabb G, Harding R (1999): Hypoxemia near mid‐gestation has long‐term effects on fetal brain development. J Neuropathol Exp Neurol 58:932–945. [DOI] [PubMed] [Google Scholar]
- Riggins T, Cacic K, Buckingham‐Howes S, Scaletti LA, Salmeron BJ, Black MM (2012): Memory ability and hippocampal volume in adolescents with prenatal drug exposure. Neurotoxicol Teratol 34:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ (2005): Recall memory in the first three years of life: A longitudinal study of preterm and term children. Dev Med Child Neurol 47:653–659. [DOI] [PubMed] [Google Scholar]
- Seress L (2001): Morphological changes of the human hippocampal formation from midgestation to early childhood In: Nelson C, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: Massachusetts Institute of Technology Press; pp 45–58. [Google Scholar]
- Shi Y, Thompson PM, de Zubicaray GI, Rose SE, Tu Z, Dinov I, Toga AW (2007): Direct mapping of hippocampal surfaces with intrinsic shape context. Neuroimage 37:792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Gerig G, Lieberman J, Jones D, Weinberger D (2003): Statistical shape analysis of neuroanatomical structures based on medial models. Med Image Anal 7:207–220. [DOI] [PubMed] [Google Scholar]
- Styner M, Oguz I, Xu S, Brechbuehler C, Pantazis D, Levitt JJ, Shenton ME, Gerig G (2006): Framework for the statistical shape analysis of brain structures using SPHARM‐PDM. The Insight Journal. Available at: http://hdl.handle.net/1926/215 (2006 MICCAI Open Science Workshop). [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Adamson C, Roberts G, Faggian N, Wood SJ, Warfield SK, Doyle LW, Anderson PJ, Egan GF, Inder TE (2013): Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: Perinatal predictors and functional significance at age 7. NeuroImage 70:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Ahmadzai ZM, Wood SJ, Inder TE, Warfield SK, Doyle LW, Egan GF (2012): Optimizing hippocampal segmentation in infants utilizing MRI post‐acquisition processing. Neuroinformatics 10:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, Egan GF, Inder TE (2008): Neonate hippocampal volumes: Prematurity, perinatal predictors, and 2‐year outcome. Ann Neurol 63:642–651. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Egan GF, Inder TE (2009): MR‐determined hippocampal asymmetry in full‐term and preterm neonates. Hippocampus 19:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012): Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 7:e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A (1999): Development of the temporal lobe in infants and children: Analysis by MR‐based volumetry. Am J Neuroradiol 20:717–723. [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2000. Neurology of the Newborn. London: W.B. Sanders. [Google Scholar]
- Volpe JJ (2009): Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jonesgotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G (1992): Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic‐resonance‐imaging. Neurology 42:1743–1750. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999): Wechsler abbreviated scale of intelligence (WASI). New York, NY: The psychological corporation. [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J (2008): Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14:1022–1033. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE (2005): Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain 128:2578–2587. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Hanaway J, Gado MH (2008): The Brain Atlas: A Visual Guide to the Human Central Nervous System Hoboken, NJ: Wiley. [Google Scholar]


