INTRODUCTION
Each year in the United States there are at least 1.7 million people who sustain a Traumatic Brain Injury (TBI). Over 1.4 million of these are treated and released from emergency departments across the country.1 Research in the field of TBI has long been dominated by research on severe brain injuries. However, over 90% of all TBI’s are considered either “mild” (GCS 13–15) or “moderate” (GCS 9–12) and far outnumber severe injuries.2–4 Mild and moderate TBI are often difficult to assess and distinguish clinically during the first hours after injury because neurological examinations are of restricted value. Traumatic brain injury is a leading cause of combat casualty. An estimated 15–25% of all injuries sustained in 20th century conflicts are to the head.5–7 Tools to diagnose and triage brain injury victims would be useful in both civilian and military settings. Accurate diagnosis in acute care environments is critical to patient outcome. Such decisions include performing Computed Tomography (CT) scans of the brain, seeking neurosurgical consultation, admitting or transferring to a higher level of care, returning to play or duty, and averting the consequences of “second impact syndrome,”8, 9 when repeated concussions in a short period become potentially debilitating or fatal.
According to recent estimates, 62 million CT scans are performed annually in the US10 and this has raised concern over unnecessary exposure to ionizing radiation.11–14 In the United States, the high rate of ordering CT scans for mild TBI (also known as concussion) is fostered by the nature of ED practice: high case volumes, brief physician-patient contact, uncertain follow-up, and fear of medicolegal repercussions.15 Yet, emergency departments with a high ordering rate of head CT scans can still miss intracranial injuries.16, 17 In a study by Stiell et al. 5% of “missed” hematomas occurred at the institutions with the highest and third highest rates of CT use.16 Moreover, CT may not demonstrate subtle lesions or diffuse injury acutely.18–20
There are a number of organ-based diseases that use rapid serum-based biomarkers to guide diagnosis and treatment but no such rapid diagnostic markers exist for TBI. A number of biomarkers have been investigated for TBI.21, 22 The most extensively studied among these include glial protein S-100 beta(β), neuron-specific enolase (NSE), and myelin basic protein (MBP). Although some of these published studies suggest that these biomarkers correlate with degree of injury; conflicting results exist.23–28 Ubiquitin C-terminal hydrolase (UCH-L1) was previously used as a histological marker for neurons due to its high abundance and specific expression in neurons.29 This study follows the bench to bedside approach to translational research in TBI biomarkers. Previously, UCH-L1 was identified as a protein with a two-fold increase in abundance in the injured cortex 48 hours after controlled cortical impact in a rat model of TBI.30 Subsequently, a UCH-L1 sandwich enzyme-linked immunosorbent assay quantitatively showed that CSF and serum UCH-L1 levels in rats were significantly elevated as early as 2 hours following both traumatic and ischemic injury.31 Clinical studies in humans with severe TBI confirmed, using ELISA analysis, that the UCH-L1 protein was significantly elevated in human CSF32, 33 and was detectable very early after injury and remained significantly elevated for 168 hours post-injury.33, 34
Based on the important function of UCH-L1 in neurons, its high specificity and abundance in the central nervous system and its association with magnitude of TBI in human CSF33, 34 this study assessed whether UCH-L1 was significantly elevated in the serum of mild and moderate TBI patients compared to participants in the uninjured and non-head injured trauma control groups. Additionally, this study examined the relationship between UCH-L1 levels and measures of acute injury severity such as GCS, traumatic intracranial lesions on CT scan and the need for neurosurgical intervention.
MATERIALS AND METHODS
Study Design, Setting and Population
This prospective controlled cohort study enrolled a convenience sample of adult patients with blunt head trauma followed by either loss of consciousness, amnesia, or disorientation35 and presenting to the emergency department within 4 hours of injury with a GCS of 9 to 15. Head CT Scans were performed at the discretion of the treating physician.
Study sites included the Emergency Departments (ED) of three Level I Trauma Centers; Shands at University of Florida in Gainesville, Florida; Orlando Regional Medical Center in Orlando, Florida; and Washington University in St. Louis, Missouri.
Eligibility for suspected mild TBI was determined by the treating physician. Patients were excluded if: 1) they were less than 18 years old; 2) there was no history of trauma as their primary event (e.g. syncope or seizure); 3) they had known dementia, chronic psychosis or active CNS pathology; 4) were pregnant, or 5) were incarcerated.
There were two control groups: 1) normal adult volunteers without any acute injuries who responded to advertisements in a local flyer; 2) non-head injured patients presenting to the emergency department with either a single limb orthopedic injury or following a motor vehicle collision without blunt head trauma. Participants in the trauma control groups had a normal mental status at the time of enrollment and had no evidence of acute brain injury or hemodynamic instability and were enrolled during the same period as TBI patients were enrolled. The participants in these control groups were carefully screened to ensure they had no blunt head trauma and no symptoms of brain injury. We used very strict clinical criteria for defining control group patients. The study team carefully assessed every patient at the time of enrollment to ensure each patient met inclusion/exclusion. Our definition specified that the participants in the control groups have no blunt head trauma and have no loss of consciousness, no amnesia, and no alteration in mental status or sensorium at any time after trauma (such as disorientation or confusion). Neuroimaging studies such as CT scan and MRI were left to the discretion of the treating physician and did not impact the group assignment.
The purpose of including non-head injured trauma controls was to examine biomarker levels in patients who were exposed to the acceleration and deceleration forces without blunt trauma.
Protocol
All initial patient assessments were made by board certified emergency medicine physicians trained by a 1-hour session to evaluate patient eligibility. Blood samples were obtained from each TBI and non-head injured trauma control participant shortly after arrival to the ED and within 4 hours of the reported time of injury. A single vial of approximately 5mL of blood was collected and placed in clot tubes with a serum separator and allowed to clot at room temperature. The blood was centrifuged within 30 minutes and the serum was placed in bar-coded aliquot containers and stored in a freezer at −70 degrees Celsius until it was transported to a central laboratory (Banyan Biomarkers Inc.). There, the samples were analyzed using a sandwich enzyme-linked immunosorbent assay (ELISA) to UCH-L1 as described below. After assessment and treatment in the emergency department, patients were either discharged home or admitted to hospital based on severity of their injuries and patient management was not altered by the study. Blood sampling and handling in uninjured controls was conducted in the same manner.
Patients underwent standard CT scan of the head according to the judgment of the treating physician. The CT scan ordering pattern at the participating Level I trauma centers is such that most patients with blunt head injury with subsequent symptoms have a head CT scan performed as part of usual care. In some instances, physicians ordered CT scans of the head on trauma control patients based on mechanism or clinical circumstances. CT examinations of each TBI patient were interpreted by board-certified radiologists who recorded location, extent and type of brain injury. Radiologists were blinded to the study protocol.
This study was approved by the respective Institutional Review Boards (IRB) of the University of Florida, Orlando Regional Medical Center and Washington University. Written informed consent was obtained from all patients and/or legal authorized representatives prior to enrollment.
Outcome measures and assessment
The primary outcome measure tested the ability of UCH-L1 to distinguish patients with mild and moderate TBI from those without TBI (uninjured control participants) and assessed the relationship to non-head injured trauma control participants. The secondary outcome measures tested the ability of UCH-L1 to distinguish between different levels of injury severity. These severity measures included: 1) Glascow Coma Score (GCS) scores36, 37 obtained at presentation to the emergency department, 2) the presence of intracranial lesions on initial CT scan; and 3) the need for neurosurgical intervention.
Intracranial lesions on CT included any acute traumatic intracranial lesions visualized on CT scan. Neurosurgical intervention was defined as either death within 7 days secondary to head injury or the need for any of the following procedures within 7 days: craniotomy, elevation of skull fracture, intracranial pressure monitoring, or intubation for head injury.38, 39
Biomarker sandwich ELISA Analysis Method
The serum samples were measured using a UCH-L1 sandwich enzyme-linked immunosorbent assay (ELISA) version 1b modified from a protocol previously reported.31, 33 Both mouse monoclonal antibody (capture antibody) and rabbit polyclonal antibody (detection antibody) were made in-house against recombinant human UCH-L1 full length protein and partial protein, respectively. Both are affinity purified using a target-protein-based affinity column. Their specificity to the target protein (UCH-L1) was confirmed by immunoblotting. Reaction wells were coated with capture antibody (5 μg/mL purified mouse monoclonal anti-human UCHL1) in 0.05 M sodium bicarbonate, pH 9.6 and incubated overnight at 4°C. Plates were then washed with 350 μL/well blocking buffer (Tris buffer saline with 0.02% Tween-20 (v/v); [TBST]) and incubated further with 300 μL/well TBST for 30 minutes at ambient temperature with gentle shaking. Antigen standard (UCH-L1 standard curve 0.06–15 ng/mL), unknown samples (20 μL of serum), and assay internal control samples were incubated with detection antibody [rabbit polyclonal antihuman UCH-L1, made in-house; 0.72 μg/mL; 100 μL total volume] overnight. Afterward the capture antibody coated plate was incubated with detection antibody-sample mixture for 1.5 hours at room temperature and was washed using an automatic plate washer (each well rinsed with 350 μL of wash buffer [TBST]). Secondary anti-rabbit-IgG HRP (Amersham Biosciences; 1/2000 dilution) in blocking buffer was then added to wells (100 μL) at 100 μL/well, and the plates were further incubated at room temperature for 1 hour. Finally, the wells were developed with substrate solution: Ultra-TMB ELISA 100 μL/well (Pierce #34028) with incubation for 10 minutes and the plate was read at 450 nm with a 96-well spectrophotometer (Molecular Device Spectramax 190). The intra-assay CV was 2.1% to 7.9% while inter-assay CV was 0.9% to 10.6 % within the assay dynamic range. The lower limit of detection (LOD) was determined to be 0.030 ng/mL. Samples with undetectable (ND) levels of UCH-L1 were assigned a value of 50% of the LOD (i.e. ND=0.015 ng/mL). Any sample yielding a signal over the quantification range was diluted and re-assayed. As negative controls, we noted that if anti-UCH-L1 capture or detection antibodies were substituted with non-immune normal IgG (mouse) or (rabbit) respectively, no target signals were detected.
Data Analysis
Descriptive statistics with means and proportions were used to describe the data. For statistical analysis, biomarker levels were treated as continuous data, measured in ng/ml and expressed as means (±SEM). Data were assessed for equality of variance and distribution. Logarithmic transformations were conducted on non-normally distributed data. Statistical significance was set at a p-value of 0.05. Group comparisons for different GCS Scores were performed using analysis of variance with multiple comparisons using Games-Howell post-hoc test. Receiver Operating Characteristics (ROC) curves were created to explore the ability of the biomarker to distinguish between injured and uninjured control participants and TBI patients within 4 hours of injury, as well as for intracranial lesions on CT scan and need for neurosurgical intervention. Estimates of the area under these curves (AUC) were obtained (AUC=0.5 indicates no discrimination and an AUC=1.0 indicates a perfect diagnostic test). Classification performance was assessed by sensitivity, specificity, positive and negative predictive values with 95% confidence intervals. All analyses were performed using the statistical software package PASW 17.0 (IBM Corporation®, Somers NY).
In accordance with the primary outcome measure, sample size was based on the ability of UCH-L1 to distinguish mild TBI patients with a GCS 13–15 from non-head injured trauma control patients. Using data from a feasibility study40 sample sizes of 22 mild TBI cases and 22 trauma control cases achieved an 80% power to detect a difference of 1.03 ng/ml between the mild TBI group (GCS 13–15) and the non-head injured trauma control patient with a significance level of 0.05.
RESULTS
A total of 295 patients were enrolled in the study and had serum samples drawn for analysis. There were 96 TBI patients: 86 with GCS 13–15 and 10 with GCS 9–12; and 199 control participants: 176 uninjured control patients with no injuries and 23 trauma control patients who had peripheral injuries without TBI (16 MVC control cases and 7 orthopedic control cases). The flow diagram in Figure 1 describes the distribution of enrolled patients. CT scan of the head scan was performed in all TBI patients and traumatic intracranial lesions on CT scan were evident in 28 (29%): 23% of patients with GCS 13–15 and 80% of those with GCS 9–12. A CT scan was also performed in 9 trauma control patients despite the patients’ lack of signs or symptoms of TBI – no blunt trauma, no loss of consciousness, no amnesia and no alteration in sensorium at any time after injury. These CT’s were performed at the discretion of the treating physician based on mechanism or clinical circumstances and none of them showed any signs of traumatic intracranial lesions. Neurosurgical intervention was performed on 14 (14%) patients: 6 (43%) presented with GCS 13–15 and 8 (57%) with GCS 9–12.
Figure 1.
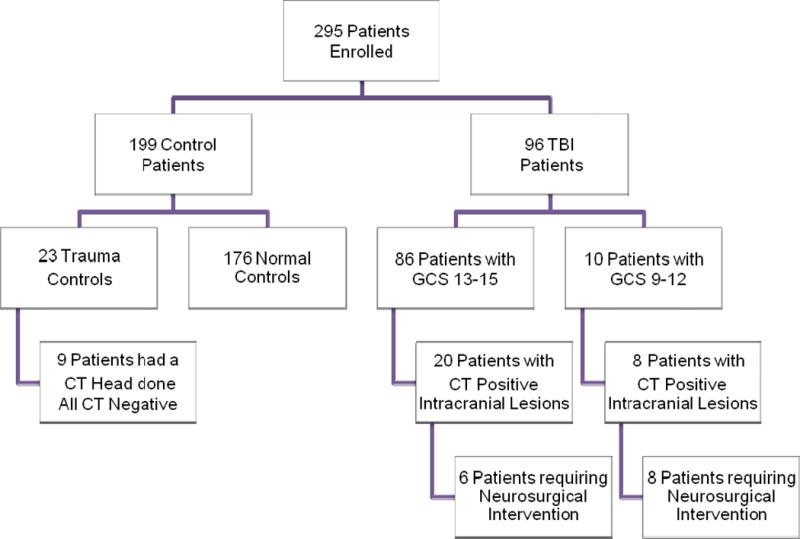
Flow diagram of enrolled patients
There were 26 TBI patients enrolled from the University of Florida, 52 from Orlando Regional Medical Center and 18 from Washington University. The mean age of TBI patients was 39 years (range 18–89) with 64% males and the mean age of control participants was 37 years (range 18–83) with 60% males. The 3 most common injury mechanisms were motor vehicle crashes (45%), falls (15%) and motorcycle crashes (15%). Characteristics of participants in the control groups (uninjured and trauma) and TBI patients were similar for age (p=0.325) and gender (p=0.17) (Table 1).
Table 1.
Characteristics of Enrolled Patients
| TBI | CONTROL | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | Total | University of Florida | Regional Orlando Medical Center | Washington University | Normal Controls | Trauma Controls |
| N=96 | N=26 | N=52 | N=18 | N=176 | N=23 | |
|
| ||||||
| Mean age (yrs±SD) | 39 (±15) | 36 (±15) | 39 (±15) | 46 (±15) | 37 (±14) | 44 (±17) |
| Range | (18–89) | (18–61) | (19–70) | (23–89) | (18–65) | (19–83) |
|
| ||||||
| Gender (%) | ||||||
| male/female | 64/36 | 62/39 | 64/36 | 67/33 | 53/47 | 70/30 |
|
| ||||||
| GCS Score in ED (%) | ||||||
| GCS 9–12 | 10 (10) | 5 (19) | 3 (6) | 2 (11) | 0 (0) | 0 (0) |
| GCS 13–15 | 86 (90) | 21 (81) | 49 (94) | 16 (89) | 176 (100) | 23 (100) |
|
| ||||||
| Mechanism of Injury | ||||||
| Motor Vehicle Crash | 43 (45) | 16 (61) | 25 (47) | 2 (11) | 0 (0) | 16 (70) |
| Fall | 14 (15) | 2 (8) | 6 (13) | 6 (33) | 0 (0) | 2 (9) |
| Motorcycle | 14 (15) | 2 (8) | 11 (21) | 1 (6) | 0 (0) | 0 (0) |
| Pedestrian Struck | 7 (7) | 3 (11) | 3 (6) | 1 (6) | 0 (0) | 1 (4) |
| Bicycle | 6 (6) | 0 (0) | 4 (7) | 2 (11) | 0 (0) | 0 (0) |
| Hit with blunt object | 5 (5) | 1 (4) | 1 (2) | 3 (17) | 0 (0) | 0 (0) |
| Assault | 4 (4) | 0 (0) | 2 (4) | 2 (11) | 0 (0) | 0 (0) |
| Sports/Other | 3 (3) | 2 (8) | 0 (0) | 1 (6) | 0 (0) | 4 (17) |
|
| ||||||
| Loss of Consciousness (%) | ||||||
| Yes | 70 (73) | 18 (69) | 42 (81) | 10 (56) | 0 (0) | 0 (0) |
| No | 22 (23) | 6 (23) | 10 (19) | 6 (33) | 176 (100) | 9 (100) |
| Unknown | 4 (4) | 2 (8) | 0 (0) | 2 (11) | 0 (0) | 0 (0) |
|
| ||||||
| Amnesia (%) | 37 (39) | 10 (38) | 22 (42) | 5 (28) | 0 (0) | 0 (0) |
|
| ||||||
| Admitted to Hospital (%) | 58 (60) | 24 (92) | 26 (50) | 8 (44) | 0 (0) | 10 (43) |
|
| ||||||
| Intoxicated (Alcohol or Drugs) | 13 (14) | 7 (27) | 4 (2) | 2 (11) | – | 1 (4) |
|
| ||||||
| Neurosurgical Intervention | 14 (15) | 5 (19) | 8 (15) | 1 (6) | – | 0 (0) |
|
| ||||||
| Traumatic Intracranial Lesions on CT Scan of Head | 28 (29) | 11 (42) | 11 (21) | 6 (33) | – | 0 (0) |
|
| ||||||
| Lesion Types* | ||||||
| Epidural hematoma | 2 (7) | 1 (9) | 1 (9) | 0 (0) | 0 (0) | |
| Subdural hematoma | 5 (18) | 0 (0) | 1 (9) | 4 (66) | 0 (0) | |
| Subarachnoid hemorrhage | 6 (21) | 3 (27) | 2 (18) | 1 (17) | – | 0 (0) |
| Contusion | 3 (11) | 3 (27) | 0 (0) | 0 (0) | 0 (0) | |
| Intracerebral hemorrhage | 1 (4) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | |
| Pneumocephalus | 2 (7) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | |
| Diffuse Axonal Lesions | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Combination of lesions§ | 9 (32) | 4 (37) | 4 (37) | 1 (17) | 0 (0) | |
Isolated lesions
Includes any combination of the above lesions
Both the TBI and trauma control patients had serum samples drawn within 4 hours of injury. The average time to serum collection for TBI patients was 2.7 hours (95%CI 2.4–2.9); for orthopedic control patients it was 2.5 hours (95%CI 1.9–3.2); and for MVC control patients it was 3.2 (95%CI 2.7–3.7). UCH-L1 demonstrated a rapid appearance in serum post-injury with levels detectible in less than an hour of injury. Overall mean levels of UCH-L1 in all TBI patients was 0.955 (±0.248) (range 0.015–19.25) compared to 0.083 (±0.005) (range 0.015–0.490) in all controls (p<0.001).
In Figure 2a levels of UCH-L1 in uninjured and in the trauma control group (participants in the orthopedic and MVC control groups) are shown relative to 3 groups of GCS score divided as GCS 15, GCS 13–14, and GCS 9–12. There were significant differences between the groups overall (p<0.001). In particular when patients with an ED GCS score of 15 were isolated from the TBI group (2b) early UCH-L1 levels demonstrated significant differences between patients with a GCS 15 versus uninjured control patients (p=0.001), and between patients with a GCS 15 versus trauma control patients (p=0.022). Area under the curve (AUC) was calculated from the ROC curves constructed to assess the performance of early UCH-L1 levels in distinguishing TBI from control patients. ROC curves demonstrated that early UCH-L1 levels were able to distinguish TBI from uninjured control participants with an AUC 0.87 (95%CI 0.82–0.92) (Figure 3a). More specifically, UCH-L1 was able to differentiate TBI patients with a GCS 15 from uninjured control participants 0.87 (95%CI 0.81–0.93) (Figure 3b).
Figure 2.
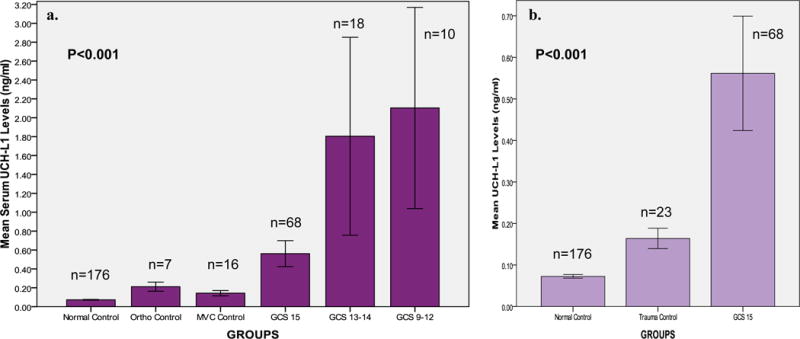
a & b UCH-L1 Levels among the different GCS groups versus Control groups
Figure 3.
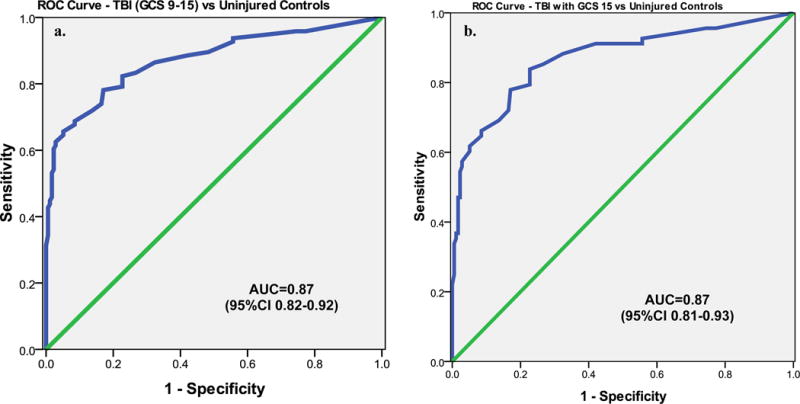
a & b ROC Curve for distinguishing TBI patients versus Uninjured Controls
When serum levels of UCH-L1 were compared in patients with traumatic intracranial lesions on CT scan (CT positive) to those without CT lesions (CT negative), levels were significantly higher in those with lesions on CT scan (P<0.001) (Figure 4a). Patients with GCS 15 were assessed independently and serum UCH-L1 levels were appreciably more elevated in those with CT scan lesions than those without (P=0.013) (Figure 4b). The area under the curve for discriminating between CT scan positive and CT scan negative intracranial lesions was 0.73 (95%CI 0.62–0.83) (Figure 4c). Figure 4d shows UCH-L1 levels in the 16 trauma control patients having no CT performed versus the 9 trauma control patients who had CT scans of the head ordered by their treating physician despite lack of TBI symptoms. There was no difference in UCH-L1 levels between the trauma control patients who did or did not have CT scans performed. TBI patients with a negative CT had higher levels of UCH-L1 than trauma control patients with a negative CT (p=0.057). UCH-L1 levels were significantly elevated in patients with traumatic intracranial lesions on CT (CT positive) than those without CT lesions (CT negative) regardless of whether they were trauma control or TBI patients (p<0.001).
Figure 4.
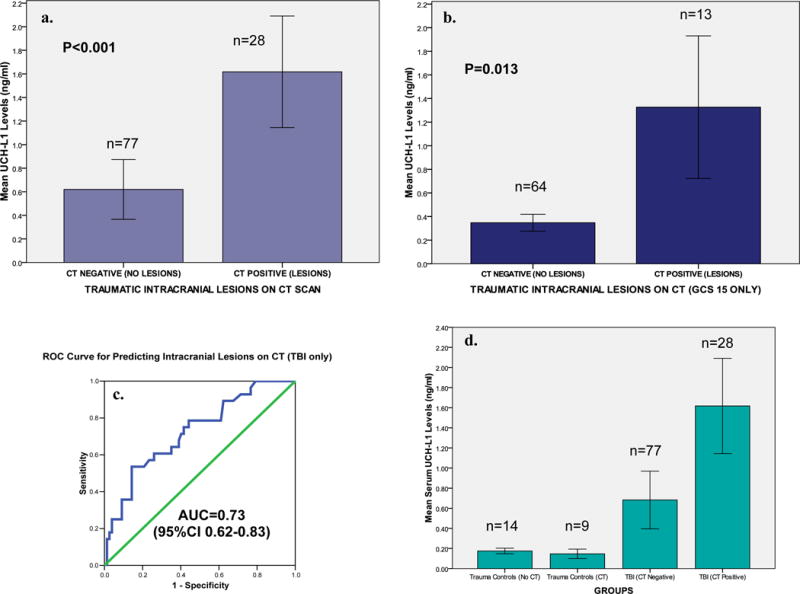
a, b, c & d Bar Graphs comparing serum UCH-L1 Levels in patients with and without traumatic intracranial lesions on CT in all TBI patients and the subgroup with GCS 15 AND an ROC Curve for distinguishing CT positive versus CT negative
Additionally, we compared serum levels of UCH-L1 in patients who had a neurosurgical intervention versus those who received no such intervention. Substantially higher serum levels were detected in those who had a neurosurgical intervention (P<0.001) (Figure 5a). When the subgroup of patients with GCS 15 was assessed separately, serum UCH-L1 levels were significantly elevated in those having a neurosurgical intervention versus those who did not (P<0.001) (Figure 5b). The ROC curve for discriminating between those having and not having a neurosurgical intervention yielded an AUC of 0.86 (95%CI 0.76–0.94) (Figure 5c).
Figure 5.
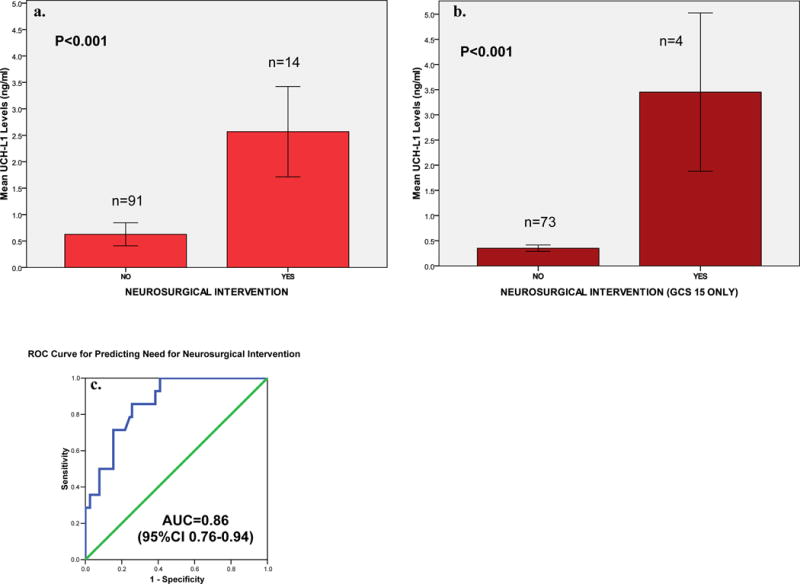
a, b & c Bar Graph comparing serum UCH-L1 Levels in patients with and without neurosurgical intervention in all TBI patients and in those with GCS 15 only AND an ROC Curve for distinguishing patients with and without neurosurgical intervention
Cutoff points for UCH-L1 were derived from the ROC Curves for detecting intracranial lesions on CT scan and having neurosurgical intervention. The aim of this exploratory analysis was to maximize the sensitivity and correctly classify all CT positive lesions and all those with neurosurgical intervention. Classification performance for detecting intracranial lesions on CT at a UCH-L1 cutoff level of 0.09 ng/ml yielded a sensitivity of 100% (95%CI 88–100), a specificity of 21% (95%CI 13–32) and a negative predictive value of 100% (76–100) (Table 2a). Classification performance for predicting neurosurgical intervention at a UCH-L1 cutoff level of 0.21 ng/ml yielded a sensitivity of 100% (95%CI 73–100), a specificity of 57% (95%CI 46–67) and a negative predictive value of 100% (95%CI 91–100) (Table 2b).
Table 2.
a & b Contingency table and Classification Performance of Serum UCH-L1 in detecting Intracranial Lesions on CT and Contingency table and Classification Performance of Serum UCH-L1 in detecting Neurosurgical Intervention
| 2a. | |||||
|---|---|---|---|---|---|
| CT positive | CT negative | Sensitivity | 100% (88–100) | ||
| UCH-L1 positive ≥0.09 ng/ml | 28 | 61 | Specificity | 21% (13–32) | |
| UCH-L1 negative <0.09 ng/ml | 0 | 16 | NPV | 100% (76–100) | |
| PPV | 31% (22–42) | ||||
| 2b. | |||||
| Neurosurgical Intervention | No Neurosurgical Intervention | Sensitivity | 100% (73–100) | ||
| UCH-L1 positive ≥0.21 ng/ml | 14 | 39 | Specificity | 57% (46–67) | |
| UCH-L1 negative <0.21 ng/ml | 0 | 52 | NPV | 100% (91–100) | |
| PPV | 26% (16–41) | ||||
DISCUSSION
Studies assessing UCH-L1 in human serum following a TBI are limited at this time. This clinical study is among the first to systematically measure early levels of UCH-L1 in human serum in TBI patients with GCS 9–15. UCH-L1 is appealing as a candidate biomarker for several reasons. UCH-L1 is highly expressed in neurons29 with tissue distribution almost exclusively in the brain. It is a small protein with a molecular weight of about 24 kDa and has a compact and almost globular shape.41 Western blots of CSF fluids show that it remains as an intact protein with no detectable breakdown product, a feature that facilitates its crossing of the brain-blood barrier and stability in biofluid. UCH-L1 has a rapid appearance in serum with levels detected within 1 hour of injury and can distinguish control groups from TBI groups and shows a graded response to severity of injury. Finally, it is associated with clinically relevant endpoints such as traumatic intracranial lesions on CT scan and neurosurgical intervention.
We elected to study both mild and moderate injury because initial GCS scores in the ED in this population can be surprisingly deceptive. The classification of a TBI as a mild or a moderate can change based on neuroimaging results and the presence of factors altering mental status such as intoxication, medications and other injuries. A patient with a GCS of 15 who has an acute bleed on CT scan can be classified as moderate. Conversely, a patient with a GCS of 11 who has no evidence of intracranial injury on CT scan can be classified as a mild. Although we studied TBI patients from GCS 9–15 we included focused analyses of those with a so-called “mild TBI” (concussion) and those presenting with a GCS score of 15.
Much of the previous work on biomarkers in mild TBI has been limited by factors such as wide variations in sample collection times and inadequate control groups. When we designed the study we carefully considered these limitations. To overcome the sample schedule shortfall we restricted sample collection to within 4 hours of injury to reflect actual clinical practice and measure UCH-L1 as soon after injury as possible. Additionally, we incorporated two different types of control groups in this study. Participants in the uninjured control group represented the general population and participants in the non-head injured trauma control group had either orthopedic injuries or exposure to the forces of motor vehicle crashes. The use of these control groups is unique to this study. The uninjured participants helped to establish normative UCH-L1 values. The trauma control groups provided an initial exploration of biomarker release in trauma patients without head injury, mimicking the clinical setting in which the biomarker could eventually be applied if validated. Many trauma control patients were exposed to significant trauma including the acceleration-deceleration vectors of motor vehicle crashes and falls from heights over 5 feet. Their injuries paralleled TBI patients except for their lack of both blunt head trauma and TBI symptoms.
To our advantage, in nine of the trauma control patients the mechanism was so significant that physicians actually ordered head CT’s as part of their clinical care despite the lack of blunt head injury and the lack of signs and symptoms of brain injury. This provided a unique opportunity to assess levels of UCH-L1 in CT negative trauma control patients against trauma control patients without CT and CT negative TBI patients. TBI patients with a negative CT had higher levels than trauma control patients with negative CT’s. More importantly, the largest elevation in UCH-L1 occurred in those with traumatic intracranial lesions on CT, regardless of GCS or the type of trauma control group.
More importantly, serum UCH-L1 was able to distinguish patients with a mild TBI patients, otherwise known as concussion, from both uninjured and trauma control patients. From a patient management perspective, distinguishing trauma control patients presenting with a GCS 15 from TBI patients with a GCS 15 is critical.
UCH-L1 demonstrated a rapid appearance in serum post-injury with levels detectible in less than an hour of injury. There was an incremental rise in serum levels with severity of injury: undetectable or in very low levels in participants in the uninjured control group, slightly higher in participants in the trauma control group, significantly elevated in TBI patients with a GCS 15 and highest levels in those with intracranial lesions on CT. Moreover, markedly higher serum levels of UCH-L1 were found in those who had a neurosurgical intervention. This finding was also sustained among those with a GCS of 15.
When ROC Curves were constructed a cutoff level of 0.09 ng/ml yielded the highest sensitivity (100%) for detecting intracranial lesions on CT and a cutoff level of 0.21 ng/ml provided the best sensitivity (100%) for predicting those who had a neurosurgical intervention. If these findings can be validated serum UCH-L1 could have several important clinical applications in managing TBI acutely. It could help with more judicious use of CT scans of the head for which there are concerns over exposure to ionizing radiation from CT scans, it could be incorporated into guidelines for return to duty, work or sports activities, and guide decisions to transfer patients to neurosurgical facilities. They could also provide opportunities for early counseling of patients and for helping to avert the consequences of “second impact syndrome.”8, 9
LIMITATIONS
While these data are promising, the authors recognize there are major limitations to this study. The current study was performed in a limited cohort of patients with mild and moderate TBI, a disease that tends to be heterogeneous in nature. Patients were enrolled as a convenience sample because research team members could not be on duty 24/7. Despite this, patients were recruited consecutively when research assistants were on duty including on weekends and nights so a representative sample could be enrolled.
We know that UCH-L1 is highly abundant in the central nervous system and has shown considerable specificity to neurons. Even though injured control participants were exposed to significant trauma, this study cannot confirm that UCH-L1 is not released from other injured tissues. Further study is required to assess release of UCH-L1 in the setting of multiple organ injury. Additionally, UCH-L1 should be examined in spinal cord injury and in peripheral nerve injury where UCH-L1 may potentially be present. Studies are currently underway to evaluate extracranial injuries on UCH-L1 values.
The study included a limited number of trauma control patients. Although a power analysis for this preliminary study revealed an adequate sample size to make a statistically significant distinction between trauma control patients and TBI patients, clinical validation of these findings will require a much larger sample. Future studies will require TBI and non-TBI injured patients in a variety of settings with multiple organ injuries.
This study addressed severity of injury in the acute care setting and did not describe long-term outcome in these patients. Outcome data will be assessed as these data become available in our ongoing studies.
Due to the limited sample size, multivariate analysis assessing the impact of other factors affecting mental status such as intoxicants and medications were not conducted. Prospective studies are ongoing that will allow these type of analyses to be performed with adequate power.
CONCLUSION
This study is among the first to systematically assess UCH-L1 in human serum following mild and moderate TBI. This present work follows the bench to bedside approach to translational research in TBI biomarkers by extending the findings collected in human CSF following a severe TBI. We confirmed that the UCH-L1 protein is present in human serum and that its levels are significantly elevated in this population using ELISA analysis, including those with a GCS of 15. UCH-L1 is detectable in serum within an hour of injury and is associated with measures of injury severity including GCS score, CT lesions and neurosurgical intervention. These results will require further validation in a larger cohort of TBI and non-TBI injured trauma patients before clinical application.
Acknowledgments
Grant Support
This study was supported in part by Department of Defense Award number DoD W81XWH-06-1-0517. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of Department of the Army or Department of Defense.
The project described was supported in part by Award Number R01NS057676 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke or the National Institutes of Health.
Footnotes
Author Disclosure Statement
Drs. Papa, Brophy and Demery are consultants of Banyan Biomarkers, Inc. but receive no stocks or royalties from the company and will not benefit financially from this publication. Drs. Liu, Mo, Akinyi and Mondello are employees of Banyan Biomarkers, Inc. Drs. Wang and Hayes own stock, receive royalties from, and are officers of Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
Contributor Information
Linda Papa, Director of Academic Clinical Research, Attending Emergency Physician, Department of Emergency Medicine, Orlando Regional Medical Center, Adjunct Professor, Department of Emergency Medicine, University of Florida Associate Professor, Florida State University, College of Medicine, Associate Professor, University of Central Florida, College of Medicine, 86 W. Underwood (S-200), Orlando, Florida, 32806, Tel.: 407-237-6329, Fax: 407-649-3083.
Lawrence M. Lewis, Associate Professor of Emergency Medicine and Medicine, Washington University School of Medicine, Division of Emergency Medicine, 660 South Euclid Avenue, Campus Box 8072 St. Louis, MO 63110, Tel.: (314) 758-6787, Fax: (314) 362-0478.
Salvatore Silvestri, Program Director, Emergency Medicine Residency, Attending Emergency Physician, Department of Emergency Medicine, Orlando Regional Medical Center, 86 W. Underwood (S-200), Orlando, Florida, 32806, Tel.: 407-237-6324, Fax: 321-843-6058.
Jay L. Falk, Chief Academic Medical Officer, Attending Emergency Physician, Department of Emergency Medicine, Orlando Regional Medical Center, 86 W. Underwood (S-200), Orlando, Florida, 32806, Tel.: 407-237-6324, Fax: 321-843-6058.
Philip Giordano, Corporate Director, Research Operations, Attending Emergency Physician, Department of Emergency Medicine, Orlando Regional Medical Center, 86 W. Underwood (S-200), Orlando, Florida, 32806, Tel.: 407-237-6324, Fax: 321-843-6058.
Gretchen M. Brophy, Professor of Pharmacotherapy & Outcomes Science and Neurosurgery, Virginia Commonwealth University, Medical College of Virginia Campus, 410 N. 12th Street, PO Box 980533, Richmond, VA 23298-0533, Tel.: (804) 828-1201, Fax: (804) 828-8359.
Jason A. Demery, Assistant Professor of Psychiatry, University of Florida Forensic Institute, Licensed Psychologist & Forensic Neuropsychologist, UF Springhill Health Center, 8491 NW 39th Ave., Gainesville, Florida 32606, Tel.: (352) 265-3284, Fax: (352) 265-3285.
Ming Cheng Liu, Center of Innovative Research, Banyan Biomarkers Inc., 12085 Research Dr., Alachua, FL 32615, Tel.: (386) 518-6757, Fax: (386) 518-6811.
Jixiang Mo, Center of Innovative Research, Banyan Biomarkers Inc., 12085 Research Dr., Alachua, FL 32615, Tel.: (386) 518-6757, Fax: (386) 518-6811.
Linnet Akinyi, Center of Innovative Research, Banyan Biomarkers Inc., 12085 Research Dr., Alachua, FL 32615, Tel.: (386) 518-6757, Fax: (386) 518-6811.
Stefania Mondello, Fellow, Center of Innovative Research, Banyan Biomarkers Inc., 13400 Progress Blvd., Alachua, FL 32615, Tel.: (386) 518-6713, Fax: (386) 518-6776, Alachua, Florida.
Kara Schmid, Walter Reed Army Institute of Research, Department of Applied Neurobiology, Division of Psychiatry and Neuroscience, Silver Spring, Maryland 20910, Tel.: (301) 319-9376.
Claudia S. Robertson, Professor, Department of Critical Care and Neurosurgery, Baylor College of Medicine, One Baylor Plaza, Houston, Texas 77030, Tel.: (713) 873-2792, Fax: (713) 798-8063.
Frank C. Tortella, Walter Reed Army Institute of Research, Department of Applied Neurobiology, Division of Psychiatry and Neuroscience, Silver Spring, Maryland 20910, Tel.: (301) 319-9687.
Ronald L. Hayes, Banyan Biomarkers Inc., 13400 Progress Blvd., Alachua, FL 32615, Tel.: (386) 518-6713, Fax: (386) 518-6776.
Kevin K. W. Wang, University of Florida, Department of Psychiatry, 100 S. Newell Drive, McKnight Brain Institute, Gainesville, FL 32610, Tel.: (352) 328-7663, Fax: (352)-392-2579.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. Accessed November 11, 2010. [Google Scholar]
- 2.Yealy DM, Hogan DE. Imaging after head trauma. Who needs what? Emerg Med Clin North Am. 1991 Nov;9(4):707–717. [PubMed] [Google Scholar]
- 3.Vollmer DG, Dacey RG., Jr The management of mild and moderate head injuries. Neurosurg Clin N Am. 1991 Apr;2(2):437–455. [PubMed] [Google Scholar]
- 4.Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. Jama. 1999;282(10):974–983. [PubMed] [Google Scholar]
- 5.Carey ME. Analysis of wounds incurred by U.S. Army Seventh Corps personnel treated in Corps hospitals during Operation Desert Storm, February 20 to March 10, 1991. J Trauma. 1996 Mar;40(3 Suppl):S165–169. doi: 10.1097/00005373-199603001-00036. [DOI] [PubMed] [Google Scholar]
- 6.Sapsford W. Penetrating brain injury in military conflict: does it merit more research? J R Army Med Corps. 2003 Mar;149(1):5–14. doi: 10.1136/jramc-149-01-02. [DOI] [PubMed] [Google Scholar]
- 7.Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005 May 19;352(20):2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- 8.Cantu RC. Return to play guidelines after a head injury. Clin Sports Med. 1998 Jan;17(1):45–60. doi: 10.1016/s0278-5919(05)70060-0. [DOI] [PubMed] [Google Scholar]
- 9.Erlanger DM, Kutner KC, Barth JT, Barnes R. Neuropsychology of sports-related head injury: Dementia Pugilistica to Post Concussion Syndrome. Clin Neuropsychol. 1999 May;13(2):193–209. doi: 10.1076/clin.13.2.193.1963. [DOI] [PubMed] [Google Scholar]
- 10.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008 Nov;95(5):502–507. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 12.Fayngersh V, Passero M. Estimating radiation risk from computed tomography scanning. Lung. 2009 May-Jun;187(3):143–148. doi: 10.1007/s00408-009-9143-9. [DOI] [PubMed] [Google Scholar]
- 13.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008 May;81(965):362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 14.Heilbrun ME, Chew FS, Tansavatdi KR, Tooze JA. The role of negative CT of the abdomen and pelvis in the decision to admit adults from the emergency department after blunt trauma. J Am Coll Radiol. 2005 Nov;2(11):889–895. doi: 10.1016/j.jacr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Long AE. Radiographic decision-making by the emergency physician. Emerg Med Clin North Am. 1985;3:437–446. [PubMed] [Google Scholar]
- 16.Stiell IG, Wells GA, Vandemheen K, et al. Variation in ED use of computed tomography for patients with minor head injury. Ann Emerg Med. 1997 Jul;30(1):14–22. doi: 10.1016/s0196-0644(97)70104-5. [DOI] [PubMed] [Google Scholar]
- 17.Ryu WH, Feinstein A, Colantonio A, Streiner DL, Dawson D. Regional variability in the use of CT for patients with suspected mild traumatic brain injury. Can J Neurol Sci. 2009 Jan;36(1):42–46. doi: 10.1017/s0317167100006296. [DOI] [PubMed] [Google Scholar]
- 18.Mirvis SE, Shanmuganathan K. Trauma radiology: Part IV. Imaging of acute craniocerebral trauma. J Intensive Care Med. 1994 Nov-Dec;9(6):305–315. doi: 10.1177/088506669400900605. [DOI] [PubMed] [Google Scholar]
- 19.Lobato RD, Gomez PA, Alday R, et al. Sequential computerized tomography changes and related final outcome in severe head injury patients. Acta Neurochir (Wien) 1997;139(5):385–391. doi: 10.1007/BF01808871. [DOI] [PubMed] [Google Scholar]
- 20.Gaetz M. The neurophysiology of brain injury. Clin Neurophysiol. 2004 Jan;115(1):4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 21.Papa L, Robinson G, Oli M, et al. Use of Biomarkers for Diagnosis and Management of Traumatic Brain Injury Patients. Expert Opinion on Medical Diagnostics. 2008;2(8):937–945. doi: 10.1517/17530059.2.8.937. [DOI] [PubMed] [Google Scholar]
- 22.Papa L, Lewis LM, Falk JL, et al. Elevated Levels of Serum Glial Fibrillary Acidic Protein Breakdown Products in Mild and Moderate Traumatic Brain Injury Are Associated With Intracranial Lesions and Neurosurgical Intervention. Ann Emerg Med. 2011 Nov 7; doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piazza O, Storti MP, Cotena S, et al. S100B is not a reliable prognostic index in paediatric TBI. Pediatr Neurosurg. 2007;43(4):258–264. doi: 10.1159/000103304. [DOI] [PubMed] [Google Scholar]
- 24.Martens P. Serum neuron-specific enolase as a prognostic marker for irreversible brain damage in comatose cardiac arrest surviviors. Acad Emerg Med. 1996;3:126–131. doi: 10.1111/j.1553-2712.1996.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 25.Rainey T, Lesko M, Sacho R, Lecky F, Childs C. Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24h) time-point. Resuscitation. 2009 Mar;80(3):341–345. doi: 10.1016/j.resuscitation.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 2006 Jun;20(7):759–765. doi: 10.1080/02699050500488207. [DOI] [PubMed] [Google Scholar]
- 27.Watt SE, Shores EA, Baguley IJ, Dorsch N, Fearnside MR. Protein S-100 and neuropsychological functioning following severe traumatic brain injury. Brain Inj. 2006 Sep;20(10):1007–1017. doi: 10.1080/02699050600909698. [DOI] [PubMed] [Google Scholar]
- 28.Morochovic R, Racz O, Kitka M, et al. Serum S100B protein in early management of patients after mild traumatic brain injury. Eur J Neurol. 2009 Oct;16(10):1112–1117. doi: 10.1111/j.1468-1331.2009.02653.x. [DOI] [PubMed] [Google Scholar]
- 29.Jackson P, Thompson RJ. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci. 1981 Mar;49(3):429–438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- 30.Kobeissy FH, Ottens AK, Zhang Z, et al. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics. 2006 Oct;5(10):1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Liu MC, Akinyi L, Scharf D, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci. 2010 Feb;31(4):722–732. doi: 10.1111/j.1460-9568.2010.07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siman R, Toraskar N, Dang A, et al. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J Neurotrauma. 2009 Nov;26(11):1867–1877. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010 Jan;38(1):138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brophy G, Mondello S, Papa L, et al. Biokinetic Analysis of Ubiquitin C-Terminal Hydrolase-L1 (Uch-L1) in Severe Traumatic Brain Injury Patient Biofluids. J Neurotrauma. Feb 10; doi: 10.1089/neu.2010.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medicine MTBICACoR. Definition of Mild Traumatic Brain Injury. J Head Trauma Rehabil 1993. 1993;8(3):86–87. [Google Scholar]
- 36.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34(1–4):45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 38.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005 Sep 28;294(12):1511–1518. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 39.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001 May 5;357(9266):1391–1396. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 40.Papa L, Diaz L, Akinyi L, et al. The association between levels of a novel serum biomarker and severity of injury in patients with mild and moderate traumatic brain injury [Abstract] Acad Emerg Med. 2009 Apr;16(4 (Suppl.1)) [Google Scholar]
- 41.Johnston SC, Riddle SM, Cohen RE, Hill CP. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J. 1999 Jul 15;18(14):3877–3887. doi: 10.1093/emboj/18.14.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]


