Abstract
Aim of the study
To evaluate the usefulness of ultrasonographic acromion-greater tuberosity distance measurement and Shoulder ratio in detecting post-stroke inferior shoulder subluxation.
Material and methods
Forty-five hemiplegic stroke patients and 45 controls underwent shoulder sonography to measure their acromion-greater tuberosity distance. Side-to-side acromion-greater tuberosity distance differences and Shoulder ratios were derived from the acromion-greater tuberosity distance values. The long head of biceps tendon, subscapularis tendon, supraspinatus tendon, and the infraspinatus tendon were also evaluated to exclude full thickness tendon tears. Data were analyzed using the Statistical Package for Social Sciences version 20.0 for windows. Normality of data distribution was checked using the Kolmogorov–Smirnov test. Mann–Whitney U test and Chi-square tests were utilized.
Results
Hemiplegic and control shoulders’ acromion-greater tuberosity distance values were 2.8 ± 0.6 cm and 2.4 ± 0.4 cm, respectively (p = 0.001). Hemiplegic and control shoulder ratios were 1.3 ± 0.3 and 1.1 ± 0.1, respectively; p < 0.001. Point biserial correlation showed that the presence of subluxation correlated moderately with higher shoulder ratios in all the hemiplegics (rpb = 0.520; p < 0.001).
Conclusion
Our results suggest that acromion-greater tuberosity distance measurement is useful for detecting inferior shoulder subluxation. Shoulder ratio may be of complementary or supplemental value to acromion-greater tuberosity distance difference.
Keywords: stroke, hemiplegia, shoulder subluxation, ultrasonography, acromion-greater tuberosity distance
Introduction
Hemiplegia is a devastating/debilitating complication of stroke. The hemiplegic limb usually progresses through the stages of flaccidity, spasticity and synergy, but these stages may also occur simultaneously in the affected limb(1). Glenohumeral subluxation (GHS) most commonly occurs in the flaccid stage – a stage marked by areflexia, atonia, and the loss of volitional activity(1).
GHS has been variably defined as “increased translation of the humeral head relative to the glenoid fossa”(2) or as “each non-traumatic, partial or total change of relationship between the scapula and the humerus in all directions and in all planes, as compared with the non-affected shoulder, that appeared after stroke”(3) or as “a partial or incomplete dislocation that usually stems from changes in the mechanical integrity of the joint” (4). Inferior, superior, anterior, and posterior types of GHS have been described(3,4).
When present, early and accurate detection/treatment of GHS is crucial to the rehabilitation of the affected limb. Neglected GHS may become irremediable in the long term. Furthermore, the presence of GHS often constitutes a stumbling block to rehabilitation because it impairs normal shoulder function, prolongs hospital stay, and has adverse psychological effects(5). GHS has also been implicated as a contributory factor to hemiplegic shoulder pain(3).
Finger-breadth palpation, anthropometry with caliper/tape measure, anthropometry with thermoplastic jig, and plain radiography (qualitative and quantitative methods) are established methods of diagnosing GHS(3,6). However, these methods have significant limitations including suboptimal capacity for early detection and use of ionizing radiation(6,7).
The use of shoulder ultrasound (USS) for detecting GHS in post-stroke hemiplegic shoulders may be a way to overcome these limitations. Ultrasonographic measurement of acromion-greater tuberosity distance (AGTD) has been employed for assessing post-stroke inferior GHS as it is a fairly good reflection of the changes described in the first definition of subluxation above(6,8–12).
The purpose of this study is to evaluate the usefulness of AGTD and shoulder ratio for detecting subluxation in post-stroke hemiplegic shoulders.
Material and methods
This was a prospective, case-control study approved by the Ethics and Research Committee of our institution. Conscious, first-time stroke patients with hemiplegia were included in the study. Patients with previous or current shoulder trauma, cervical disk disease, glenohumeral osteoarthritis, inflammatory arthritis, adhesive capsulitis, and full-thickness rotator cuff tears were excluded. Forty-five hemiplegic subjects and 45 age- and sex-matched controls were enrolled.
The unaffected/contralateral/non-hemiplegic upper limbs of the stroke patients served as the primary control while age- and sex-matched, asymptomatic, non-hemiplegic volunteers aged ≥40 years served as secondary controls. The secondary controls were without previous history of shoulder trauma. Informed consent was obtained from all the participants.
Stroke was diagnosed based on clinical and neuroradiological findings. Data regarding age, gender, hemiplegic side, time from the onset of stroke (stroke duration), and stroke type (ischemic or hemorrhagic) were collected.
The motor status of the shoulder girdle was assessed using the Medical Research Council (MRC) scale as follows(6):
Grade 5 – Normal power, moves against full resistance;
Grade 4 – Reduced power but can move against gravity and resistance;
Grade 3 – Active movement against gravity;
Grade 2 – Active movement with gravity eliminated;
Grade 1 – Flicker or trace of contraction/movement;
Grade 0 – No contraction/movement.
Using the MRC scores, the hemiplegic shoulders were grouped into poor motor status (score = 0–2) and good motor status (score = 3–5).
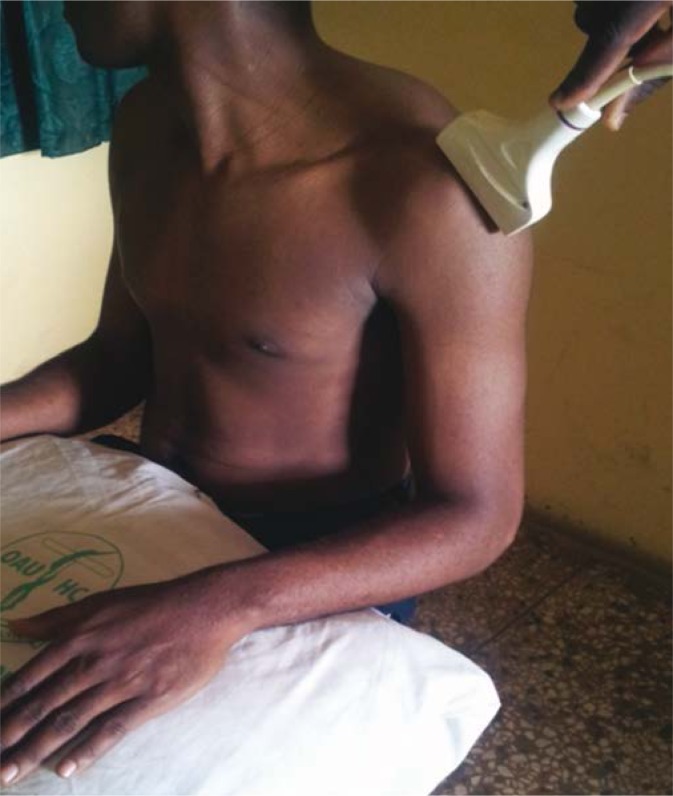
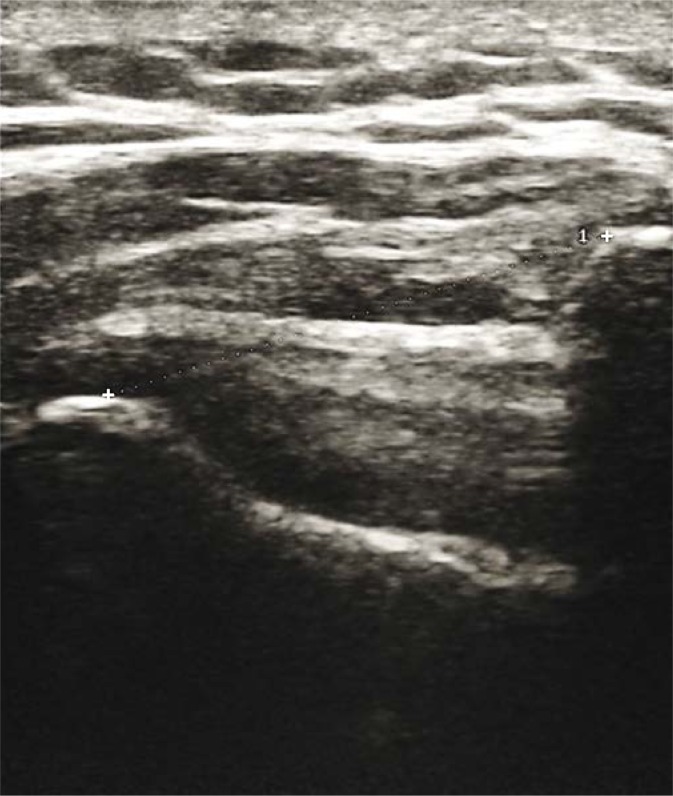
Shoulder sonography was performed prior to commencement of physical rehabilitation; within 3 weeks of stroke with hemiplegia. Shoulder sonography for subluxation was performed by the first author (who was blinded to the subjects’ clinical status and has 3 years’ experience with shoulder sonography) with a 7–12 MHz transducer of MINDRAY® ultrasound machine DC-7 (Shenzhen Mindray Bio-medical Electronics, Nanshan, Shenzhen, China) using the protocol described by Kumar et al.(10) The shoulder subluxation distance was measured by determining the Acromion-Greater Tuberosity Distance (AGTD) (6–11). The subject was seated with their hips and knees flexed to 90° and feet resting flat on the ground. The shoulder was in neutral rotation, with the elbow at 90° of flexion and forearm in pronation. The forearms were rested on a pillow (placed on the patient’s lap), but the elbow itself remained unsupported to ensure that the shoulder girdle was not elevated (Fig 1)(10). Once in this position, the lateral border of the acromion was palpated, and the ultrasonographic transducer head placed over the acromion along the vertical/longitudinal axis of the humerus to scan the shoulder. These two bony reference points were then identified on the frozen image, and the AGTD measured from the lateral edge of the acromion process of scapula to the nearest margin of the superior part of greater tuberosity of the humerus (Fig 2)(10). A dark linear acoustic shadow beneath the acromion helped to identify its lateral edge. The hyperechoic appearance of the supraspinatus tendon at its insertion site helped in identifying the greater tuberosity(10). Sonographic measurements were obtained once. AGTD for shoulder subluxation was interpreted relative to the contralateral shoulder. The previously reported range of AGTD difference between normal shoulders is 0 – 0.36 cm(13). Therefore, AGTD difference between shoulders of >0.4 cm was taken to indicate presence of subluxation(13).
Fig. 1.
Patient and probe positioning for measuring the Acromion – Greater Tuberosity Distance (AGTD)
Fig. 2.
Corresponding sonographic image showing the Acromion – greater tuberosity distance (AGTD) measurement (between cursors)
Shoulder subluxation ratio was also determined as the ratio of the AGTD measurement in the hemiplegic shoulder divided by that of the unaffected shoulder of the stroke patients(6). In the controls, the corresponding ratio was obtained by dividing the larger AGTD measurement by the smaller.
The long head of biceps tendon (LHBT), subscapularis (SCT) tendon, supraspinatus (SST) tendon, and the infraspinatus tendon (IST) were also evaluated to exclude full thickness tendon tears.
Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) software, version 20.0 for windows was used for analysis. Normality of data distribution was checked using the Kolmogorov-Smirnov test. Scheffe post-hoc analysis was used to detect intergroup differences. Point biserial correlation was used to assess the relationship between shoulder ratio and the presence of subluxation. The intrarater reliability of ultrasonographic measurements of AGTD was assessed using intraclass correlation coefficients (ICC) with 95% confidence intervals. The standard error of measurement (SEM) was further used to define the 95% confidence limits of each measurement. A p value <0.05 was considered statistically significant.
Results
The mean age of the hemiplegics was 62.0 ± 11.3 years while that of controls was 65.8 ± 11.3 years, p = 0.115. There were 24 male and 21 female hemiplegics and the same gender proportion in controls.
Thirty-nine (86.7%) hemiplegics had ischemic stroke while 6 (13.3%) had hemorrhagic stroke. Twenty – six (57.8%) hemiplegics had right shoulder hemiplegia while 19 (42.2%) had left shoulder hemiplegia. AGTD and Shoulder ratio showed normal distribution but side-to-side AGTD difference was not normally distributed.
AGTD
The hemiplegic shoulders of the stroke patients had significantly greater (p = 0.001) mean AGTD than all the shoulders of controls (Tab. 1 and 2). Similarly, the hemiplegic shoulder AGTD of 2.8 ± 0.6 cm was significantly greater than the AGTD of the unaffected/contralateral side 2.3 ± 0.4 cm in the stroke patients (p < 0.001). Furthermore, hemiplegics with shoulder subluxation had statistically greater AGTD of 3.0 ± 0.8 than hemiplegics without subluxation (2.3 ± 0.5 cm); p = 0.003; (Tab. 3).
Tab. 1.
Comparison of AGTD, AGTD difference, and Shoulder ratio of Hemiplegics and Controls
| Variables | Hemiplegics (n = 45) | Controls (n = 45) | t | P value |
|---|---|---|---|---|
| Hemiplegic vs. Controls AGTD (cm) | 2.8 ± 0.6 | 2.4 ± 0.4 | 3.381 | 0.001 |
| Hemiplegic vs. Controls AGTD Difference (cm) | 0.6 ± 0.6 | 0.2 ± 0.2 | 4.333 | <0.001 |
| Hemiplegic vs. Controls Shoulder ratio | 1.3 ± 0.3 | 1.1 ± 0.1 | 3.992 | <0.001 |
Tab. 2.
Comparison of the AGTDs of Hemiplegic shoulders, Contralateral/Unaffected shoulders, and the bilateral shoulders of Controls
| Variables | N | AGTD (cm) [Mean (SD)] | F | df | P value |
|---|---|---|---|---|---|
| Hemiplegic side | 45 | 2.8 ± 0.6 | 9.368 | 3. 176 | <0.001 |
| Unaffected side | 45 | 2.3 ± 0.4 | |||
| Right Shoulders of Controls | 45 | 2.5 ± 0.5 | |||
| Left Shoulders of Controls | 45 | 2.4 ± 0.4 | |||
| Scheffe post-hoc analysis for intergroup differences | |||||
| Group | P value | ||||
| Hemiplegic vs. Unaffected side | <0.001 | ||||
| Hemiplegic vs. Right Control | 0.031 | ||||
| Hemiplegic vs. Left control | 0.004 | ||||
| Unaffected side vs. Right Controls | 0.216 | ||||
| Unaffected side vs. Left Controls | 0.561 | ||||
| Right Control vs. Left Controls | 0.925 | ||||
Tab. 3.
Comparison of AGTD, AGTD difference, and Shoulder ratio of hemiplegics and hemiplegics without subluxation
| Variables | Shoulder subluxation | t | df | P value | |
|---|---|---|---|---|---|
| Present (n = 20) | Absent (n = 25) | ||||
| Hemiplegic AGTD (cm) | 3.0 ± 0.8 | 2.3 ± 0.5 | 3.211 | 43 | 0.003 |
| AGTD difference (cm) | 1.1 ± 0.8 | 0.4 ± 0.2 | 6.486 | 43 | 0.000* |
| Shoulder ratio | 1.4 ± 0.5 | 1.00 ± 0.0 | 3.991 | 43 | 0.000 |
Mann-Whitney U test applied
There was no statistically significant difference in the mean AGTD of the unaffected/contralateral shoulders of hemiplegics (2.3 ± 0.4 cm), the right shoulders of controls
(2.5 ± 0.5 cm) and the left shoulders of controls (2.4 ± 0.4 cm); p = 0.062; (Tab. 2). No statistically significant difference was detected between the mean AGTD of hemiplegics with poor motor status (2.6 ± 0.7 cm) and those with good motor status (2.6 ± 0.7 cm); p > 0.99. In addition, there were no statistically significant differences in the mean AGTD of the hemiplegic shoulders across the different power grades on the MRC scale. There were no statistically significant differences between the mean AGTD of ischemic stroke (2.6 ± 0.7 cm) and hemorrhagic stroke (2.3 ± 0.8 cm), p = 0.335; as well as dominant shoulder hemiplegia (2.6 ± 0.7 cm) versus non-dominant shoulder hemiplegia (2.6 ± 0.8 cm), p = 0.934.
Side-to-Side AGTD Difference
The mean side-to-side AGTD difference in the hemiplegics was significantly greater (p < 0.001) than that of controls (Tab. 1). Hemiplegics with subluxation had statistically greater (p < 0.001) mean side-to-side AGTD difference than those without subluxation (Tab. 3). No statistically significant difference was detected between the mean side-to-side AGTD difference of hemiplegics with poor motor status (0.53 cm) and those with good motor status (0.50 cm); p = 0.891. Furthermore, one-way ANOVA for comparing means showed no significant differences in mean side-to-side AGTD difference values across the different power grades of the MRC scale.
Shoulder Ratio
The hemiplegic shoulders also had significantly greater (p < 0.001) shoulder ratio than the control shoulders (Tab. 1). Furthermore, subluxed hemiplegic shoulders had significantly greater (p < 0.001) mean shoulder ratio than non-subluxed hemiplegic shoulders (Tab. 3). Further statistical analysis of differences in shoulder ratios among various subgroups is shown in Tab. 4. The mean shoulder ratio was 1.3 ± 0.5 in hemiplegics with poor motor status and 1.1 ± 0.4 in those with good motor status; (p = 0.281).
Tab. 4.
Differences in Shoulder ratios of Hemiplegics and Controls by gender
| Variables | Shoulder ratio [Mean (SD)] | t | df | P value |
|---|---|---|---|---|
| Male Controls | 1.1 ± 0.1 | -0.642 | 43 | 0.524 |
| Female Controls | 1.1 ± 0.1 | |||
| Male Hemiplegics | 1.2 ± 0.2 | 2.422 | 46 | 0.019 |
| Male Controls | 1.1 ± 0.1 | |||
| Female Hemiplegics | 1.4 ± 0.4 | 3.401 | 40 | 0.002 |
| Female Controls | 1.1 ± 0.1 |
One-way ANOVA did not detect any statistically significant differences in the mean shoulder ratio across the different power grades of the MRC scale. Point biserial correlation showed that the presence of subluxation correlated moderately with higher shoulder ratios in all the hemiplegics (rpb = 0.520; p < 0.001).
The Intraclass Correlation Coefficients (ICC values) for intrarater reliability were 0.78, 0.80, and 0.86 for the affected shoulders, unaffected shoulders, and the shoulders of controls, respectively. The standard error of measurement (SEM) for AGTD measurements was < 0.2 cm for all the three categories of shoulders.
Discussion
Inferior shoulder subluxation, which is also unknown as “drooping shoulder”(14,15) is relatively common in post-stroke hemiplegic shoulders. Besides hemiplegia, other recognized causes of inferior subluxation include: fracture of the humeral surgical neck with axillary nerve damage, tumor (Pancoast tumor) infiltration of the brachial plexus, glenohumeral septic arthritis, shoulder replacement surgery, and hemarthrosis (secondary to hemophilia or trauma)(14,15).
None of these potentially confounding factors was present in our study population.
Twenty (44.4%) of the hemiplegic shoulders were subluxed. This is higher than the 25.3% and 37% reported by Pop (in Poland)(16) and Suethanapornkul et al.(17) (in Thailand), respectively, but lower than the 58% reported by Kumar et al.(7) (in the United Kingdom). However, it lies well within the often quoted range of 17–81%(18). These differences are likely due to the fact that inferior shoulder subluxation mainly affects acute hemiplegic shoulders(1), which suggests that research with a higher proportion of acute hemiplegic shoulders in its sample size is likely to record higher prevalence of subluxation and vice versa.
The arm position significantly affects the measured value of AGTD(18). A pillow-supported forearm position of Kumar et al.(7,10) was adopted in this study over the gravity-dependent free-hanging position of Park et al.(6) (which reportedly could further worsen soft tissue injuries in the hemiplegic shoulders). Our results show hemiplegic side mean AGTD value of 2.8 ± 0.6 cm compared to 3.15 ± 0.69 cm reported by Park et al.(6) using the free hanging position. Kumar et al.(7,10) reported hemiplegic side mean AGTD values of 2.2 ± 0.6 cm(7) and 2.3 ± 0.6 cm10 in two separate studies. Our results and those of Kumar et al.(7,10) are significantly lower than that of Park et al.(6) This significant disparity may lend credence to the observation by Kumar et al.(10) that the forearm supported position may be preferable for detecting inferior subluxation, especially in stroke patients.
Apart from arm position during measurement, AGTD is also reportedly reduced when there are proliferative changes within the greater tubercle or the acromion process and in shoulders with full thickness rotator cuff tear (FTRCT)(13). However, no case of FTRCT was seen in our study population.
The mean shoulder ratios in the hemiplegic subjects and controls were 1.3 ± 0.3 and 1.1 ± 0.1, respectively. Further analysis showed that hemiplegics with subluxation had shoulder ratio of 1.4 ± 0.5 while those without subluxation had mean shoulder ratio of 1.0 ± 0.0. These results are lower than the shoulder ratio in hemiplegics of 1.45 ± 0.28 reported by Park et al.(6) Since shoulder ratio is a derivative of AGTD, their higher shoulder ratio value could have been due to the fact that they used the free hanging arm position for AGTD measurement. Other researchers that were reviewed did not explore the value of shoulder ratio in their studies.
The point biserial correlation is a standardized measure of the strength of a relationship between two variables when one of the two variables is dichotomous. A point biserial correlation (rpb) analysis between subluxation (dichotomous variable measured as present or absent) and shoulder ratio (metric continuous) showed a significant relationship between the two variables.
Suethenapornkul et al.(17) reported that shoulder subluxation was significantly associated with hemorrhagic type of stroke. Such an association was not observed in our study. The reason for this disparity is not immediately evident.
Ultrasonography is a relatively established tool for assessing post-stroke hemiplegic shoulders(19). Furthermore, it has important comparative advantages in evaluating shoulder subluxation(6,7). These include possibility of serial measurement without exposure to ionizing radiation; the fact that it can be done at the bedside with a portable scanner, it can measure distances directly without the need to correct for radiographic magnification, it can diagnose concurrent soft tissue injuries; sensitivity to small changes, and the fact that it can be used to monitor interventions for GHS.
The limitations of this study include a relatively small sample size (though the number used was obtained from calculation using the prevalence of stroke in our environment) and non-evaluation of anterior and posterior components of subluxation (though the inferior subluxation evaluated seems to be the commonest variant).
In conclusion, ultrasonographic AGTD measurement is a recommended tool for detecting inferior shoulder subluxation in post-stroke hemiplegic shoulders. Shoulder ratio may be a complementary tool to AGTD difference but this requires further evaluation and validation. Shoulder ultrasound would also be potentially of use in the follow-up of hemiplegic patients’ response to treatment/rehabilitation of the dislocated shoulder.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Gould R, Barnes SS. Shoulder pain in hemiplegia. Available from: http://emedicine.medscape.com/article/328793-overview.
- 2.Huang SW, Liu SY, Tang HW, Wei TS, Wang WT, Yang CP. Relationship between severity of shoulder subluxation and soft-tissue injury in hemiplegic stroke patients. J Rehabil Med. 2012;44:733–739. doi: 10.2340/16501977-1026. [DOI] [PubMed] [Google Scholar]
- 3.Paci M, Nannetti N, Rinaldi LA. Glenohumeral subluxation in hemiplegia: an overview. J Rehabil Res Dev. 2005;42:557–568. doi: 10.1682/jrrd.2004.08.0112. [DOI] [PubMed] [Google Scholar]
- 4.Moreels B, Walker W, Beckers J, Buxton S, O’Reilly N. Shoulder subluxation. Available from: http://www.physio-pedia.com/Shoulder_subluxation.
- 5.Kumar P, Kassam J, Denton C, Taylor E, Chatterley A. Risk factors for inferior shoulder subluxation in patients with stroke. Phys Ther Rev. 2010;15:3–11. [Google Scholar]
- 6.Park GY, Kim JM, Sohn SI, Shin IH, Lee MY. Ultrasonographic measurement of shoulder subluxation in patients with post-stroke hemiplegia. J Rehabil Med. 2007;39:526–530. doi: 10.2340/16501977-0099. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Mardon M, Bradley M, Gray S, Swinkels A. Assessment of glenohumeral subluxation in poststroke hemiplegia: Comparison between ultrasound and fingerbreadth palpation methods. Phys Ther. 2014;94:1622–1631. doi: 10.2522/ptj.20130303. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Bradley M, Swinkels A. Within-day and day-to-day intra-rater reliability of ultrasonographic measurements of acromion-greater tuberosity distance in healthy people. Physiother Theory Pract. 2010;26:347–351. doi: 10.3109/09593980903059522. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P, Chetwynd J, Evans A, Wardle G, Crick C, Richardson B. Interrater and intrarater reliability of ultrasonographic measurements of acromion-greater tuberosity distance in healthy people. Physiother Theory Pract. 2011;27:172–175. doi: 10.3109/09593985.2010.481012. [DOI] [PubMed] [Google Scholar]
- 10.Kumar P, Bradley M, Gray S, Swinkels A. Reliability and validity of ultrasonographic measurements of acromion-greater tuberosity distance in poststroke hemiplegia. Arch Phys Med Rehabil. 2011;92:731–736. doi: 10.1016/j.apmr.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Kumar P, Cruziah R, Bradley M, Gray S, Swinkels A. Intra-rater and inter-rater reliability of ultrasonographic measurements of acromion-greater tuberosity distance in patients with post-stroke hemiplegia. Top Stroke Rehabil. 2016;23:147–153. doi: 10.1080/10749357.2015.1120455. [DOI] [PubMed] [Google Scholar]
- 12.Duerr M. Reliability and accuracy of distance measurements between shoulder bony landmarks evaluated by ultrasound in asymptomatic subjects. Auckland, New Zealand: Auckland University of Technology; 2010. [Master of Philosophy Thesis] [Google Scholar]
- 13.Cholewinski JJ, Kusz DJ, Wojciechowski P, Cielinski LS, Zoladz MP. Ultrasound measurement of rotator cuff thickness and acromiohumeral distance in the diagnosis of subacromial impingement syndrome of the shoulder. Knee Surg Sports Traumatol Arthrosc. 2008;16:408–414. doi: 10.1007/s00167-007-0443-4. [DOI] [PubMed] [Google Scholar]
- 14.Lev-Toaff AS, Karasick D, Rao VM. “Drooping shoulder” – nontraumatic causes of glenohumeral subluxation. Skeletal Radiol. 1984;12:34–36. doi: 10.1007/BF00373173. [DOI] [PubMed] [Google Scholar]
- 15.Resnik CS. Septic arthritis: a rare cause of drooping shoulder. Skeletal Radiol. 1992;21:307–309. doi: 10.1007/BF00241770. [DOI] [PubMed] [Google Scholar]
- 16.Pop T. Subluxation of the shoulder joint in stroke patients and the influence of selected factors on the incidence of instability. Ortop Traumatol Rehabil. 2013;15:259–267. doi: 10.5604/15093492.1058421. [DOI] [PubMed] [Google Scholar]
- 17.Suethanapornkul S, Kuptniratsaikul PS, Kuptniratsaikul V, Uthensut P, Dajpratha P, Wongwisethkarn J. Post stroke shoulder subluxation and shoulder pain: a cohort multicenter study. J Med Assoc Thai. 2008;91:1885–1892. [PubMed] [Google Scholar]
- 18.Kumar P, Bourke C, Flanders J, Gorman T, Patel H. The effect of arm position on the ultrasonographic measurements of the acromion-greater tuberosity distance. Physiother Theory Pract. 2014;30:171–177. doi: 10.3109/09593985.2013.834490. [DOI] [PubMed] [Google Scholar]
- 19.Idowu BM, Ayoola OO, Adetiloye VA, Komolafe MA. Sonographic evaluation of structural changes in post-stroke hemiplegic shoulders. Pol J Radiol. 2017;82:141–148. doi: 10.12659/PJR.899684. [DOI] [PMC free article] [PubMed] [Google Scholar]