Abstract
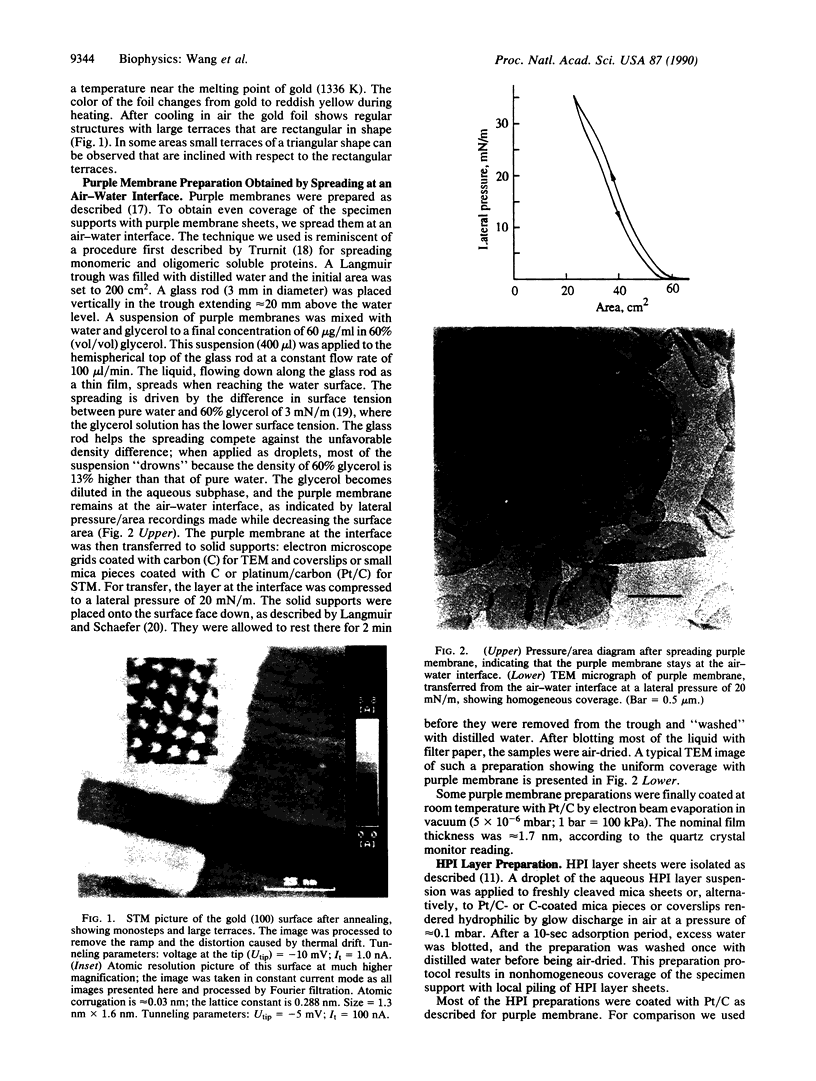
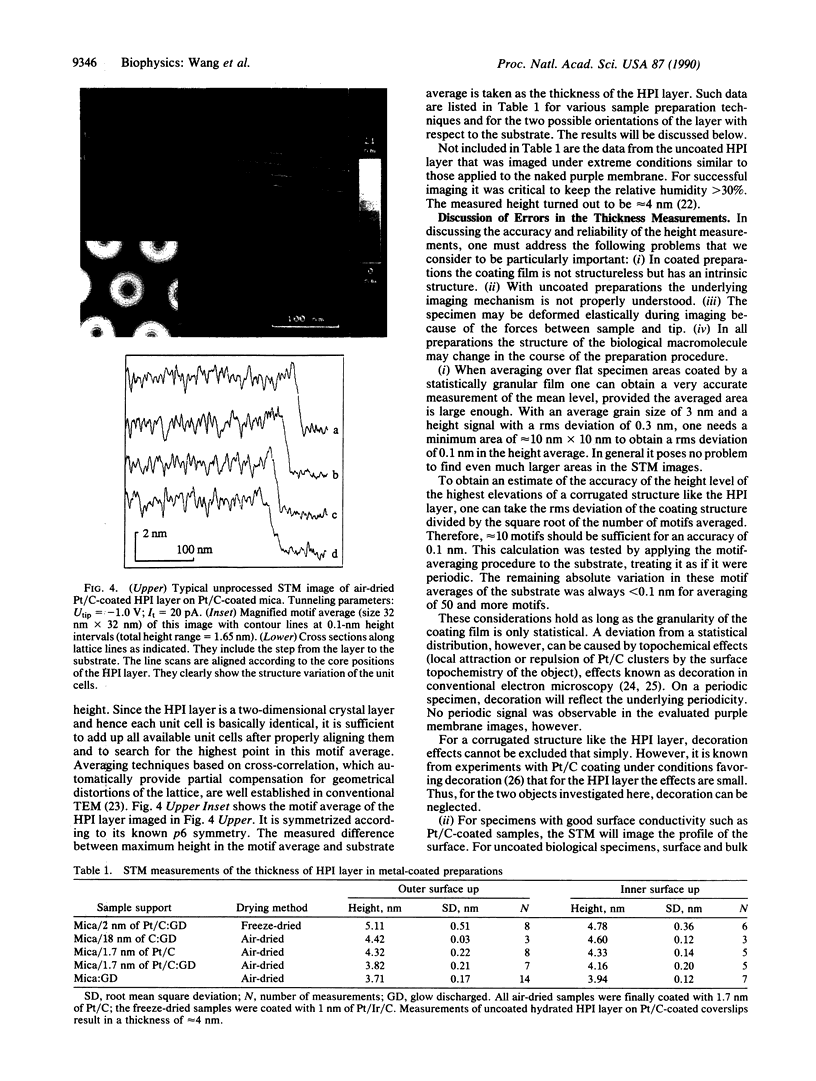
A single-tube scanning tunneling microscope has been zeta-calibrated by using atomic steps of crystalline gold and was used for measuring the thickness of two biological samples, metal-coated as well as uncoated. The hexagonal surface layer of the bacterium Deinococcus radiodurans with an open network-type structure shows thickness values that are strongly influenced by the substrate and the preparation method. In contrast, the thickness of the purple membrane of Halobacterium halobium with its densely packed less-corrugated structure exhibits very little variation in thickness in coated preparations and the values obtained are in good agreement with x-ray data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein M., Stasiak A., Gross H., Stoll E., Travaglini G. Scanning tunneling microscopy of recA-DNA complexes coated with a conducting film. Science. 1988 Apr 22;240(4851):514–516. doi: 10.1126/science.3358130. [DOI] [PubMed] [Google Scholar]
- Bachmann L., Weinkauf S., Baumeister W., Wildhaber I., Bacher A. Electron microscopy of subnanometer surface features on metal-decorated protein crystals. J Mol Biol. 1989 Jun 5;207(3):575–584. doi: 10.1016/0022-2836(89)90466-x. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Barth M., Hegerl R., Guckenberger R., Hahn M., Saxton W. O. Three-dimensional structure of the regular surface layer (HPI layer) of Deinococcus radiodurans. J Mol Biol. 1986 Jan 20;187(2):241–250. doi: 10.1016/0022-2836(86)90231-7. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Guckenberger R., Engelhardt H., Woodcock C. L. Metal shadowing and decoration in electron microscopy of biological macromolecules. Ann N Y Acad Sci. 1986;483:57–76. doi: 10.1111/j.1749-6632.1986.tb34497.x. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Karrenberg F., Rachel R., Engel A., ten Heggeler B., Saxton W. O. The major cell envelope protein of Micrococcus radiodurans (R1). Structural and chemical characterization. Eur J Biochem. 1982 Jul;125(3):535–544. doi: 10.1111/j.1432-1033.1982.tb06715.x. [DOI] [PubMed] [Google Scholar]
- Guckenberger R., Kösslinger C., Gatz R., Breu H., Levai N., Baumeister W. A scanning tunneling microscope (STM) for biological applications: design and performance. Ultramicroscopy. 1988;25(2):111–121. doi: 10.1016/0304-3991(88)90218-5. [DOI] [PubMed] [Google Scholar]
- Hallmark VM, Chiang S, Rabolt JF, Swalen JD, Wilson RJ. Observation of atomic corrugation on Au(111) by scanning tunneling microscopy. Phys Rev Lett. 1987 Dec 21;59(25):2879–2882. doi: 10.1103/PhysRevLett.59.2879. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Jésior J. C., Wade R. H. Electron-irradiation-induced flattening of negatively stained 2D protein crystals. Ultramicroscopy. 1987;21(4):313–319. doi: 10.1016/0304-3991(87)90029-5. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Downing K. H. Two-dimensional structure of plant light-harvesting complex at 3.7 A [corrected] resolution by electron crystallography. J Mol Biol. 1989 Jun 20;207(4):823–828. doi: 10.1016/0022-2836(89)90247-7. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Sass H. J., Büldt G., Beckmann E., Zemlin F., van Heel M., Zeitler E., Rosenbusch J. P., Dorset D. L., Massalski A. Densely packed beta-structure at the protein-lipid interface of porin is revealed by high-resolution cryo-electron microscopy. J Mol Biol. 1989 Sep 5;209(1):171–175. doi: 10.1016/0022-2836(89)90180-0. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]







