Abstract
The acquisition and assimilation of inorganic C have been investigated in several of the 15 clades of the Ochrophyta other than diatoms, with biochemical, physiological and genomic data indicating significant mechanistic variation. Form ID Rubiscos in the Ochrophyta are characterized by a broad range of kinetics values. In spite of relatively high K0.5CO2 and low CO2 : O2 selectivity, diffusive entry of CO2 occurs in the Chrysophyceae and Synurophyceae. Eustigmatophyceae and Phaeophyceae, on the contrary, have CO2 concentrating mechanisms, usually involving the direct or indirect use of 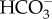 . This variability is possibly due to the ecological contexts of the organism. In brown algae, C fixation generally takes place through a classical C3 metabolism, but there are some hints of the occurrence of C4 metabolism and low amplitude CAM in a few members of the Fucales. Genomic data show the presence of a number of potential C4 and CAM genes in Ochrophyta other than diatoms, but the other core functions of many of these genes give a very limited diagnostic value to their presence and are insufficient to conclude that C4 photosynthesis is present in these algae.
. This variability is possibly due to the ecological contexts of the organism. In brown algae, C fixation generally takes place through a classical C3 metabolism, but there are some hints of the occurrence of C4 metabolism and low amplitude CAM in a few members of the Fucales. Genomic data show the presence of a number of potential C4 and CAM genes in Ochrophyta other than diatoms, but the other core functions of many of these genes give a very limited diagnostic value to their presence and are insufficient to conclude that C4 photosynthesis is present in these algae.
This article is part of the themed issue ‘The peculiar carbon metabolism in diatoms'.
Keywords: CO2 concentrating mechanism, Rubisco, inorganic carbon, photosynthesis, brown algae, diffusive CO2 entry
1. Introduction
Stramenopile eukaryotes comprise a diverse clade with a great range of nutritional modes [1] (table 1). Ochrophyta (i.e. Ochrista) is a diverse phylum of stramenopiles; most of the ochrophytan clades (16 in [5]) are photosynthetic or have photosynthetic members. The rest of the stramenopiles, the ecologically and economically very significant oomycetes and some others clades (a total of 8 in [2]), are all non-photosynthetic. The phylogenetic relations among these organisms are discussed by Baurain et al. [6], Brown & Sorhannus [7], Beakes et al. [2], Schmidt et al. [8] and Yang et al. [5].
Table 1.
Examples of modes of nutrition in the Ochrophyta. See Raven et al. [1] for definition of modes of nutrition.
| nutritional mode | example | references |
|---|---|---|
| free-living osmoorganotrophy | most Saprolegniales, e.g. Saprolegnia (oomycetes) | [2] |
| symbiotic (all parasitic)a osmoorganotrophy | perenosporalean clades, e.g. Phytophthorab (oomycetes); bicoecids; Cafeteria | [2] |
| phagoorganotrophy | some Chrysophyceae, e.g. Paraphysomonas | [2] |
| photolithotrophy | examples: Bacillariophyceae, Eustigmatophyceae, Phaeophyceae, Synurophyceae | [3] |
| phagophotomixotrophy | many Chrysophyceae | [3,4] |
aSymbiosis is used in the broad sense, including mutualism and parasitism.
bMajor parasite of flowering plants.
The Bacillariophyceae is the best-investigated class of ochrophytes with respect to inorganic C acquisition and assimilation, showing the widespread occurrence of CO2 concentrating mechanisms (CCMs) and of pyrenoids, energized transport of 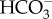 and limited support for C4 or C4-like photosynthetic metabolism [9–12]. The emphasis on diatoms is reasonable in view of their phylogenetic and ecological diversity [13] and their contribution to global biogeochemical cycles (e.g. a marine diatom net primary productivity of about 20 Pg C per year [14]). However, there are 15 (possibly more) other ochrophyte classes with photosynthetic members [5–8,15]. The benthic marine macroalgal Phaeophyceae play a major role in primary productivity and as ecosystem engineers on rocky shores, especially in temperate and polar regions, with several species exceeding 10 m in length [16]. The Chrysophyceae and Synurophyceae are significant in the phytoplankton of acidic and often CO2-enriched freshwaters [3,17–19], the microalga Nannochloropsis (Eustigmatophyceae) is being assessed for its biotechnological potential [20–22] and members of the Pelagophyceae and Raphidophyceae can form harmful algal blooms [23]. Here we synthesize what is known about inorganic carbon acquisition and assimilation in these non-diatom ochrophytes for comparison with what is known for diatoms.
and limited support for C4 or C4-like photosynthetic metabolism [9–12]. The emphasis on diatoms is reasonable in view of their phylogenetic and ecological diversity [13] and their contribution to global biogeochemical cycles (e.g. a marine diatom net primary productivity of about 20 Pg C per year [14]). However, there are 15 (possibly more) other ochrophyte classes with photosynthetic members [5–8,15]. The benthic marine macroalgal Phaeophyceae play a major role in primary productivity and as ecosystem engineers on rocky shores, especially in temperate and polar regions, with several species exceeding 10 m in length [16]. The Chrysophyceae and Synurophyceae are significant in the phytoplankton of acidic and often CO2-enriched freshwaters [3,17–19], the microalga Nannochloropsis (Eustigmatophyceae) is being assessed for its biotechnological potential [20–22] and members of the Pelagophyceae and Raphidophyceae can form harmful algal blooms [23]. Here we synthesize what is known about inorganic carbon acquisition and assimilation in these non-diatom ochrophytes for comparison with what is known for diatoms.
2. Mechanisms of inorganic C influx: CO2 diffusion and biophysical CO2 concentrating mechanisms
Diffusive CO2 entry from the bulk phase to ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) is driven by a concentration difference produced by the photosynthetic consumption of CO2 that produces a lower CO2 concentration in the proximity of Rubisco active sites than in the bulk phase supplying the CO2. Here the energization of the flux is indirect, coming from the use of absorbed photons for the reduction of NADP+ to NADPH and the phosphorylation of ADP to ATP; the NADPH and ATP are then used in CO2 assimilation producing, in the first instance, carbohydrate. Under these circumstances, the CO2 concentration and the CO2 : O2 ratio at the Rubisco site typically allow significant Rubisco oxygenase activity and a corresponding operation of a photorespiratory C oxidation cycle that converts the 2-P-glycolate from Rubisco oxygenase into triose phosphate, with input of energy, directly or indirectly, from the thylakoid reactions of photosynthesis [24].
The functioning of CCMs, by definition, involves a higher steady state concentration of CO2 at the site of Rubisco than occurs in the bulk medium. This transport of CO2 against the free energy (concentration) gradient is energized independently of the energy input to the conversion of CO2 to carbohydrate. This is the case for both the biophysical (energized, uphill transport of 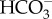 or CO2 or H+ across a cell membrane) and the biochemical (C4 photosynthesis or Crassulacean acid metabolism, CAM) CCMs. This parallel input of energy comes ultimately from the thylakoid reactions of photosynthesis, but sometimes indirectly via photosynthate production and its catabolism through glycolysis and the mitochondrial reactions [24]. Generally, despite the occurrence of CCMs, the CO2 : O2 ratio at the Rubisco active site is not higher enough to prevent a small flux of organic C through Rubisco oxygenase and a photorespiratory C oxidation cycle that adds to the energy cost [25], as does the inevitable leakage of some of the CO2 accumulated at the Rubisco site [24,26,27].
or CO2 or H+ across a cell membrane) and the biochemical (C4 photosynthesis or Crassulacean acid metabolism, CAM) CCMs. This parallel input of energy comes ultimately from the thylakoid reactions of photosynthesis, but sometimes indirectly via photosynthate production and its catabolism through glycolysis and the mitochondrial reactions [24]. Generally, despite the occurrence of CCMs, the CO2 : O2 ratio at the Rubisco active site is not higher enough to prevent a small flux of organic C through Rubisco oxygenase and a photorespiratory C oxidation cycle that adds to the energy cost [25], as does the inevitable leakage of some of the CO2 accumulated at the Rubisco site [24,26,27].
3. Inorganic C acquisition in Ochrophyta other than diatoms
(a). Introduction
There is substantial physiological evidence on the mechanism of inorganic carbon acquisition for a number of classes of the Ochrophyta, i.e. Chrysophyceae, Eustigmatophyceae, Phaeophyceae and Synurophyceae, with a little information available for a few other classes; genomic data are also available for the Eustigmatophyceae and the Phaeophyceae. We also discuss the occurrence of pyrenoids, showing that pyrenoids are not always required for stramenopile CCMs [9,28], and there can be pyrenoids but no obvious CCM activity [3].
A common rationale for the occurrence of diffusive CO2 entry or of a CCM is the natural external CO2 and O2 concentrations and the kinetics and intracellular content of Rubisco. There is considerable variation in the kinetics, e.g. K0.5 for CO2, K0.5 for O2 and CO2 : O2 selectivity, among the form ID Rubiscos in the Ochrophyta (table 2), as well as variations in the CO2-saturated rate of CO2 fixation per gram of Rubisco protein, with high CO2-saturated specific reaction rates being generally associated with high K0.5 for CO2 and a low CO2 : O2 selectivity (= Srel or τ) [35]. However, significant variations of K0.5(CO2) and CO2 : O2 selectivity may occur within the mechanistic possibilities suggested by Tcherkez et al. [35]; this is the case for diatoms [29], in which there is evidence of positive selection of Rubisco [36]. Diffusive CO2 entry is more likely in organisms whose Rubiscos have a relatively low K0.5(CO2) and a high CO2 : O2 selectivity; diffusive CO2 entry would be particularly favoured if there is a CO2 subsidy from the terrestrial catchment groundwater supply to rivers and some small lakes [37,38]. By contrast, Rubiscos with a high K0.5(CO2) and a low CO2 : O2 selectivity combined with no CO2 subsidy and approximately air-equilibrium CO2 and O2 concentrations would be compatible with the occurrence of a CCM. The data in table 2 show that this expectation is not always met, with diffusive entry of CO2 in the Chrysophyceae and Synurophyceae, and CCMs in the Eustigmatophyceae and Phaeophyceae (see below). The occurrence of diffusive CO2 entry in organisms with relatively high Rubisco K0.5(CO2) could be rationalized by the availability of sufficient energy, N and P (for the RNA needed for protein synthesis) to produce more Rubisco per cell and/or a large CO2 subsidy.
Table 2.
In vitro kinetics of ochrophytan Rubiscos. n.d., not determined.
| species | class | CCM | K0.5CO2 (mmol m−3) | K0.5O2 (mmol m−3) | CO2/O2 selectivity | references |
|---|---|---|---|---|---|---|
| 11 species | Bacillariophyceae | + | 23–68 | 413–2032 | 57–116 | [29] |
| Nannochlopsis sp. | Eustigmatophyceae | + | 7–10 | about 1000 | 27 | [30] |
| Mallomonas papillosa | Synurophyceae | − | 18.2 | n.d. | n.d. | [31] |
| Synura petersenii | Synurophyceae | − | 28.4 | n.d. | n.d. | [31] |
| Synura uvella | Synurophyceae | − | 41.8 | n.d. | n.d. | [31] |
| 4 species | Phaeophyceae | + | 12–43 | n.d. | n.d. | [32] |
| Olisthodiscus luteus | Raphidophyceae | ? | 45–59 | 692 | 101 | [9,33,34] |
(b). Chrysophyceae and Synurophyceae
The sister classes Chrysophyceae and Synurophyceae both have diffusive CO2 transport from the bulk medium to Rubisco. Most of the data relate to the Synurophyceae [3,17,31,39–42], with some for the Chrysophyceae [3,41], which, as indicated in table 1, are frequently phagomixotrophic [43].
The evidence on inorganic C entry includes pH drift. Through this method, it was possible to show that the photosynthetic activity of these algae can increase the pH and deplete CO2 to values attributable to a diffusive CO2 entry. More direct estimates of relatively high CO2 compensation concentrations from net photosynthetic rates as a function of inorganic C concentration, as well as estimates of photorespiration, support the view of inorganic carbon acquisition in Chrysophyceae and Synurophyceae as mostly due to CO2 diffusive entry driven by consumption of CO2 in the chloroplasts. For the two cases investigated (one Chrysophyceae and one Synurophyceae), there is no external carbonic anhydrase activity [3]. Vegetative cells of the Synurophyceae and cysts of the Chrysophyceae and Synurophyceae have extraprotoplasmic silicification, in common with several other clades of the Ochrophyta, especially the Bacillariophyceae and Bolidophyceae [44,45]. Among other roles of silicification is that as a pH buffer facilitating H+ movement related to extracellular carbonic anhydrase activity [46]; this cannot be the case in the Chrysophyceae and Synurophyceae with no extracellular carbonic anhydrase. Despite the lack of CCMs, pyrenoids occur in some Chrysophyceae (Chromulina, but not Ochromonas) [3,9] and Synurophyceae (e.g. Synura) [3,47].
For the Synurophyceae, there are also comparisons of the in vitro K0.5 of Rubisco and in vivo photosynthesis. The form ID Rubiscos of the Synurophyceae have K0.5 values (mmol m−3) in vitro of 18.2 (Mallomonas papulosa), 28.4 (Synura petersenii) and 41.8 (Synura uvella) [31]. The photosynthetic K0.5(CO2) are 92.0–440.5 mol m−3 for M. papillosa (varying with the buffer used to maintain the pH at 7.0), 40.4–43.7 mol m−3 (varying with pH 6–7) for S. petersenii and 44.9–209 mol m−3 for S. uvella (varying with pH 6–7) [31]. The in vivo photosynthetic K0.5 values can be accommodated by the in vitro Rubisco K0.5 with diffusive CO2 entry, granted a Rubisco content that gives a Vmax for Rubisco equal to the Vmax for in vivo photosynthesis, and allowing for the necessary decrease in CO2 concentration along the diffusion pathway from the bulk medium to Rubisco [41]. The Rubisco assays used unpurified cell extracts [31], so the CO2-saturated Rubisco specific reaction rate cannot be calculated.
(c). Eustigmatophyceae
Eustigmatophyceae appear to all have a CCM, when cells are grown in culture media in equilibrium with the present atmosphere. The freshwater Eustigmatos vischeri and Vischeria stellata can take up both CO2 and 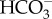 [48–50], while the soil-dwelling Monodus subterraneus can only use CO2 [48,49]. Most of the data are available for the marine Nannochloropsis spp. (N. gaditana, N. oceanica and N. salina) and Monallatus sp., where mass spectrometric and other evidence show
[48–50], while the soil-dwelling Monodus subterraneus can only use CO2 [48,49]. Most of the data are available for the marine Nannochloropsis spp. (N. gaditana, N. oceanica and N. salina) and Monallatus sp., where mass spectrometric and other evidence show 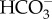 influx powered by mitochondrial respiration, with significant simultaneous efflux of CO2 [48,49,51–58]. Merrett et al. [53] showed that
influx powered by mitochondrial respiration, with significant simultaneous efflux of CO2 [48,49,51–58]. Merrett et al. [53] showed that 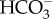 influx was inhibited by DIDS (4′4′-diisothiocyantostilbene-2,2-disulfonic acid), an inhibitor of anion exchangers, and by the absence of Cl−, consistent with the occurrence of a
influx was inhibited by DIDS (4′4′-diisothiocyantostilbene-2,2-disulfonic acid), an inhibitor of anion exchangers, and by the absence of Cl−, consistent with the occurrence of a  : Cl− antiport of unknown stoichiometry. The energized
: Cl− antiport of unknown stoichiometry. The energized 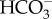 influx is downregulated when cells are grown at high CO2 [58], consistent with decreased CCM expression at high CO2 when diffusive CO2 entry can provide as high a CO2 concentration at the active site of Rubisco as does the CCM at lower external CO2 concentrations. However, Merrett et al. [53] were unable to demonstrate a higher internal than external inorganic C concentration using silicone oil centrifugation, just as Huertas et al. [48,49] were unable to do from CO2 efflux kinetics just after cessation of illumination. Huertas et al. [48] suggest that the intracellular pool occupies a small fraction of the cell volume and/or that Nannochloropsis Rubisco had, like red algal Rubiscos, a low K0.5 for CO2. This latter suggestion was verified by Tchernov et al. [30], who found a K0.5 for CO2 of extracted Nannochloropsis sp. Rubisco of 7–10 mol m−3, the lowest values in table 2. This notwithstanding, the need for a CCM exists in Nannochloropsis due to a very low CO2 : O2 selectivity of 27 [30] and incomplete suppression of Rubisco oxygenase activity in vivo [59]. Expressing the photosynthetic K0.5 for
influx is downregulated when cells are grown at high CO2 [58], consistent with decreased CCM expression at high CO2 when diffusive CO2 entry can provide as high a CO2 concentration at the active site of Rubisco as does the CCM at lower external CO2 concentrations. However, Merrett et al. [53] were unable to demonstrate a higher internal than external inorganic C concentration using silicone oil centrifugation, just as Huertas et al. [48,49] were unable to do from CO2 efflux kinetics just after cessation of illumination. Huertas et al. [48] suggest that the intracellular pool occupies a small fraction of the cell volume and/or that Nannochloropsis Rubisco had, like red algal Rubiscos, a low K0.5 for CO2. This latter suggestion was verified by Tchernov et al. [30], who found a K0.5 for CO2 of extracted Nannochloropsis sp. Rubisco of 7–10 mol m−3, the lowest values in table 2. This notwithstanding, the need for a CCM exists in Nannochloropsis due to a very low CO2 : O2 selectivity of 27 [30] and incomplete suppression of Rubisco oxygenase activity in vivo [59]. Expressing the photosynthetic K0.5 for  in terms of CO2 gives K0.5 values of 0.27 mmol m−3 for Monallantus sp. and 0.63 mmol m−3 for Nanochloropsis gaditana [52], i.e. an order of magnitude higher affinity than for the Rubisco of Nannochloropsis sp. [30], indicating the involvement of a CCM. Furthermore, the occurrence of a net CO2 efflux in the light with all inorganic C entering as
in terms of CO2 gives K0.5 values of 0.27 mmol m−3 for Monallantus sp. and 0.63 mmol m−3 for Nanochloropsis gaditana [52], i.e. an order of magnitude higher affinity than for the Rubisco of Nannochloropsis sp. [30], indicating the involvement of a CCM. Furthermore, the occurrence of a net CO2 efflux in the light with all inorganic C entering as 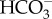 and high affinities for inorganic C, a close approximation to inorganic C saturation at seawater inorganic C concentration, a low (zero) and O2-insensitive CO2 compensation point, a high ability to photosynthesize at high pH and no inhibition of photosynthesis by 21 kPa O2 relative to 2 kPa O2 means that there has to be a higher internal than external CO2 concentration. Pyrenoids are apparently universal in the Eustigmatophyceae (but see [60]), in parallel with the occurrence of CCMs [61,62].
and high affinities for inorganic C, a close approximation to inorganic C saturation at seawater inorganic C concentration, a low (zero) and O2-insensitive CO2 compensation point, a high ability to photosynthesize at high pH and no inhibition of photosynthesis by 21 kPa O2 relative to 2 kPa O2 means that there has to be a higher internal than external CO2 concentration. Pyrenoids are apparently universal in the Eustigmatophyceae (but see [60]), in parallel with the occurrence of CCMs [61,62].
The genomic data show probable 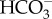 transporters of the anion exchanger family at the plasmalemma (Nga00165.01) and the chloroplast envelope (Nga06584) of Nannochloropsis gaditana CCMP526 [21]; see www.nannochloropsis.org/gene/Naga_10007g30 and www.nannochloropsis.org/gene/Naga_10007g124. The Nannochloropsis oceanica CCMP1779 genome has two genes that resemble the LCIA 1595 in Chlamydomonas [63,64]: one is a chloroplast envelope carrier protein (CCP), which is induced by low CO2 (CCMP1779_7325-mRNA-1); the other is the LCIA protein (CCMP1779_6536-mRNA-1), which belongs to the formate/nitrite transporter family, has an unknown location and is also induced under low CO2 [22]. Using the same strain of N. oceanica, Poliner et al. [65] found two SL4
transporters of the anion exchanger family at the plasmalemma (Nga00165.01) and the chloroplast envelope (Nga06584) of Nannochloropsis gaditana CCMP526 [21]; see www.nannochloropsis.org/gene/Naga_10007g30 and www.nannochloropsis.org/gene/Naga_10007g124. The Nannochloropsis oceanica CCMP1779 genome has two genes that resemble the LCIA 1595 in Chlamydomonas [63,64]: one is a chloroplast envelope carrier protein (CCP), which is induced by low CO2 (CCMP1779_7325-mRNA-1); the other is the LCIA protein (CCMP1779_6536-mRNA-1), which belongs to the formate/nitrite transporter family, has an unknown location and is also induced under low CO2 [22]. Using the same strain of N. oceanica, Poliner et al. [65] found two SL4 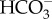 transporters. The SLC4 family from metazoans catalyses a variety of
transporters. The SLC4 family from metazoans catalyses a variety of 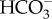 transport processes, i.e. 1
transport processes, i.e. 1 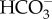 : 1 Cl− exchanger, 1
: 1 Cl− exchanger, 1 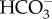 : 1 Na+ cotransporter, 2
: 1 Na+ cotransporter, 2 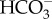 : 1 Na+ cotransporter, 3
: 1 Na+ cotransporter, 3 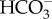 : 1 Na+ cotransporter and a (2
: 1 Na+ cotransporter and a (2 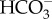 + 1 Na+) : 1 Cl− exchanger [66]. Of these, only the 1
+ 1 Na+) : 1 Cl− exchanger [66]. Of these, only the 1 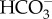 : 1 Na+ cotransporter could lead to inorganic carbon accumulation in the cytosol, granted the probable gradients of Na+ and Cl− across the plasmalemma [67,68]. SLC4
: 1 Na+ cotransporter could lead to inorganic carbon accumulation in the cytosol, granted the probable gradients of Na+ and Cl− across the plasmalemma [67,68]. SLC4 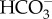 transporters are also known from the diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana and the brown alga Ectocarpus siliculosus [65]. The occurrence of SL4
transporters are also known from the diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana and the brown alga Ectocarpus siliculosus [65]. The occurrence of SL4 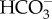 transporters is not diagnostic of CCMs since they are found in the C3 terrestrial flowering plant Arabidopsis thaliana [65] and metazoans [66]. Li et al. [69] found two low CO2-induced putative formate/nitrite transporters in the plastid envelope of N. oceanica IMET1.
transporters is not diagnostic of CCMs since they are found in the C3 terrestrial flowering plant Arabidopsis thaliana [65] and metazoans [66]. Li et al. [69] found two low CO2-induced putative formate/nitrite transporters in the plastid envelope of N. oceanica IMET1.
(d). Phaeophyceae
The largest ochristan algae occur in the Phaeophyceae [16]. The final pH and corresponding equilibrium CO2 concentration achieved in pH drift experiments with Phaeophyceae show 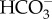 use or, less likely, energized accumulation of CO2 in almost all cases [70–78], with the proviso that the final pH may be restricted by pH per se rather than by limitations on the removal of inorganic C from seawater [41,79]. Similar conclusions arise from the meta-analysis by Stepien [80] of δ13C natural abundance of organic C of Phaeophyceae collected from their natural habitats, with the proviso that the 13CO2 : 12CO2 discrimination of the isolated Rubisco of the only ochristan tested, the diatom Skeletonema costatum [81], differs from that assumed by Stepien [80] and the authors that she cites.
use or, less likely, energized accumulation of CO2 in almost all cases [70–78], with the proviso that the final pH may be restricted by pH per se rather than by limitations on the removal of inorganic C from seawater [41,79]. Similar conclusions arise from the meta-analysis by Stepien [80] of δ13C natural abundance of organic C of Phaeophyceae collected from their natural habitats, with the proviso that the 13CO2 : 12CO2 discrimination of the isolated Rubisco of the only ochristan tested, the diatom Skeletonema costatum [81], differs from that assumed by Stepien [80] and the authors that she cites.
Of the intertidal and subtidal Phaeophyceae from the northeast Atlantic examined by Johnston & Raven [82–84], Surif & Raven [85,86] and Johnston [32], the intertidal species (Fucales) show high affinities for inorganic C, a close approximation to inorganic C saturation at seawater inorganic C concentration, a low (zero) and O2-insensitive CO2 compensation concentration, a high ability to photosynthesize at high pH and no inhibition of photosynthesis by 21 kPa O2 relative to 2 kPa O2. By contrast, the subtidal Laminariales have a lower affinity for inorganic C, lack of inorganic C saturation at seawater inorganic C concentration, a higher and O2-sensitive CO2 compensation concentration, a smaller ability to photosynthesize at high pH and some inhibition of photosynthesis by 21 kPa O2 relative to 2 kPa O2. These characteristics indicate the occurrence of a CCM in all species; the CCM of the Laminariales appears to be less developed in terms of inorganic C affinity and O2 insensitivity of photosynthesis than that of the Fucales. The work of Johnston & Raven [82–84], Surif & Raven [85,86] and Johnston [32] involved experiments under submersed and under emersed conditions with (where identical treatments were used) closely similar results, although the external availability of both 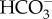 and CO2 in submersed experiments and external availability of only CO2 in emersed experiments leaves unanswered questions about the mechanism of inorganic C entry to cells.
and CO2 in submersed experiments and external availability of only CO2 in emersed experiments leaves unanswered questions about the mechanism of inorganic C entry to cells.
Why, in ecological and evolutionary terms, is there this difference between intertidal and subtidal brown algae in the northeast Atlantic? We need to look at other possible determinants of zonation, such as tolerance of desiccation, especially for high-intertidal algae, which can be emersed for long periods during neap tides [16]. One would expect that desiccation tolerance would be important for high-intertidal algae, especially when emersed for long periods at spring tides. Surif & Raven [86] suggest that a high affinity for inorganic C of intertidal brown algae in the NE Atlantic increases the photosynthetic C gain per emersion period. However, there are several intertidal (some very high intertidal) red algae lacking CCMs [41], e.g. Bostrychia arbuscula (formerly Stictosiphonia arbuscula) in New Zealand in the high-intertidal zone occupied by Pelvetia canaliculata, a fucoid with a CCM, on NE Atlantic coasts [16,87].
The extent of suppression of Rubisco oxygenase and hence of photorespiration by CCMs of Phaeophyceae has been investigated by Surif & Raven [86] using a comparison of photosynthetic rates as a function of CO2 from 50 to 950 µmol mol−1 total gas with O2 at 20 or 210 mmol mol−1 total gas. The photosynthetic rates of the five intertidal species of the Fucaceae (Fucales) were unaffected by the different O2 levels [84] while those of the normally submerged Laminariales and the normally submerged member of the Cystoseiraceae (Fucales) were lower in 210 than in 20 mmol mol−1 total gas at all the CO2 levels tested [86]. The O2 inhibition of the normally submerged algae differs from that of typical C3 physiology plants by the absence of CO2–O2 competition.
Other data on O2 inhibition of photosynthesis in the Phaeophyceae [88–92] show increased glycine and serine production (as markers for the photorespiratory carbon oxidation cycle) as a fraction of total inorganic C assimilated with increasing O2 [90]. The presence of the photorespiratory carbon oxidation cycle in the Phaeophyceae has been shown by enzyme assays [93,94].
Much of the information on other members of the Phaeophyceae show characteristics more like those of normally submerged algae than the intertidal algae studied by Johnston & Raven [82–84], Surif & Raven [85,86] and Johnston [32]. Examples of the inorganic C affinity and O2 sensitivity of photosynthesis by other members of the Phaeophyceae are provided by Black et al. [88], Downton et al. [89], Burris [90], Dromgoole [91,92] and Raven et al. [71–73]: see review by Raven & Hurd [95].
The general assumption is that the CCM is based on the direct or indirect use of external 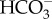 rather than on active influx of CO2.
rather than on active influx of CO2. 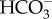 use could involve direct
use could involve direct 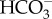 transport using an anion transport protein in the plasmalemma that is usually detected in macroalgae by inhibition of photosynthesis by DIDS and/or SITS (4′-acetamido-4′-isothiocyantostilbene-2,2-disulfonic acid). Such transport is known for the gametophyte, but not for the sporophyte phase of the kelp Undaria pinnatifida [96] and for the sporophyte phase (gametophyte not examined) of the giant kelp Macrocystis pyrifera over a wide range of pH values [28]. No inhibition of photosynthesis by DIDS was found for the members of the Desmarestiales, Fucales, Laminariales, Sphacelariales or Scytosiphonales studied by Larsson & Axelsson [97] or Zou & Gao [74]. Evidence of
transport using an anion transport protein in the plasmalemma that is usually detected in macroalgae by inhibition of photosynthesis by DIDS and/or SITS (4′-acetamido-4′-isothiocyantostilbene-2,2-disulfonic acid). Such transport is known for the gametophyte, but not for the sporophyte phase of the kelp Undaria pinnatifida [96] and for the sporophyte phase (gametophyte not examined) of the giant kelp Macrocystis pyrifera over a wide range of pH values [28]. No inhibition of photosynthesis by DIDS was found for the members of the Desmarestiales, Fucales, Laminariales, Sphacelariales or Scytosiphonales studied by Larsson & Axelsson [97] or Zou & Gao [74]. Evidence of 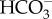 entry in E. siliculosus (Ectocarpales) comes from experiments at an external pH of 9.5, where CO2 is less than 0.23 mmol m−3 [98]. In these experiments, the photosynthetic rate at pH 9.5 was still 30% of that at pH 7.9 and was insensitive to buffering of the medium with 50 mol m−3 of either cationic or anionic pH buffers, or to inhibition of external carbonic anhydrase, thus ruling out the mechanism of
entry in E. siliculosus (Ectocarpales) comes from experiments at an external pH of 9.5, where CO2 is less than 0.23 mmol m−3 [98]. In these experiments, the photosynthetic rate at pH 9.5 was still 30% of that at pH 7.9 and was insensitive to buffering of the medium with 50 mol m−3 of either cationic or anionic pH buffers, or to inhibition of external carbonic anhydrase, thus ruling out the mechanism of 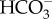 use discussed in the two following paragraphs [98].
use discussed in the two following paragraphs [98].  entry could account for part of the photosynthesis of E. siliculosus at an external pH of 7.9–8.2 ([98]; cf. [99]). At an external pH of 8 in photosynthesis-saturating red light, E. siliculosus accumulates 4–5 mol m−3 inorganic C, i.e. about twice the external concentration; this internal pool is decreased to about 2 mol m−3 when blue light is added, with photosynthetic consumption of the released inorganic C [100].
entry could account for part of the photosynthesis of E. siliculosus at an external pH of 7.9–8.2 ([98]; cf. [99]). At an external pH of 8 in photosynthesis-saturating red light, E. siliculosus accumulates 4–5 mol m−3 inorganic C, i.e. about twice the external concentration; this internal pool is decreased to about 2 mol m−3 when blue light is added, with photosynthetic consumption of the released inorganic C [100].
The alternative mechanism is based on localized acidification of the surface of the organism using energized H+ efflux, although the diffusion boundary layer of photosynthesizing cells is at a higher pH than the bulk medium [101]. The acid zones have an equilibrium CO2 : 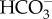 ratio that increases by an order of magnitude with each pH unit decrease, and there is also an order of magnitude increase in the rate of uncatalysed
ratio that increases by an order of magnitude with each pH unit decrease, and there is also an order of magnitude increase in the rate of uncatalysed 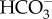 conversion to CO2 [101]. The higher concentration of CO2 at the cell surface has two fates; one is to leak back to the bulk phase, the other is a biologically useful diffusion into the cell to Rubisco. This mechanism is commonly supplemented by the presence of extracellular carbonic anhydrase [102]. The generation of surface acid areas to facilitate CO2 diffusion towards Rubisco is well characterized in freshwater Charales and some submerged freshwater flowering plants, where each acid zone has an area of more than 1 mm2. Price & Badger [103] showed that this mechanism of
conversion to CO2 [101]. The higher concentration of CO2 at the cell surface has two fates; one is to leak back to the bulk phase, the other is a biologically useful diffusion into the cell to Rubisco. This mechanism is commonly supplemented by the presence of extracellular carbonic anhydrase [102]. The generation of surface acid areas to facilitate CO2 diffusion towards Rubisco is well characterized in freshwater Charales and some submerged freshwater flowering plants, where each acid zone has an area of more than 1 mm2. Price & Badger [103] showed that this mechanism of 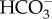 use is inhibited by higher concentrations (20–50 mol m−3) of pH buffers of an appropriate pKa. Such attempts that have been made to identify acid zones on the surface of the Phaeophyceae during photosynthesis have not identified areas in the square millimetres [95], although the techniques used were not suited to identifying smaller acid areas. Transient surface acidification occurring with blue light added to photosynthesis-saturating red light in a range of brown algae [104] shows that surface acidification can occur during photosynthesis in the Phaeophyceae.
use is inhibited by higher concentrations (20–50 mol m−3) of pH buffers of an appropriate pKa. Such attempts that have been made to identify acid zones on the surface of the Phaeophyceae during photosynthesis have not identified areas in the square millimetres [95], although the techniques used were not suited to identifying smaller acid areas. Transient surface acidification occurring with blue light added to photosynthesis-saturating red light in a range of brown algae [104] shows that surface acidification can occur during photosynthesis in the Phaeophyceae.
In the absence of direct measurements of sustained localized surface acidification, the occurrence of a CCM of the kind proposed by Walker et al. [101] has been suggested (see discussion above) on the basis of inhibition of photosynthesis by pH buffers and by inhibitors of extracellular carbonic anhydrases. Sometimes the absence of inhibition by the 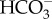 transporter inhibitors DIDS and SITS has also been used as negative evidence for acid zone-dependent CCMs. Support for a Walker et al. style [101] CCM by the use of purported inhibitors of H+ efflux catalysed by plasmalemma H+-ATPase is equivocal because the H+ gradient generated could also be used to indirectly energize a Na+–HCO3− symport influx of
transporter inhibitors DIDS and SITS has also been used as negative evidence for acid zone-dependent CCMs. Support for a Walker et al. style [101] CCM by the use of purported inhibitors of H+ efflux catalysed by plasmalemma H+-ATPase is equivocal because the H+ gradient generated could also be used to indirectly energize a Na+–HCO3− symport influx of 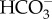 . With these provisos, the mechanism that occurs in the Laminariales other than gametophytes of U. pinnatifida and sporophytes of M. pyrifera is consistent with a mechanism based on that described by Walker et al. [101] for Chara ([105–107]; see also [108,109], as is some evidence for this mechanism in a member of the Scytosiphonales [74], but not for a member of the Fucales [77]).
. With these provisos, the mechanism that occurs in the Laminariales other than gametophytes of U. pinnatifida and sporophytes of M. pyrifera is consistent with a mechanism based on that described by Walker et al. [101] for Chara ([105–107]; see also [108,109], as is some evidence for this mechanism in a member of the Scytosiphonales [74], but not for a member of the Fucales [77]).
Despite the occurrence of CCMs, most brown algae lack pyrenoids although these structures have evolved independently in several clades of the Phaeophyceae [9,28,78,110,111].
The genome of Ectocarpus siliculosa contains a putative Na+–HCO3− transporter targeted to the chloroplast, and a putative Cl−–HCO3− transporter with no clear targeting [112,113]. Nakajima et al. [11] and Poliner et al. [65] found two SLC4 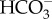 transporters in the genome of E. siliculosa; other Ochrophyta have two (in the eustigmatophycean N. oceanica and the centric diatom T. pseudonana) and four (in the pennate diatom P. tricornutum) SLC4 genes. The P. tricornutum PtSLC4-2 is targeted to the plasmalemma, is inhibited by the anion transporter inhibitor DIDS and is Na+-dependent. Ye et al. [114] report the genome sequence of the kelp (Laminariales) Saccharina japonica; the analysis by Bi & Zhou [115], however, does not mention
transporters in the genome of E. siliculosa; other Ochrophyta have two (in the eustigmatophycean N. oceanica and the centric diatom T. pseudonana) and four (in the pennate diatom P. tricornutum) SLC4 genes. The P. tricornutum PtSLC4-2 is targeted to the plasmalemma, is inhibited by the anion transporter inhibitor DIDS and is Na+-dependent. Ye et al. [114] report the genome sequence of the kelp (Laminariales) Saccharina japonica; the analysis by Bi & Zhou [115], however, does not mention 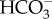 transporters or H+-ATPases, but does show a range of carbonic anhydrases. Genomic data indicate the occurrence of many of the enzymes of the photorespiratory carbon oxidation cycle [112].
transporters or H+-ATPases, but does show a range of carbonic anhydrases. Genomic data indicate the occurrence of many of the enzymes of the photorespiratory carbon oxidation cycle [112].
(e). Raphidophyceae and Tribophyceae
Little is known of the inorganic C acquisition by the Raphidophyceae, other than Rubisco kinetics [33,116]. The in vitro K0.5 for CO2 of Rubisco in the marine Olisthodiscus luteus is 45 mmol m−3 at 23°C (table 2); for the marine Heterosigma carterae, the K0.5 for inorganic C dependence of the rate of photosynthesis in vivo, expressed in terms of CO2, is 3 mmol m−3 at 16°C [117]. This difference suggests that a CCM is operative in the algae of this class. The pH drift work of Nimer et al. [118] indicated low photosynthetic rates for the marine Heterosigma akashiwo; little can be deduced from these data about the mode of inorganic C acquisition. Pyrenoids seem to be universal in the Raphidophyceae [119]. For the Tribophyceae (i.e. Xanthophyceae), the only data are those of Beardall and Entwisle [120] on the terrestrial–freshwater Botrydiopsis intercedens. In this species, internal inorganic C accumulation occurs to a greater extent than it would for diffusive CO2 entry, in accord with the pH gradient; this is suggestive of the operation of a CCM. Several members of the Tribophyceae have pyrenoids [9] though there seem to be no data on B. intercedens. There are no data on inorganic C acquisition by the sister clade to the diatoms, the Bolidiophyceae, now known to have the Parmales as the silicified cyst phase [121].
(f). Pyrenoids and CO2 concentrating mechanisms
As indicated above for individual taxa, pyrenoids seem to be essential features of some CCMs based on active transport across membranes [9,113]. However, pyrenoids occur in some species of the Chrysophyceae and Synurophyceae, classes that uniformly lack CCMs, while the Phaeophyceae, apparently with CCMs in all taxa, generally lack pyrenoids, with several independent origins of pyrenoids. In the Eustigmatophyceae, all members investigated have CCMs and pyrenoids. In the Synchromophyceae, a condensed pyrenoid is often present in the chloroplast stroma [8], although there is no evidence on the occurrence of CCMs in this class. This is also the case for the Pelagophyceae, in which Aureaumbra (at least) has pyrenoids [122].
4. Inorganic C assimilation in Ochrophyta other than diatoms
(a). Introduction
All photosynthetic, eukaryotic C assimilation pathways have the photosynthetic carbon reduction cycle (PCRC, or Calvin–Benson cycle), with Rubisco as the carboxylase, at their core. Raven et al. [25] discussed alternative inorganic C assimilation pathways found in some autotrophic Archaea and Bacteria, and energetic, inorganic C affinity and O2 damage as reasons why many of them are not appropriate for functioning in the present atmosphere. In some organisms, the PCRC is downstream of diffusive entry, in others it is downstream of a ‘biophysical’ CCM based on active transport of 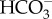 , CO2 or H+ to produce C3 photosynthetic biochemistry. In some oxygenic photosynthetic organisms, the PCRC is downstream of a C3–C4 cycle in C4 photosynthesis; this is a ‘biochemical’ CCM, although it occurs downstream of a ‘biophysical’ CCM in some aquatic flowering plants. In C4 photosynthesis, there is close temporal coupling of the C3–C4 and PCRC cycles with small pool sizes of the C4 and C3 intermediates. Another upstream C3–C4 cycle contributes to crassulacean acid metabolism (CAM); here there is a temporal lag of about 12 h between the scotophase acidication with CO2 fixation into malate, which is stored as malic acid in vacuoles, and deacidification in the photophase, where CO2 is released by decarboxylation of malic acid, and refixed by Rubisco and the PCRC, with the C3 moiety from malate decarboxylation stored until the next scotophase as mono- or polysaccharide.
, CO2 or H+ to produce C3 photosynthetic biochemistry. In some oxygenic photosynthetic organisms, the PCRC is downstream of a C3–C4 cycle in C4 photosynthesis; this is a ‘biochemical’ CCM, although it occurs downstream of a ‘biophysical’ CCM in some aquatic flowering plants. In C4 photosynthesis, there is close temporal coupling of the C3–C4 and PCRC cycles with small pool sizes of the C4 and C3 intermediates. Another upstream C3–C4 cycle contributes to crassulacean acid metabolism (CAM); here there is a temporal lag of about 12 h between the scotophase acidication with CO2 fixation into malate, which is stored as malic acid in vacuoles, and deacidification in the photophase, where CO2 is released by decarboxylation of malic acid, and refixed by Rubisco and the PCRC, with the C3 moiety from malate decarboxylation stored until the next scotophase as mono- or polysaccharide.
The biochemistry of autotrophic CO2 assimilation was determined in pre-molecular biology by two main methods. One was short-time (seconds) exposure of the organism to 14CO2 (terrestrial organisms) or 14C inorganic C (aquatic organisms) in the light, followed by rapidly killing the organism and quantification of the water- or ethanol-soluble organic compounds labelled with 14C. C3 biochemistry is characterized by 3-phosphoglycerate (PGA) as the initial labelled compound, followed by PCRC sugar phosphates and then compounds derived from the PCRC; labelling of C4 dicarboxylic acids in anaplerotic processes is only a few percent of that through the PCRC. C4 biochemistry has C4 dicarboxylic acids as the initial labelled products, followed by PGA and other PCRC compounds; a few seconds labelling (pulse) followed by a change back to unlabelled inorganic C (chase) shows a decrease in label in PGA and an increase in label in C4 dicarboxylic acids. This was the method used in the 1950s and 1960s to establish the C3 pathway of autotrophic inorganic C assimilation in green microalgae (Chlorella, Scenedesmus) and the terrestrial flowering plant Hordeum, and the C4 pathway in the terrestrial flowering plants Saccharum and Zea. The other, much less decisive, method is determination of the activity of carboxylase enzymes in cell extracts; a high Rubisco : PEPC (phosphoenolpyruvate carboxylase-oxygenase) activity ratio has been taken to indicate C3 biochemistry, and a low ratio (less than 1) is suggestive of C4 biochemistry.
(b). Phaeophyceae
Both the kinetics of labelling of organic compounds after addition of 14C inorganic C and the in vitro activity of carboxylases methods have been applied to the Phaeophyceae [32,123–128]. The kinetics of the 14C-inorganic C labelling method shows C3 biochemistry in a range of brown algae [32,123,127–129]. In the brown algae examined by Küppers & Kremer [130], Kremer [129] and Hillrichs & Schmid [127] there is significant labelling of C4 acids (aspartate, malate) in the light, and the time course of the label position within aspartate shows that the PEP comes from photosynthetic 3-PGA, with slower incorporation of 14C-inorganic C into the β-carboxyl of aspartate by phosphoenolpyruvate carboxykinase (PEPCK) or, more likely, PEPC. The ratio of 14C-inorganic C labelling of organic C (initially mainly into aspartate and malate) by carboxylation of PEP to that carried out by Rubisco is lowest in mature tissue and highest in growing tissue, consistent with an anaplerotic role of PEP carboxylation, although the rates may be higher than the anaplerotic requirement [129,130]. 14C-inorganic C labelling of organic C (mainly aspartate and malate) occurs in the dark at a rate higher than that of green and red marine macroalgae and is also probably higher than the anaplerotic requirement [129]. As indicated below, in some fucoids, a part of this dark 14C–inorganic C assimilation can be attributed to low amplitude CAM.
Possibly related to the labelling of C4 acids mentioned above is the finding of a ‘buffer system’ taking up (at high pH in the dark) and releasing (at normal seawater pH in the light) inorganic C, as indicated by stimulation of O2 production in the light in North Atlantic intertidal members of the Fucales (species of Ascophyllum, Fucus and Pelvetia; all Fucaceae) [131,132]. This ‘buffer system’ is associated with H+ exchange between seawater and the algae, is not found in other subtidal brown algae examined, i.e. Halidrys, Fucales (Cystosieraceae), Desmarestia (Desmarestiales) and Laminaria (Laminariales), and is paralleled by a particular spatial organization of organelles in the outer cell layer (meristoderm) of the thallus [131,132]. A more widespread phenomenon among brown algae is the stimulation of photosynthesis by additional blue light to already saturating red light [133,134], which involves release in blue light of at least some of the inorganic C from an intracellular pool accumulated in red light [100]. The blue light effect was not found in the only diatom examined, P. tricornutum [133]. Possibly related to the two preceding sets of data, but probably not, is the interesting finding in the work of Johnston [32] on 14C inorganic C pulse-chase incorporation in the North Atlantic intertidal Ascophyllum nodosum of a continued increase in label incorporation in organic compounds in the chase period, in algae collected in July 1998 but not in January 1988. After a 5 s pulse in July 1988, the increase in label of organic C seems to be saturated at the longest time period tested (300 s) at about eight times the organic C label at the end of the pulse period. This requires a very substantial accumulation of an acid labile pool of either inorganic C or of an acid-labile organic C compound in the 5 s pulse, with incorporation into a range of acid-stable compounds in the chase period. Kawamitsu and Boyer [135] used the North Atlantic intertidal Fucus vesiculosus and showed that, after exposure to light in seawater, photosynthetic O2 production continued for a decreasing rate over 2 h in CO2 free air. A small fraction of the C store on which this O2 production was based was inorganic C; most of it was organic, presumably (an) organic acid(s). Unlike CAM, and the work of Axelsson et al. [131,132], this C store was not filled during the scotophase [135], although there is evidence of minor CAM inorganic C assimilation in several fucoid brown algae including F. vesiculosus [136]. Axelsson et al. [131,132] comment that their ‘photosynthetic buffer’ may be related to the very low CAM activity found in the species showing the ‘photosynthetic buffer’.
The enzyme analyses showed significant activity of PEPCK, but higher activities of Rubisco [32,123,126]. PEPC activity could not be detected in these investigations, including that of Busch & Schmid [126] on E. siliculosus, although genomic evidence indicates that PEPC occurs in this alga [112].
The most recent tool for distinguishing between C3 and C4 (and CAM) photosynthetic biochemistry is genomics and transcriptomics. However, the presence of genes encoding enzymes used in C4 (and CAM) photosynthesis such as PEPC, PEPCK, PPDK (phosphate pyruvate dikinase, sometimes considered for diagnostic C4 photosynthesis), MDH (malate dehydrogenase), NADP-ME (NADP malic enzyme) and NAD-ME (NAD malic enzyme), is not of itself sufficient to show that C4 photosynthesis occurs. One reason is that the enzymes have other functions and occur in photosynthetic organisms known to use C3 biochemistry [137,138]. Another is that the enzyme might not be expressed in an intracellular location compatible with C4 photosynthesis [139]. Bi & Zhou [115] have produced a generalized diagram (their fig. 1) including C4 photosynthesis and list genes [114] compatible with the PEPCK C4 mechanism (granted appropriate intracellular localization of the enzymes), but this is not evidence demonstrating that C4 photosynthesis occurs. The conclusion of Gravot et al. [112] is that there is not clear evidence from genome analysis as to the occurrence of C4 photosynthesis in the model brown alga E. siliculosus.
The other inorganic C assimilation pathway found in the Phaeophyceae is very low amplitude CAM in some members of the Fucales, but not in other orders of the brown algae [112,136].
(c). Raphidophyceae and Eustigmatophyceae
For other photosynthetic Ochrophyta, Descolas-Gros & Oriol [140] found activity of PEPCK but not PEPC in H. akashiwo (Raphidophyceae). However, PEPC was found in the proteome of another raphidophycean, Aureococcus anophagefferens [141].
Genomic data on N. gaditana (Eustigmatophyceae) shows PEPC, MDH (NAD(P) malic dehydrogenase) and PPDK in the cytosol, and NAD(P)-ME in the chloroplast, that could be part of C4 photosynthetic biochemistry, as well as pyruvate carboxylase (PC) in the mitochondria (fig. 6 of [21]).
Genomic data on N. oceanica CCMP1779 (Eustigmatophyceae) show the presence of PEPC, MDH, PPDK and NAD(P)-ME, but the intracellular location is not indicated (table S10 of [22]). Vieler et al. [22] (their table S10) show that expression of probable inorganic C transporters is increased at low CO2 concentrations, but make no comment about the effects of CO2 on expression of the enzymes that could be involved in C4 photosynthesis. Li et al. [69] used transcriptomic data on N. oceanica IMET1 to predict the location of enzymes that could be part of a C4 photosynthesis and found that enzyme location is not entirely as required for an effective C4 pathway.
5. Conclusion
We only have a significant body of information on inorganic C acquisition and assimilation for four non-diatom classes of Ochrophyta, i.e. the Chrysophyceae, Eustigmatophyceae, Phaeophyceae and Synurophyceae, with some information on the Raphidophyceae and Tribophyceae (table 3). Diffusive CO2 entry from the bulk medium to Rubisco occurs in the Chrysophyceae and Synurophyceae, while the Eustigmatophyceae and Phaeophyceae have CCMs involving influx of 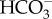 and/or CO2 (table 3).
and/or CO2 (table 3).
Table 3.
Summary of mechanisms of inorganic carbon acquisition and assimilation among classes in the Ochrophyta. No data are available for the Aureanophyceae, Bolidophyceae, Chrysomeridophyceae, Dictyochophyceae, Pelagophyceae, Phaeothamniophyceae, Pinguiophyceae, Schizocladiophyceae and Synchromophyceae. See the main text for details.
| class | Rubisco kinetics | diffusive CO2 entry | CCM | C3 biochemistry | C4 biochemistry | CAM | phagotrophy |
|---|---|---|---|---|---|---|---|
| Bacillariophyceae | yes | no | yes | yes | C3–C4 in some? | no | no |
| Chrysophyceae | no data | yes | no | assumed C3 | no data | unlikely | no |
| Eustigmatophyceae | yes | no | yes | assumed C3 | ? | unlikely | no |
| Phaeophyceae | yes | no | yes | yes | some production of C4 acids in the light | low amplitude in some species | low amplitude in some species |
| Raphidophyceae | yes | probably not | probably yes | no data | no data | unlikely | no |
| Synurophyceae | yes | yes | no | no data | no data | unlikely | no |
| Tribophyceae | no data | no | yes | no data | no data | unlikely | no |
The Phaeophyceae is the only class for which there is biochemical evidence on the pathway of inorganic C assimilation, showing that there is predominantly C3 biochemistry, but with occasional elements of C4 biochemistry and low-amplitude CAM in some members of the Fucales.
The energization of CCMs involves active influx of 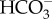 or active efflux of H+ at the plasmalemma, with little or no role for C4 biochemistry, and no net inorganic C entry in the dark in the few brown algae with low amplitude CAM. The distribution of pyrenoids does not completely parallel that of CCMs based on active transport across membranes.
or active efflux of H+ at the plasmalemma, with little or no role for C4 biochemistry, and no net inorganic C entry in the dark in the few brown algae with low amplitude CAM. The distribution of pyrenoids does not completely parallel that of CCMs based on active transport across membranes.
Molecular genetic investigation has not provided definitive evidence as to the occurrence of C4 photosynthesis and has indicated possible membrane transporters involved in CCMs. Some members of the Fucaceae, and Ectocarpus, have incompletely explained inorganic C reservoirs, as inorganic C and/or as (presumably) carboxylate C that can be readily converted to inorganic C.
Returning to the topic of the other papers in this thematic issue, i.e. C metabolism in the Bacillariophyceae, the other classes of Ochrophyta for which information is available show a greater diversity of mechanisms of inorganic C acquisition and biochemistry of autotrophic CO2 assimilation. The occurrence of CCMs is less widespread among other ochrophytes than in diatoms, as is the correlation between the occurrence of CCMs and the presence of pyrenoids. While C4 or C4-like photosynthetic metabolism is a possibility in both diatoms and other ochrophytes, no diatom is known to have the low-amplitude CAM found in some fucalean brown algae.
Acknowledgements
We acknowledge contributions of Lucy Ball, Andrew Johnston, Stephen Maberly and Misni bin Surif. Our deepest gratitude goes to Angela Falciatore for her help in the molecular and phylogenetic issues addressed in this review. The University of Dundee is a registered Scottish Charity, No SC 015096.
Data accessibility
This article has no data.
Authors' contributions
J.A.R. and M.G. contributed to the collection of information and the writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Raven JA, Beardall J, Larkum AWD, Sánchez-Baracaldo P. 2013. Interactions of photosynthesis with genome size and function. Phil. Trans. R. Soc. B 368, 20120264 ( 10.1098/rstb.2012.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beakes GW, Glockling SL, Sekimoto S. 2012. The evolutionary phylogeny of the oomycete ‘fungi’. Protoplasma 249, 3–19. ( 10.1007/s00709-011-0269-2) [DOI] [PubMed] [Google Scholar]
- 3.Maberly SC, Ball LA, Raven JA, Sűltmeyer D. 2009. Inorganic carbon acquisition by chrysophytes. J. Phycol. 49, 1052–1061. ( 10.1111/j.1529-8817.2009.00734.x) [DOI] [PubMed] [Google Scholar]
- 4.Wilken S, Schuurmans JM, Matthijs HCP. 2014. Do mixotrophs grow as photoheterotrophs? Photophysiological acclimation of the chrysophyte Ochromonas danica after feeding. New. Phytol. 204, 882–889. ( 10.1111/nph.12975) [DOI] [PubMed] [Google Scholar]
- 5.Yang EC, Boo GH, Kim HJ, Cho SM, Boo SH, Anderson RA, Yoon HW. 2012. Supermatrix data highlight the phylogenetic relationships of photosynthetic stramenopiles. Protist 163, 217–231. ( 10.1016/j.protis.2011.08.001) [DOI] [PubMed] [Google Scholar]
- 6.Baurain D, et al. 2010. Phylogenetic evidence for separate acquisition of plastids in cryptophytes, haptophytes and stramenopiles. Mol. Biol. Evol. 22, 1698–1709. ( 10.1093/molbev/msq059) [DOI] [PubMed] [Google Scholar]
- 7.Brown JW, Sorhannus U. 2010. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS ONE 5, e12759 ( 10.1371/journal.pone.0012759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Horn S, Flieger K, Ehlers K, Wilhelm C, Schnetter R. 2012. Synchroma pusillum sp. nov. and other new algal isolates with chloroplast complexes confirm the Synchromophyceae (Ochrophyta) as a widely distributed group of amoeboid algae. Protist 163, 544–559. ( 10.1016/j.protis.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 9.Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price DG. 1998. The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 76, 1052–1071. ( 10.1139/b98-074) [DOI] [Google Scholar]
- 10.Roberts K, Granum E, Leegood RC, Raven JA. 2007. Carbon acquisition by diatoms. Photosynth. Res. 93, 79–88. ( 10.1007/s11120-007-9172-2) [DOI] [PubMed] [Google Scholar]
- 11.Nakajima K, Tanaka A, Matsuda Y. 2013. SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc. Natl Acad. Sci. USA 110, 1767–1772. ( 10.1073/pnas.1216234110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clement R, Dimnot L, Maberly SC, Gontero B. 2016. The nature of the CO2-concentrating mechanisms in a marine diatom, Thalassiosira pseudonana . New Phytol. 209, 1417–1427. ( 10.1111/nph.13728) [DOI] [PubMed] [Google Scholar]
- 13.Round FE, Crawford RM, Mann DG. 1990. Diatoms: biology and morphology of the genera. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Rousseaux CS, Gregg WW. 2014. Interannual variation in phytoplankton primary production at a global scale. Remote Sens. 6, 1–19. ( 10.3390/rs6010001) [DOI] [Google Scholar]
- 15.Silberfeld T, Leigh JW, Vergruggen H, Cruaud C, de Reviers B, Rousseau F. 2014. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the ‘brown algal crown radiation’. Mol. Phylogenet. Evol. 56, 659–674. ( 10.1016/j.ympev.2010.04.020) [DOI] [PubMed] [Google Scholar]
- 16.Hurd CL, Harrison PJ, Bischof K, Lobban CS. 2014. Seaweed ecology and physiology, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Saxby-Rouen KJ, Leadbeater BS, Reynolds CS. 1997. The relationship between the growth of Synura petersenii (Synurophyceae) to photon flux density, temperature and pH. Phycologia 36, 233–243. ( 10.2216/i0031-8884-36-3-233.1) [DOI] [Google Scholar]
- 18.Nixdorf B, Mischke U. 1998. Chrysophytes and chlamydomonads: pioneer colonists in extremely acid mining lakes (pH < 3) in Lusatia (Germany). Hydrobiologia 369/370, 315–327. ( 10.1023/A:1017010229136) [DOI] [Google Scholar]
- 19.Wolfe AP, Siver PA. 2013. A hypothesis linking chrysophyte microfossils to lake carbon dynamics on ecological and evolutionary time scales. Glob. Planet. Chang. 111, 189–198. ( 10.1016/j.gloplacha.2013.09.014) [DOI] [Google Scholar]
- 20.Sukenik A, Beardall J, Kromkamp J, Kopecký J, Macojídek J, Bergegeijk S, Gabon S, Shahan E, Yamishon A. 2009. Photosynthetic performance of outdoor Nannochloropsis mass cultures under a wide range of environmental conditions. Aquat. Microb. Ecol. 56, 297–308. ( 10.3354/ame01309) [DOI] [Google Scholar]
- 21.Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Sattlage RE, Boore JL, Rosewitz MC. 2011. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana . Nat. Commun. 3, 686 ( 10.1038/ncomms1688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieler A, et al. 2012. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS ONE 8, e1003064 ( 10.1371/journal.pgen.1003064)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang YZ, Koch F, Gobler CJ. 2010. Most harmful algae are vitamin B1 and B12 auxotrophs. Proc. Natl Acad. Sci. USA 107, 20 756–20 761. ( 10.1073/pnas.1009566107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raven JA, Beardall J, Giordano M. 2014. Energy costs of concentrating mechanisms in aquatic organisms. Photosynth. Res. 121, 111–124. ( 10.1007/s11120-013-9962-7) [DOI] [PubMed] [Google Scholar]
- 25.Raven JA, Giordano M, Beardall J, Maberly SC. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Phil. Trans. R Soc. B 367, 493–507. ( 10.1098/rstb.2011.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raven JA, Beardall J. 2014. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 118, 24–37. ( 10.1016/j.aquabot.2014.05.008) [DOI] [Google Scholar]
- 27.Raven JA, Beardall J. 2016. The ins and outs of carbon dioxide. J. Exp. Bot. 67, 1–13. ( 10.1093/jxb/erv451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández PA, Hurd CL, Roleda MY. 2014. Bicarbonate uptake via an anion exchange protein is the main mechanism of inorganic carbon acquisition by the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) under variable pH. J. Phycol. 50, 998–1008. ( 10.1111/jpy.12247) [DOI] [PubMed] [Google Scholar]
- 29.Young JN, Heureux AMC, Sharwood RE, Rickaby REM, Morel FMM, Whitney SM. 2016. Large variations in the Rubisco kinetics of diatoms reveals diversity among their carbon concentrating mechanisms. J. Exp. Bot. 67, 3445–3456. ( 10.1093/jxb/erw163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchernov D, Livne A, Kaplan A, Sukenik A. 2008. The kinetic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase may explain the high apparent photosynthetic affinity of Nannochloropsis sp. to ambient inorganic carbon. Isr. J. Plant Sci. 56, 37–44. ( 10.1560/IJPS.56.1-2.37) [DOI] [Google Scholar]
- 31.Bhatti S, Colman B. 2008. Inorganic carbon acquisition in some synurophyte algae. Physiol. Plant. 133, 33–40. ( 10.1111/j.1399-3054.2008.01061.x) [DOI] [PubMed] [Google Scholar]
- 32.Johnston AM. 1991. The acquisition of inorganic carbon by marine macroalgae. Can. J. Bot. 69, 1123–1132. ( 10.1139/b91-144) [DOI] [Google Scholar]
- 33.Newman SM, Cattolico RA. 1987. Structural, functional and evolutionary analysis of Ribulose-1,5-bisphosphate carboxylase from the chromophyte alga Olisthodiscus luteus . Plant Physiol. 84, 483–490. ( 10.1104/pp.84.2.483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman SM, Derocher J, Cattolico RA. 1989. Analysis of chromophytic and rhodophytic ribulose-1.5-bisphosphonate carboxylase indicated extensive structural and functional similarities among evolutionarily diverse algae. Plant Physiol. 91, 939–946. ( 10.1104/pp.91.3.939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tcherkez GGB, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused kinetics, nearly all ribulose bisphosphate carboxylases may be nearly perfectly optimised. Proc. Natl Acad. Sci. USA 103, 7246–7251. ( 10.1073/pnas.0600605103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young JN, Rickaby REM, Kapralov MV, Filatov DA. 2012. Adaptive signals in ancient Rubisco reveal a history of ancient atmospheric carbon dioxide. Phil. Trans. R. Soc. B 367, 483–492. ( 10.1098/rstb.2011.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maberly SC. 1996. Diel, episodic and seasonal changes in pH and inorganic carbon in a productive lake. Freshwater Biol. 35, 579–598. ( 10.1111/j.1365-2427.1996.tb01770.x) [DOI] [Google Scholar]
- 38.Casper P, Maberly SC, Hall GH, Finlay BJ. 2000. Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry 49, 1–10. ( 10.1023/A:1006269900174) [DOI] [Google Scholar]
- 39.Saxby-Rouen KJ, Leadbeater BS, Reynolds CS. 1998. The growth of Synura petersenii (Synurophyceae) and components of the dissolved inorganic carbon system. Phycologia 37, 467–477. ( 10.2216/i0031-8884-37-6-467.1) [DOI] [Google Scholar]
- 40.Bhatti S, Colman B. 2005. Inorganic carbon acquisition in the chrysophyte alga Mallomonas papillosa. Can. J. Bot. 83, 891–897. ( 10.1139/b05-075) [DOI] [Google Scholar]
- 41.Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC. 2005. Algae lacking carbon-concentrating mechanisms. Can. J. Bot. 83, 879–890. ( 10.1139/b05-074) [DOI] [Google Scholar]
- 42.Bhatti S, Colman B. 2011. Evidence of the occurrence of photorespiration in synurophyte algae. Photosynth. Res. 109, 251–256. ( 10.1007/s11120-011-9639-z) [DOI] [PubMed] [Google Scholar]
- 43.Wilken S, Huisman J, Naus-Wiezer S, van Donk E. 2013. Mixotrophic organisms became more heterotrophic with rising temperatures. Ecol. Lett. 16, 225–233. ( 10.1111/ele.12033) [DOI] [PubMed] [Google Scholar]
- 44.Yamada K, Yoshikawa S, Inchinomiya M, Kuwata A, Kamiya M, Ohki K. 2014. Effects of silicon-limitation on growth and morphology of Triparma laevis NIES-2565 (Parmales, Heterokontophyta). PLoS ONE 9, e103289 (doi:10.137q/journal.pone.0103289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkel ZV. 2016. Silicification in Microalgae. In The physiology of microalgae. Developments in applied phycology 6 (eds Borowitzka MA, Beardall J, Raven JA), pp. 289–300. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 46.Milligan AJ, Morel FMM. 2002. A proton buffering role for silica in diatoms. Science 297, 1848–1850. ( 10.1126/science.1074958) [DOI] [PubMed] [Google Scholar]
- 47.Hibberd DJ. 1978. The fine structure of Synura sphagnicola (Korsch.) Korsch (Chrysophyceae). Brit. Phycol. J. 13, 403–412. ( 10.1080/00071617800650451) [DOI] [Google Scholar]
- 48.Huertas IE, Colman B, Espie GS. 2002. Mitochondrial-driven bicarbonate transport supports photosynthesis in a marine microalga. Plant Physiol. 130, 284–291. ( 10.1104/pp.004598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huertas IE, Colman B, Espie GS. 2002. Inorganic carbon acquisition and its energization in eustimatophyte algae. Funct. Plant. Biol. 29, 271–277. ( 10.1071/PP01181) [DOI] [PubMed] [Google Scholar]
- 50.Huertas IE, Bhatti S, Colman B. 2005. Characterization of the CO2-concentrating mechanism in the unicellular alga Eustigmatos vischeri . Eur. J. Phycol. 40, 409–415. ( 10.1080/09670260500342571) [DOI] [Google Scholar]
- 51.Colman A, Huertas IE, Bhati S, Dason JS. 2002. The diversity of inorganic carbon acquisition mechanisms in eukaryotic algae. Funct. Plant Biol. 29, 261–270. ( 10.1071/PP01184) [DOI] [PubMed] [Google Scholar]
- 52.Munoz J, Merrett MJ. 1989. Inorganic carbon transport in some marine eukaryotic microalgae. Planta 178, 450–455. ( 10.1007/BF00963814) [DOI] [PubMed] [Google Scholar]
- 53.Merrett MJ, Nimer MJ, Dong LF. 1996. The ulilization of bicarbonate ions by the marine microalga Nannochloropsis oculata (Droop) Hibberd. Plant Cell Environ. 19, 478–484. ( 10.1111/j.1365-3040.1996.tb00340.x) [DOI] [Google Scholar]
- 54.Sukenik A, Tchernov D, Kaplan A, Huertas E, Lubian LM, Livne A. 1997. Uptake, efflux and photosynthetic utilization of inorganic carbon by the eustigmatophyte Nannochloropsis sp. J. Phycol. 33, 969–974. ( 10.1111/j.0022-3646.1997.00969.x) [DOI] [Google Scholar]
- 55.Tchernov D, Hassidim M, Luz B., Sukenik A, Reinhold L, Kaplan A. 1997. Sustained net CO2 evolution during photosynthesis by marine microorganism. Curr. Biol. 7, 723–738. ( 10.1016/S0960-9822(06)00330-7) [DOI] [PubMed] [Google Scholar]
- 56.Huertas IE, Lubian LM. 1998. Comparative study of inorganic carbon utilization and photosynthetic responses in Nannochloris (Chlorophyceae) and Nannochloropsis (Eustigmatophyceae) species. Can. J. Bot. 76, 1104–1108. ( 10.1139/b98-068) [DOI] [Google Scholar]
- 57.Huertas IE, Espie GS, Colman B, Lubian LM. 2000. Light dependent bicarbonate transport and CO2 efflux in the marine microalgae Nannochloropsis gaditana. Planta 211, 43–49. ( 10.1007/s004250000254) [DOI] [PubMed] [Google Scholar]
- 58.Hanson DT, Collins AM, Jones HDT, Roesgen J, Lopez-Nieves S, Timlin JA. 2014. On-line stable isotope gas exchange reveals an inducible but leaky carbon concentrating mechanism in Nannochloropsis salina . Photosynth. Res. 121, 311–322. ( 10.1007/s11120-014-0001-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raso S, van Genugten B, Vermuë M, Wijffels RH. 2012. Effect of oxygen concentration on the growth of Nannochloropsis sp. at low light intensity. J. Appl. Phycol. 24, 863–871. ( 10.1007/s10811-011-9706-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maruyama I, Nakamura T, Matsubayashi T, Ando Y, Maeda T. 1986. Identification of the alga known as ‘marine Chlorella’ as a member of the Eustigmatophyceae. Jap. J. Phycol. 34, 319–325. [Google Scholar]
- 61.Hibberd DJ, Leedale GF. 1972. Observations on the cytology and ultrastructure of the new algal class, Eustigmatophyceae. Ann. Bot. 36, 49–71. ( 10.1093/oxfordjournals.aob.a084577) [DOI] [Google Scholar]
- 62.Suda S, Atsumi M, Miyashita M. 2002. Taxonomic characterization of a marine Nannochloropsis species N. oceanica sp. nov. (Eustigmatophyceae). Phycologia 4, 273–279. ( 10.2216/i0031-8884-41-3-273.1) [DOI] [Google Scholar]
- 63.Chen Z-Y, Lavigne LL, Mason CB, Moroney JV. 1997. Cloning and overexpression of two cDNAs encoding the low-CO2 inducible chloroplast envelope protein LIP-36 from Chlamydomonas reinhardtii . Plant Physiol. 114, 265–273. ( 10.1104/pp.114.1.265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miura K, et al. 2004. Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii . Plant Physiol. 135, 1595–1607. ( 10.1104/pp.104.041400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poliner E, et al. 2015. Transcriptional coordination of physiological responses in Nannochloropsis oceanica CCM1779 under light/dark cycles. Plant J. 83, 1097–1113. ( 10.1111/tpj.12944) [DOI] [PubMed] [Google Scholar]
- 66.Romero MF, Chen A-P, Parker MD, Boron WF. 2013. The SLC4 family of bicarbonate (HCO3−) transporters. Mol. Aspects Med. 34, 159–182. ( 10.1016/j.mam.2012.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raven JA. 1984. Energetics and transport in aquatic plants. New York, NY: A.R. Liss. [Google Scholar]
- 68.Raven JA. 2016. Chloride: essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 68, 359–367. ( 10.1093/jxb/erw421) [DOI] [PubMed] [Google Scholar]
- 69.Li J, Han D, Wang D, Ning K, Jia J, Jing X, Hunang S, Chen Q, Xu J. 2014. Choreography of transcriptomics and lipidomics of Nannochloropsis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 26, 1645–1665. ( 10.1105/tpc.113.121418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maberly SC. 1990. Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 26, 439–449. ( 10.1111/j.0022-3646.1990.00439.x) [DOI] [Google Scholar]
- 71.Raven JA, Beardall J, Roberts S. 1989. The ecophysiology of inorganic carbon assimilation by Durvillaea potatorum (Durvillaeales, Phaeophyta). Phycologia 28, 429–437. ( 10.2216/i0031-8884-28-4-429.1) [DOI] [Google Scholar]
- 72.Raven JA, Beardall J, Johnston AM, Kübler JE, Geoghegan I. 1995. Inorganic carbon acquisition by Hormosira banksii (Phaeophyta: Fucales) and its epiphyte Notheia anomala (Phaeophyta: Fucales). Phycologia 34, 267–277. ( 10.2216/i0031-8884-34-4-267.1) [DOI] [Google Scholar]
- 73.Raven JA, Beardall J, Johnston AM, Kübler JE, McInroy SG. 1996. Inorganic carbon acquisition by Xiphophora chondrophylla (Phaeophyta: Fucales). Phycologia 35, 83–89. ( 10.2216/i0031-8884-35-2-83.1) [DOI] [Google Scholar]
- 74.Zou D, Gao K. 2010. Acquisition of inorganic carbon by Endarachne binghamianum (Scytosiphonales, Phaeophyta). Eur. J. Phycol. 45, 117–126. ( 10.1080/09670260903383909) [DOI] [Google Scholar]
- 75.Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob. Change Biol. 17, 2488–2497. ( 10.1111/j.1365-2486.2011.02411.x) [DOI] [Google Scholar]
- 76.Marconi M, Giordano M, Raven JA. 2011. Impact of taxonomy, geography and depth on δ13C and δ15N variation in a large collection of macroalgae. J. Phycol. 47, 1023–1035. ( 10.1111/j.1529-8817.2011.01045.x) [DOI] [PubMed] [Google Scholar]
- 77.Zou D, Gao K, Chen W. 2011. Photosynthetic carbon acquisition in Sargassum henslowianum (Fucales, Phaeophyta), with special reference to the comparison between vegetative and reproductive tissue. Photosynth. Res. 107, 159–168. ( 10.1007/s11120-010-9612-2) [DOI] [PubMed] [Google Scholar]
- 78.Fernández PA, Roleda MY, Hurd CL. 2015. Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynth. Res. 124, 293–304. ( 10.1007/s11120-015-0138-5) [DOI] [PubMed] [Google Scholar]
- 79.Middelboe AL, Hansen PJ. 2007. Direct effects of pH and inorganic carbon on macroalgal photosynthesis and growth. Mar. Biol. Res. 3, 134–144. ( 10.1080/17451000701320556) [DOI] [Google Scholar]
- 80.Stepien CC. 2015. Impacts of geography, taxonomy and functional group on inorganic carbon use patterns in marine macrophytes. J. Ecol. 103, 1372–1383. ( 10.1111/1365-2745.12451) [DOI] [Google Scholar]
- 81.Boller AR, Thomas PJ, Cavenaugh M, Scott KM. 2015. Isotopic discrimination and kinetic parameters of Rubisco from the marine bloom forming diatom Skeletonema costatum . Geobiology 13, 33–43. ( 10.1111/gbi.12112) [DOI] [PubMed] [Google Scholar]
- 82.Johnston AM, Raven J.A. 1986. The utilization of bicarbonate by the macroalga Ascophylum nodosum (L.) Le Jol. Plant Cell Environ. 9, 175–184. [Google Scholar]
- 83.Johnston AM, Raven JA. 1986. The analysis of photosynthesis in air and water by the Ascophyllum nodosum (L.) Le Jol. Oeologia 69, 175–184. ( 10.1007/bf00377636) [DOI] [PubMed] [Google Scholar]
- 84.Johnston AM, Raven JA. 1987. The C4-like characteristics of the intertidal macroalgae Ascophyllum nodosum (L.) Le Jolis (Fucales, Phaeophyta). Phycologia 26, 159–166. ( 10.2216/i0031-8884-26-2-159.1) [DOI] [Google Scholar]
- 85.Surif MB, Raven JA. 1989. Exogenous inorganic carbon sources for photosynthesis in seawater by members of the Fucales and the Laminariales (Phaeophyta): ecological and taxonomic implications. Oecologia 78, 97–105. ( 10.1007/BF00377203) [DOI] [PubMed] [Google Scholar]
- 86.Surif MB, Raven JA. 1990. Photosynthetic gas exchange in eulittoral and normally submersed members of the Fucales and Laminariales: interpretation in relation to C isotope ratio and N and water use efficiency. Oecologia 82, 68–80. ( 10.1007/BF00318535) [DOI] [PubMed] [Google Scholar]
- 87.Lüning K. 1990. Seaweeds: their environment, biogeography and physiology. New York, NY: John Wiley. [Google Scholar]
- 88.Black CC, Burris JE, Everson RG. 1976. Influence of oxygen concentration of photosynthesis in marine plants. Aust. J. Plant Physiol. 3, 81–86. ( 10.1071/PP9760081) [DOI] [Google Scholar]
- 89.Downton WJS, Bishop DG, Larkum AWD, Osmond CB. 1976. Oxygen inhibition of photosynthetic O2 evolution in marine plants. Aust. J. Plant Physiol. 3, 73–78. ( 10.1071/PP9760073) [DOI] [Google Scholar]
- 90.Burris JE. 1977. Photosynthesis, photorespiration, and dark respiration in eight species of algae. Mar. Biol. 39, 371–379. ( 10.1007/BF00391940) [DOI] [Google Scholar]
- 91.Dromgoole FI. 1978. The effects of pH and inorganic carbon on photosynthesis and dark respiration of Carpophyllum (Fucales, Phaeophyceae). Aquat. Bot. 4, 11–22. ( 10.1016/0304-3770(78)90003-7) [DOI] [Google Scholar]
- 92.Dromgoole FI. 1978. The effects of O2 on dark respiration and apparent photosynthesis of marine macro-algae. Aquat. Bot. 4, 281–297. ( 10.1016/0304-3770(78)90025-6) [DOI] [Google Scholar]
- 93.Gross W. 1990. Occurrence of glycolate oxidase and hydroxypyruvate reductase in Egregia menziesii (Phaeophyta). J. Phycol. 26, 381–383. ( 10.1111/j.0022-3646.1990.00381.x) [DOI] [Google Scholar]
- 94.Iwamoto K, Ikawa T. 1997. Glycolate metabolism and subcellular distribution of glycolate oxidase in Spatoglossum pacificum (Phaeophyceae, Chromophyta). Phycol. Res. 45 77–83. ( 10.1111/j.1440-1835.1997.tb00066.x) [DOI] [Google Scholar]
- 95.Raven JA, Hurd CL. 2012. Ecophysiology of photosynthesis in macroalgae. Photosynth. Res. 113, 105–125. ( 10.1007/s11120-012-9768-z) [DOI] [PubMed] [Google Scholar]
- 96.Zhang X, Hu H, Tan T. 2006. Photosynthetic organic carbon utilization by gametophytes and sporophytes of Undaria pinnatifida (Phaeophyceae). Phycologia 45, 642–647. ( 10.2216/05-28.1) [DOI] [Google Scholar]
- 97.Larsson C, Axelsson L. 1999. Bicarbonate uptake and utilization in marine macroalgae. Eur. J. Phycol. 34, 79–86. ( 10.1080/09670269910001736112) [DOI] [Google Scholar]
- 98.Schmid R. 1998. Photosynthesis by Ectocarpus siliculosus in red light and after pulses of blue light at high pH—evidence for bicarbonate uptake. Plant Cell Environ. 21, 523–529. ( 10.1046/j.1365-3040.1998.00297.x) [DOI] [Google Scholar]
- 99.Schmid R, Forster R, Dring MJ. 1992. Circadian rhythm and fast responses in Ectocarpus (Phaeophyta, Ectocarpales): II. Light and CO2 dependence of photosynthesis. Planta 187, 60–66. ( 10.1007/BF00201624) [DOI] [PubMed] [Google Scholar]
- 100.Schmid R, Hillrichs S. 2001. Uptake and accumulation of inorganic carbon in Ectocarpus sliculosus and its relation to blue light stimulation of photosynthesis. Eur. J. Phycol. 36, 257–264. ( 10.1080/09670260110001735408) [DOI] [Google Scholar]
- 101.Walker NA, Smith FA, Cathers IR. 1980. Bicarbonate assimilation by fresh-water charophytes and higher plants: I. Membrane transport of bicarbonate is not proven. J. Membr. Biol. 57, 51–58. ( 10.1007/BF01868985) [DOI] [Google Scholar]
- 102.Price GD, Badger MR, Bassett ME, Whitecross MI. 1985. Involvement of plasmalemmasomes and carbonic anhydrase in photosynthetic utilization of bicarbonate in Chara corallina . Aust. J. Plant Biol. 12, 241–256. ( 10.1071/PP9850241) [DOI] [Google Scholar]
- 103.Price GD, Badger MR. 1985. Inhibition by proton buffers of photosynthetic utilization of bicarbonate in Chara corallina . Aust. J. Plant Biol. 12, 257–267. ( 10.1071/PP9850257) [DOI] [Google Scholar]
- 104.Schmid R, Dring MJ. 1993. Rapid, blue light-induced acidifications at the surface of Ectocarpus and other marine macroalgae. Plant Physiol. 101, 907–913. ( 10.1104/pp.101.3.907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Axelsson L, Mercado JM, Figueroa FL. 2000. Utilization of HCO3− at high pH by the brown macroalga Laminaria saccharina . Eur. J. Phycol. 35, 53–59. ( 10.1080/09670260010001735621) [DOI] [Google Scholar]
- 106.Klenell M, Snoejis P, Pedersén M. 2004. Active carbon uptake in Laminaria digitata and L. saccharina (Phaeophyta) is driven by a proton pump in the plasma membrane. Hydrobiologia 514, 41–53. ( 10.1023/B:hydr.0000018205.80186.3e) [DOI] [Google Scholar]
- 107.Mercado J.M, Andria J.R, Pérez-Llorens JL, Vergara JJ, Axelsson L. 2006. Evidence for a plasmalemma-based CO2 concentrating mechanism in Laminaria saccharina . Photosynth. Res. 88, 259–268. ( 10.1007/s11120-006-9039-y) [DOI] [PubMed] [Google Scholar]
- 108.Giordano M, Maberly SC. 1989. Distribution of carbonic anhydrase in British marine macroalgae. Oecologia 81, 534–539. ( 10.1007/BF00378965) [DOI] [PubMed] [Google Scholar]
- 109.Haglund K, Ramazanov Z, Mtolera M, Pedersén M. 1992. Role of external carbonic anhydrase in light-dependent alkalization by Fucus serratus L. and Laminaria saccharina (L.) Lamour. (Phaeophyta). Planta 188, 1–6. ( 10.1007/BF01160705) [DOI] [PubMed] [Google Scholar]
- 110.Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, De Reviers B. 2010. A multilocus time-calibrated phylogeny of the brown algae (Heterokonotophyta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the ‘brown algal crown radiation’. Mol. Phylogenet. Evol. 56, 659–674. ( 10.1016/j.ympev.2010.04.020) [DOI] [PubMed] [Google Scholar]
- 111.Silberfeld T, Racault M-FLP, Fletcher RL, Couloux A, Rousseau F, de Reviers B. 2011. Systematics and evolutionary history of pyrenoid-bearing taxa in the brown algae (Phaeophyceae). Eur. J. Phycol. 46, 361–377. ( 10.1080/09670262.2011.628698) [DOI] [Google Scholar]
- 112.Gravot A, Dittami SM, Rousvoal S, Lugan R, Eggert A, Collén J, Boyen C, Bouchereau A, Tonon T. 2010. Diurnal oscillations of metabolite abundances and gene analysis prove new insights into central carbon metabolic processes of the brown alga Ecocarpus siliculosus . New Phytol. 188, 98–110. ( 10.1111/j.1469-8137.2010.03400.x) [DOI] [PubMed] [Google Scholar]
- 113.Meyer M, Griffiths H. 2013. Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. J. Exp. Bot. 64, 769–786. ( 10.1093/jxb/ers390) [DOI] [PubMed] [Google Scholar]
- 114.Ye N, et al. 2015. Saccharina genomes provide novel insights into kelp biology. Nat. Commun. 6, 6986 ( 10.1038/ncomms7986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bi Y, Zhou Z. 2016. Absorption and transport of inorganic carbon in kelps with emphasis on Saccharina japonica. In Applied photosynthesis—new progress (ed. MM Najafpour), pp. 111–131. Rijeka, Croatia: InTech Open Publishers. [Google Scholar]
- 116.Newman S, Deracher TS, Cattolico RA. 1989. Analysis of chromophytic and rhodophyte ribulose-1,5-bisphosphate indicates extensive structural and functional similarities among evolutionarily diverse algae . Plant Physiol. 91, 939–946. ( 10.1104/pp.91.3.939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clark DR, Flynn KJ. 2000. The relationship between dissolved inorganic carbon concentration and growth rate in marine phytoplankton. Proc. R. Soc. B. 267, 953–959. ( 10.1098/rspb.2000.1096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nimer NA, Iglesias-Rodriguez MD, Merrett MJ. 1997. Bicarbonate utilization by marine phytoplankton species. J. Phycol. 33, 625–631. ( 10.1111/j.0022-3646.1997.00625.x) [DOI] [Google Scholar]
- 119.Yamaguchi H, Nakayama T, Murakami A, Inouye I. 2010. Phylogeny and taxonomy of the Raphidophyceae (Heterokontophyta) and Chlorinolomas sublosa gen. et sp. nov., a new marine sand-dwelling raphidophyte. J. Plant Res. 123, 333–342. ( 10.1007/s10265-009-0281-1) [DOI] [PubMed] [Google Scholar]
- 120.Beardall J, Entwisle L. 1984. Evidence for a CO2 concentrating mechanism in Botrydiopsis (Tribophyceae). Phycologia 23, 511–513. ( 10.2216/i0031-8884-23-4-511.1) [DOI] [Google Scholar]
- 121.Ichinomiya M, et al. 2016. Diversity and oceanic distribution of the Parmales (Bolidiphyceae), a picoplanktonic group closely related to the diatoms. ISME J. 10, 2419–2434. ( 10.1038/ismej.2016.38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DeYoe HR, Stockwell DA, Bidigare RR, Latasa M, Johnston PW, Hargreaves PE, Suttle CA. 1997. Description and characterization of the algal species Aureoumbra lagunensis gen. et sp. nov, and referral of Aureoumbra and Aureococcus to the Pelagophyceae. J. Phycol. 33, 1042–1048. ( 10.1111/j.0022-3646.1997.01042.x) [DOI] [Google Scholar]
- 123.Kremer BP, Küppers U. 1977. Carboxylating enzymes and the pathway of photosynthetic carbon assimilation in different marine algae—evidence for the C4 pathway? Planta 133, 191–196. ( 10.1007/BF00391918) [DOI] [PubMed] [Google Scholar]
- 124.Kremer BP. 1980. Taxonomic implications of algal photoassimilate patterns. Brit. Phycol. J. 15, 399–409. ( 10.1080/00071618000650401) [DOI] [Google Scholar]
- 125.Kremer BP. 1980. Photorespiration and β-carboxylation in brown macroalgae. Planta 150, 189–190. ( 10.1007/BF00582365) [DOI] [PubMed] [Google Scholar]
- 126.Busch S, Schmid R. 2001. Enzymes associated with β-carboxylation in Ectocarpus siliculosus (Phaeophyceae): are they involved in net carbon acquisition? Eur. J. Phycol. 36, 61–70. ( 10.1017/s0967026201003067) [DOI] [Google Scholar]
- 127.Hillrichs S, Schmid R. 2001. Activation by blue light of inorganic carbon acquisition for photosynthesis in Ectocarpus siliculosus: organic acid pools and short-term carbon fixation. Eur. J. Phycol. 36, 71–79. ( 10.1080/09670260110001735218) [DOI] [Google Scholar]
- 128.Koch M, Bowes G, Ross C, Zhang X-H. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 19, 103–132. ( 10.1111/j.1365-2486.2012.02791.x) [DOI] [PubMed] [Google Scholar]
- 129.Kremer BP. 1981. C4-metabolism in marine brown macrophytic algae. Z. Naturforsch. 39C, 840–847. [Google Scholar]
- 130.Küppers U, Kremer BP. 1978. Longitudinal profiles of carbon dioxide fixation capacities in marine macroalgae. Plant Physiol. 62, 49–53. ( 10.1104/pp.62.1.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Axelsson L, Carlberg S, Ryberg H. 1989. Adaptations by macroalgae to low carbon availability. I. A buffer system in Ascophyllum nodosum, associated with photosynthesis. Plant Cell Environ. 12, 765–770. ( 10.1111/j.1365-3040.1989.tb01637.x) [DOI] [Google Scholar]
- 132.Axelsson L, Carlberg S, Ryberg H. 1989. Adaptations by macroalgae to low carbon availability. II. Ultrastructural specializations, related to the function of a photosynthetic buffer system in the Fucaceae. Plant Cell Environ. 12, 771–778. ( 10.1111/j.1365-3040.1989.tb01638.x) [DOI] [Google Scholar]
- 133.Forster RM, Dring MJ. 1994. Influence of blue light on the photosynthetic capacity of marine plants from different taxonomic, ecological and morphological groups. Eur. J. Phycol. 29, 21–27. ( 10.1080/09670269400650441) [DOI] [Google Scholar]
- 134.Dring MJ, Forster RM, Schmid R. 1994. Ecological significance of blue light stimulation of photosynthetic capacity in Laminaria spp. and other brown algae. Mar. Ecol. Progr. Ser. 113, 271–277. ( 10.3354/meps113271) [DOI] [Google Scholar]
- 135.Kawamatsu Y, Boyer JS. 1999. Photosynthesis and carbon storage between tides in a brown alga, Fucus vesiculosus . Mar. Biol. 133, 361–369. ( 10.1007/s002270050475) [DOI] [Google Scholar]
- 136.Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. Bot. Rev. 64, 121–175. ( 10.1007/BF02856581) [DOI] [Google Scholar]
- 137.Aubry S, Brown NJ, Hibberd JM. 2011. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J. Exp. Bot. 62, 3049–3059. ( 10.1093/jxb/err012) [DOI] [PubMed] [Google Scholar]
- 138.Chi S, Wu S, Yu J, Wang X, Tang X, Liu T. 2014. Phylogeny of C4-photosynthesis enzymes based on algal transcriptomic and genomic data supports an archaeal/proteobacterial origin and multiple duplication for most C4-related genes. PLoS ONE 9, e110154 ( 10.1371/journal.pone.0110154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kroth PG, et al. 2008. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from whole genome analysis. PLoS ONE 3, e1426 ( 10.1371/journal.pone.0001426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Descolas-Gros C, Oriol L. 1992. Variations in carboxylase activity in marine phytoplankton cultures. Mar. Ecol. Progr. Ser. 85, 163–169. ( 10.3354/meps085163) [DOI] [Google Scholar]
- 141.Wurch LL, Bertrand EH, Saito MA, van Mooy BAS, Dyhrman ST. 2011. Proteome changes driven by phosphorus deficiency in the brown tide-forming alga Aureococcus anophagefferens . PLoS ONE 6, e28949 ( 10.1371/journal.pone.0028949) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no data.


