Abstract
Since methylmalonyl-CoA epimerase appears to be absent in the majority of photosynthetic organisms, including diatoms, (S)-methylmalonyl-CoA, the intermediate of isoleucine (Ile) catabolism, cannot be metabolized to (R)-methylmalonyl-CoA then to succinyl-CoA. In this study, propionyl-CoA carboxylase (PCC) RNAi silenced strains and 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) overexpression strains were constructed to elucidate the Ile degradation pathway and its influence on lipid accumulation in Phaeodactylum tricornutum based on growth, neutral lipid content and metabolite profile analysis. Knockdown of PCC disturbed the metabolism of Ile through propionyl-CoA to methylmalonyl-CoA, as illustrated by much higher Ile content at day 6. However, Ile decreased to comparable levels to the wild-type at day 10. PCC silencing redirected propionyl-CoA to acetyl-CoA via a modified β-oxidation pathway, and transcript levels for some branched-chain amino acid (BCAA) degradation-related genes, especially HIBCH, significantly upregulated in the PCC mutant, which enhanced the BCAA degradations and thus resulted in higher triacylglycerol (TAG) content. Overexpression of HIBCH accelerates Ile degradation and results in a lowered Ile content in the overexpression strains, thus enhancing carbon skeletons to the tricarboxylic acid cycle and giving rise to increasing TAG accumulation. Our study provides a good strategy to obtain high-lipid-yield transgenic diatoms by modifying the propionyl-CoA metabolism.
This article is part of the themed issue ‘The peculiar carbon metabolism in diatoms’.
Keywords: diatom, propionyl-CoA carboxylase, branched-chain amino acid catabolism, β-oxidation
1. Introduction
Diatoms, found in waters worldwide, are one of the most successful groups of unicellular eukaryotic algae and are believed to be responsible for up to 20% of the global primary production [1,2]. They are autotrophic secondary endosymbionts and possess a remarkable number of genes with diverse origins [3,4]. As a consequence, diatoms display a flexible metabolism, as illustrated by the presence of an unexpected metazoan-like ornithine–urea cycle [5,6]. Phaeodactylum tricornutum, as a model species of diatoms, is widely used for ecology, physiology, biochemistry and molecular biology studies, especially to investigate biofuel production by algae. It has been well documented that triacylglycerol (TAG) accumulation in P. tricornutum under nitrogen stress is a consequence of re-allocation of carbon mainly from the intermediates of the tricarboxylic acid (TCA) cycle [7,8]. Furthermore, carbon skeletons from enhanced branched-chain amino acid (BCAA) degradation under nitrogen deficiency feed into the TCA cycle and contribute to TAG biosynthesis in this diatom [7].
The BCAAs leucine (Leu), isoleucine (Ile) and valine (Val) are essential amino acids for animals and humans. The first two steps of their degradation pathways are catalysed by the same branched-chain amino acid transaminase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKDH). Then, the degradation pathways of the three BCAAs diverge into separate enzyme-catalysed steps that eventually lead into the TCA cycle in mitochondria. Carbon originating from Leu converts into acetyl-CoA, whereas Ile and Val provide carbon for the conversion of propionyl-CoA to succinyl-CoA [9]. Unlike animals, photosynthetic organisms are able to synthesize BCAAs de novo and these amino acids can also interconvert each other [9]. However, methylmalonyl-CoA derived from carboxylation of propionyl-CoA cannot be metabolized to succinyl-CoA due to the absence of methylmalonyl-CoA epimerase (MCEE) in the majority of photosynthetic organisms including P. tricornutum (see electronic supplementary material, Data S1). Besides MCEE, methylmalonyl-CoA mutase (MCM) appears to be absent in plants [10]. It is indicated that catabolism of Val takes place first through conversion to Leu in Arabidopsis thaliana [10] and P. tricornutum [7]. It seems that it might be Ile degradation rather than Val degradation that gives rise to propionyl-CoA in photosynthetic organisms. Plants are believed to use a modified β-oxidation pathway for the propionyl-CoA degradation in peroxisomes [10], but it is not clear how P. tricornutum metabolizes propionyl-CoA.
In this report, propionyl-CoA carboxylase (PCC) RNAi silencing strains were constructed to elucidate propionyl-CoA metabolic pathways in P. tricornutum by transcript level and metabolome analysis. Although a distinct slowdown of Ile degradation was observed, the β-oxidation pathway was feedback activated and Ile degradation was not inhibited in the PCC knockdown lines during TAG accumulation. Furthermore, 3-hydroxyisobutyryl-CoA hydrolase (HIBCH), a single-copy gene involved in the β-oxidation of propionyl-CoA, was overexpressed in P. tricornutum, and a slightly enhanced BCAA degradation was found in the overexpression lines. It is also interesting that increasing TAG accumulation was obtained without significantly compromising cell biomass in PCC1 knockdown mutants and HIBCH overexpression lines.
2. Material and methods
(a). Strains and growth conditions
Axenic cultures of P. tricornutum Bohlin (CCMP2561) were obtained from the culture collection of the Provasoli-Guillard National Center for Culture of Marine Phytoplankton, Bigelow Laboratory for Ocean Sciences, USA. For the growth experiment and metabolite analysis, cells (2 × 105 cells ml−1) from mid-logarithmic phase cultures were inoculated in artificial seawater enriched with f/2 (nitrate concentration was reduced to 500 µM) [11] and cultivated at 22°C under continuous illumination of 60 µmol photons m−2 s−1 on a shaking table with continuous shaking at 60 r.p.m. For quantitative real-time PCR analysis, cultures with 4 × 105 cells ml−1 were grown under continuous illumination of 100 µmol photons m−2 s−1 and bubbled with filtrated air.
(b). Growth and lipid content analysis
Sampling was performed every 2 days for determinations of cell number, nitrate concentration, neutral lipid content and TAG accumulation. Nitrate concentration in the medium was evaluated spectrophotometrically at 220 nm [12]. The relative neutral lipid content was monitored by fluorometric assay using the dye Nile Red (Sigma-Aldrich) [13]. A total of 6 × 106 cells for each sample were stained with Nile Red as described by Ge et al. [7] and the fluorescence was measured at 572 nm using an LS 55 Fluorescence Spectrometer (Perkin-Elmer). Total lipid was extracted from the cell pellet according to Bligh & Dyer [14] and analysed using thin-layer chromatography (TLC) for comparison of the TAG content. TLC was performed as described by Reiser & Somerville [15] by one-dimensional TLC on silica gel plates 60 F254 (Merck KgaA, Darmstadt, Germany) with triolein as the standard (Sigma-Aldrich).
(c). Construction of PCC RNAi strains
Two PCC (PCC1 and PCC2, corresponding to α- and β-subunit of PCC) silencing vectors were generated according to De Riso et al. [16]. Briefly, a 201-bp fragment (corresponding to the PCC1 gene sequence from 1006 to 1206 bp) and a 412-bp fragment (corresponding to the PCC1 gene sequence from 1006 to 1417 bp) were amplified from the P. tricornutum cDNA, respectively, with the primers pcc1_fw (containing an EcoRI site) and pcc1_rv1 (containing an XbaI site), and pcc1_fw and pcc1_rv2 (containing an XbaI site). The fragments were digested with EcoRI and XbaI and ligated in sense and antisense orientations to the EcoRI site of the linearized phir-PtGUS vector, replacing the GUS gene fragments. The same procedure was followed for the generation of the PCC2 silencing vector. A short fragment of 240-bp (from 955 to 1194 bp of the PCC2 gene sequence) and a long one of 439 bp (corresponding to the gene sequence from 955 to 1393 bp) were amplified from the P. tricornutum cDNA, respectively, using the primers pcc2_fw (containing an EcoRI site) and pcc2_rv1 (containing an XbaI site), and pcc2_fw and pcc2_rv2. PCC silencing vectors (containing a bleomycin-resistance gene) were introduced into P. tricornutum by electroporation according to Zhang & Hu [17]. The transformants were screened by checking the integration of the sh ble gene with the primers ble_fw and ble_rv. All primers for the PCR are listed in the electronic supplementary material (Data S2).
(d). HIBCH GFP fusion and overexpression constructs
The full-length cDNA sequence of HIBCH was identified based on expressed sequence tag libraries [18] and is 1182 bp without intron (ProtID_12599). For the localization analysis of HIBCH, a construct was generated to express C-terminal green fluorescent protein (GFP) fusion proteins in P. tricornutum cells. The full-length coding regions of HIBCH were amplified by PCR using primers hgfp-fw and hgfp-rv (electronic supplementary material, Data S2) from the cDNA and inserted into the KpnI site (immediately upstream of the egfp sequence) of the pPhaT1-eGFP vector [17]. To generate the HIBCH overexpression construct, the open reading frame of HIBCH was amplified by PCR using the hoe-fw and hoe-rv primers (electronic supplementary material, Data S2) and ligated into the pPhaT1 vector (between the XbaI and HindIII sites). Inserts of all constructs were sequenced to confirm that no unintended nucleotide changes were present. The vector was introduced into wild-type P. tricornutum and transformants were screened as mentioned above. For staining of the mitochondria of GFP-positive clones, cells were harvested and then incubated for 25 min in 190 µM MitoTracker Orange (Invitrogen) with shaking in the dark. After staining, cells were washed once with f/2 medium and then observed using a Leica TCS SP8 laser scanning confocal microscope. Fluorescence of eGFP and plastid autofluorescence was excited at 488 nm, and was detected with 500–550 nm and 630–690 nm, respectively. Fluorescence of MitoTracker Orange was excited at 552 nm and detected at a bandwidth of 560–590 nm.
(e). Metabolite analysis
Cells were harvested and quickly frozen in liquid nitrogen, then were freeze-dried. Freeze-dried cells were treated using a standard process for metabolite extraction and the extracts were detected by NMR as described by Ge et al. [7]. Spectral analysis was done using Chenomx Inc NMR-suite software v. 8.0. A total of 65 metabolites were identified and quantified (electronic supplementary material, Data S3), and principle component analysis (PCA) was applied to metabolites' concentration data to visualize inherent clustering between the samples.
(f). Quantitative real-time PCR
Cells grown for 36 (before TAG accumulation), 48, 60, 84 and 108 h were harvested by centrifugation (3000 r.p.m., 15 min) and used for RNA extraction with TRIzol Reagent (Invitrogen). Contaminating DNA was removed with DNase I (Invitrogen) and RNA was then reverse transcribed into first-strand cDNA with the High-capacity cDNA Reverse Transcription Kits (Invitrogen). Gene transcription was measured using the SYBR Green PCR Master Mix (Applied Biosystems) and LightCycler 480 Real-Time PCR System (Roche). Primers used for real-time PCR are shown in the electronic supplementary material (Data S2). The Histone H4 gene was used as the endogenous control gene for normalizing expression of the target gene [19]. ΔCT values were obtained by subtracting the average values of experimental genes from an average of the control gene for each sample.
3. Results and discussion
(a). Putative BCAA degradation pathway
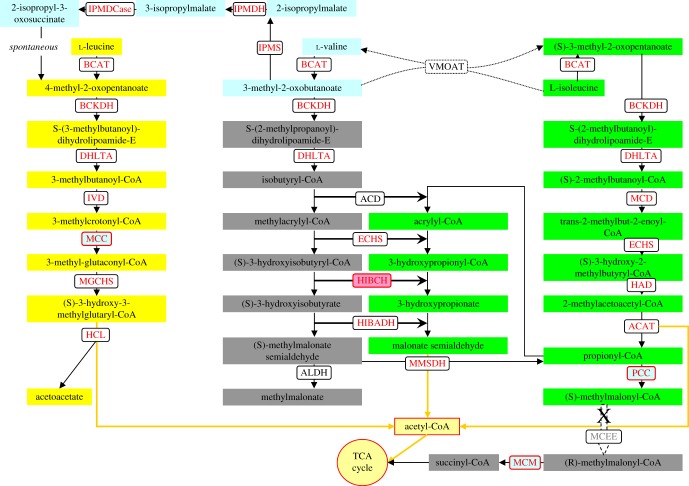
Based on the KEGG (human) Leu, Val and Ile degradation pathways, most of BCAA degradation-related genes were annotated in P. tricornutum's genome on the JGI database (http://genome.jgi.doe.gov/Phatr2/Phatr2.home.html). For Leu degradation, although dihydrolipoyllysine-residue (2-methylpropanoyl) transferase (DHLTA), isovaleryl-CoA dehydrogenase (IVD) and methylglutaconyl-CoA hydratase (MGCHS) encoding genes were not annotated in the JGI database, we found the corresponding coding genes with the Protein ID 54219, 22019 and 11811, respectively, in the P. tricornutum genome by using homology BLAST searching. Knockdown of β-subunit of methylcrotonyl-CoA carboxylase (MCC2), a key gene involved in Leu degradation, disturbed Leu catabolism and led to increased accumulation of Leu in P. tricornutum [7]. Mutation in MCC of Arabidopsis also blocks Leu catabolism and increases Leu accumulation [20]. It is evident that the Leu degradation pathway in these two photosynthetic organisms at least is the same as that in humans (figure 1).
Figure 1.
Pathways of BCAA degradation in P. tricornutum. Transcriptionally upregulated genes during TAG accumulation are in red. BCAT, branched-chain amino acid transaminase; BCKDH, branched-chain α-keto acid dehydrogenase; DHLTA, dihydrolipoyllysine-residue (2-methylpropanoyl) transferase; IVD, isovaleryl-CoA dehydrogenase; MCC, methylcrotonyl-CoA carboxylase; MGCHS, methylglutaconyl-CoA hydratase; HCL, hydroxymethylglutaryl-CoA lyase; IPMS, 2-isopropylmalate synthase; IPMDH, isopropylmalate dehydratase; IPMDCase, 3-isopropylmalate dehydrogenase; MCD, 2-methylacyl-CoA dehydrogenase; ECHS, enoyl-CoA hydratase; HAD, 3-hydroxyacyl-CoA dehydrogenase; ACAT, acetyl-CoA C-acyltransferase; ACD, acyl-CoA dehydrogenase; HIBCH, 3-hydroxyisobutyryl-CoA hydrolase; HIBADH, 3-hydroxyisobutyrate dehydrogenase; MMSDH, methylmalonate-semialdehyde dehydrogenase; ALDH, aldehyde dehydrogenase; PCC, propionyl-CoA carboxylase; MCM, methylmalonyl-CoA mutase; MCEE, methylmalonyl-CoA epimerase, VMOAT, valine-3-methyl-2-oxovalerate transaminase.
For Val degradation, two un-annotated genes encoding short-chain acyl-CoA dehydrogenase (ACD) and HIBCH were found in the P. tricornutum genome with the Protein ID 25932 and 12599. The oxidative degradation of Val is known to proceed through a (S)-methylmalonate semialdehyde intermediate to propionyl-CoA then eventually to succinyl-CoA in humans. In addition, (S)-methylmalonate semialdehyde can also be converted to methylmalonate by aldehyde dehydrogenase (ALDH). However, no methylmalonate was detected during amino acid degradation in P. tricornutum in our previous [7] and present study (electronic supplementary material, Data S3). In this work, we found that the mRNA levels of two ALDH genes were downregulated during nitrogen starvation (electronic supplementary material, Data S4), while transcript levels of genes encoding enzymes involved in conversion of Val to Leu were upregulated [7]. MCC2 lesion in P. tricornutum [7] and Arabidopsis [20] also disturbs Val catabolism. In addition, it has been reported that incubation of A. thaliana seedlings with uniformly labelled [13C]Val results in an accumulation of [13C]Leu not [13C]hydroxypropionate, showing that the major pathway for Val metabolism was not through a modified β-oxidation pathway, but through the conversion to Leu [10]. Taken together, unlike in humans, Val and Leu degradation share the same pathway in this diatom and Arabidopsis, which converts Val into acetyl-CoA rather than succinyl-CoA finally (figure 1).
According to the human Ile degradation pathway, all genes encoding Ile catabolism enzymes except for the MCEE gene were found in the P. tricornutum genome, including an un-annotated gene encoding 2-methylacyl-CoA dehydrogenase (MCD, ProtID_20310). MCEE is also absent in other sequenced diatoms. Furthermore, MCEE is missing in fungi and almost all of the photosynthetic organisms (electronic supplementary material, Data S1). MCEE exists in Chlorella variabilis and Galdieria sulphuraria, and the former is a photosynthetic endosymbiont of protozoans while the latter is an extremophilic species with broad metabolic capacities (heterotrophy and photoautotrophy), suggesting that MCEE is derived from horizontal gene transfer [21,22]. Physcomitrella patens, a transitional form between lower and higher plants [23], also has an MCEE encoding gene. Besides MCEE, methylmalonyl-CoA mutase (MCM) appears to be absent in plants [10]. Therefore, methylmalonyl-CoA, the product of propionyl-CoA carboxylation, cannot be metabolized to succinyl-CoA. Plants and some fungi can degrade propionyl-CoA via a modified β-oxidation pathway [10,24]. All genes encoding the β-oxidation pathway of propionyl-CoA have been found in the P. tricornutum genome, indicating that its Ile degradation pathway is different from that in humans. No phenotype was observed in the MCM knockdown lines of P. tricornutum [7], which further suggests that propionyl-CoA metabolism is not a biotin and B12-dependent pathway (figure 1).
(b). Silencing of PCC activated β-oxidation pathway of propionyl-CoA
PCC, a mitochondrial biotin-dependent enzyme, catalyses the carboxylation of propionyl-CoA to produce (S)-methylmalonyl-CoA. The holoenzyme of PCC is an α6β6 dodecamer, with biotin carboxylase and biotin carboxyl carrier protein domains in the α-subunit and carboxyltransferase domains in the β-subunit [25]. Two putative genes, protein IDs 51245 (PCC1) and 45886 (PCC2), encoding PCC α- and β-subunits, respectively, have been annotated in the genome of P. tricornutum. The α- and β-subunits of P. tricornutum (PtPCC) and human PCC share 44% and 65% sequence identity, respectively, showing highly conserved amino acid sequences with functional domains (electronic supplementary material, figures S1 and S2).
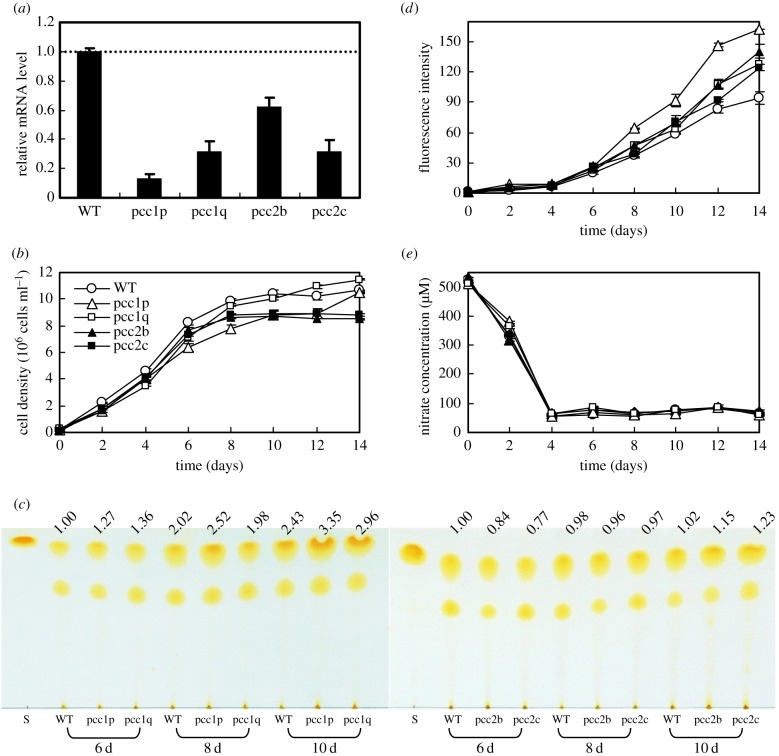
In order to clarify propionyl-CoA degradation pathways, PCC1 and PCC2 short hairpin RNA knockdown strains were constructed in P. tricornutum. Two PCC1 silenced strains (pcc1p and pcc1q) and two PCC2 silenced strains (pcc2b and pcc2c) showed 69–87% and 38–68% reductions, respectively, in transcript levels compared with wild-type (figure 2a). During exponential growth phase the growth of the four PCC mutants was not significantly different from that of wild-type, while compared with wild-type the PCC2 knockdown lines exhibited 13–20% lower cell density in the stationary phase and the PCC1 knockdown lines showed the comparable cell density at day 14 (figure 2b). The four mutants showed comparable or increased TAG contents relative to wild-type cells by TLC analysis (figure 2c), especially at day 10 when their TAG content is significantly higher. Neutral lipid content per cell detected by Nile Red at day 14 in the four PCC mutants increased by 32–73% compared with what was observed in wild-type cells (figure 2d). In addition, there was no difference in nitrate utilization between these PCC mutants and wild-type (figure 2e).
Figure 2.
Effect of PCC gene silencing on growth and neutral lipid accumulation. (a) Relative mRNA levels of PCC (error bars represent s.e. of triplicate technical replicates of duplicate cultures), (b) growth, (c) accumulation of TAGs detected by thin-layer chromatography (TLC), (d) accumulation of neutral lipid detected by Nile Red assay (fluorescence intensity normalized to cell number) and (e) nitrate utilization of wild-type (WT) and four RNAi silenced lines (pcc1p, pcc1q, pcc2b and pcc2c) grown in f/2 (NaNO3 concentration was reduced to 500 µM) enriched artificial seawater medium. mRNA levels were performed at day 8. The values above the TLC panel indicate the relative TAGs normalized to the WT at day 6, which was set as 1. Error bars in (b), (d) and (e) represent s.e. of three biological replicates.
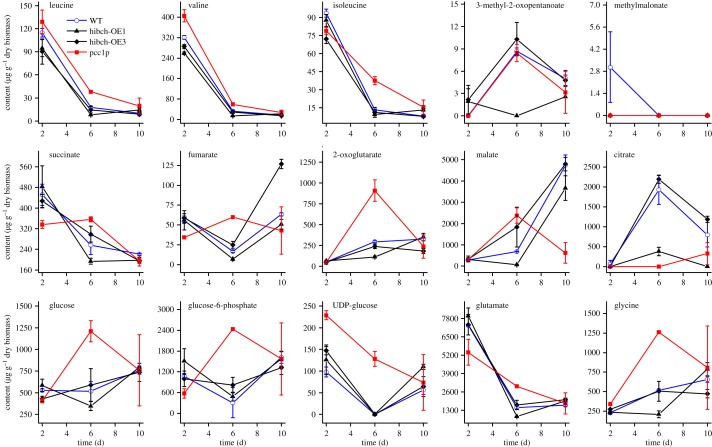
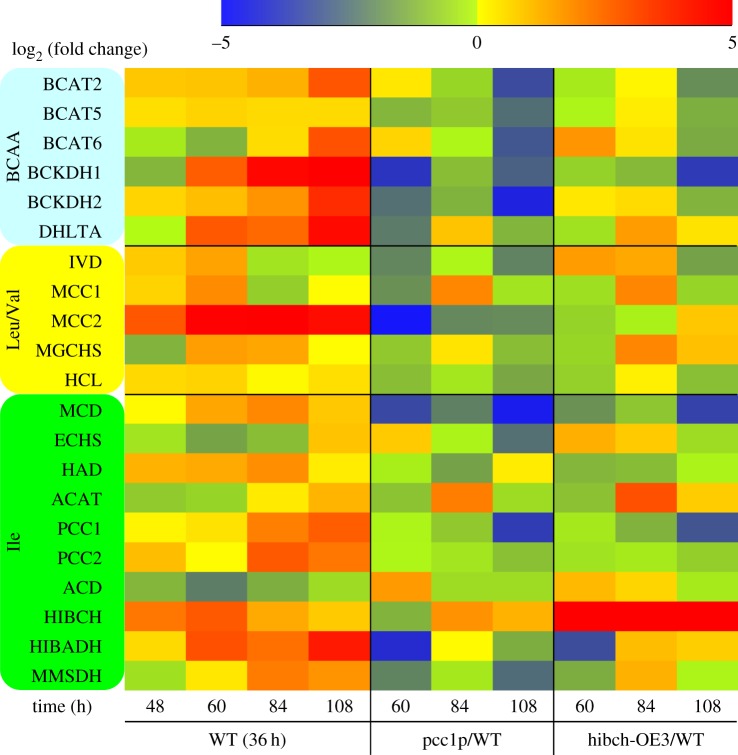
Knockdown of MCC2 disturbs BCAA catabolism and decreases the carbon flux towards the TCA cycle, thus resulting in decreased TAG accumulation. MCC2 inhibition also gave rise to incomplete utilization of nitrogen and lowered biomass in P. tricornutum [7]. However, silencing of PCC enhanced lipid accumulation and had no influence on nitrate utilization. In particular, a comparable cell biomass with wild-type was reached in the strain of pcc1p, but its TAG and neutral lipid content were significantly higher. This mutant was used to carry out the comparison of metabolome and transcript level with wild-type. Metabolite analysis showed that silencing of PCC1 slowed down the 3 BCAA degradation, and in particular, Ile content at day 6 was much higher than that of wild-type. Nevertheless, all the 3 BCAA contents decreased to a very low level in the mutant at day 10, which is comparable to that of wild-type (figure 3). PCC lesion disturbs carboxylation of propionyl-CoA to methylmalonyl-CoA, and thus might change Ile metabolism. An interconversion between Ile and Val catalysed by valine-3-methyl-2-oxovalerate transaminase (VMOAT) was reported in pea [26], and Val can convert into Leu in P. tricornutum and Arabidopsis, which might account for the fact that PCC silencing affected all three BCAA metabolisms. Quantitative real-time PCR analysis showed a significantly lower mRNA level for many BCAA degradation-related genes in strain pcc1p at 60 h compared with wild-type (figure 4). However, the transcript levels of most of the BCAA degradation-related genes recovered to levels comparable to those in wild-type at 84 h, while an about threefold increase was observed in acetyl-CoA C-acyltransferase (ACAT), α-subunit of methylcrotonyl-CoA carboxylase (MCC1) and HIBCH. HIBCH was also upregulated (twofold) at 108 h. It is suggested that silencing of PCC activated propionyl-CoA metabolism to acetyl-CoA via a modified β-oxidation pathway, thus resulting in carbon flux towards the TCA cycle and increased TAG accumulation. In addition, strongly upregulated ACAT could enhance conversion of 2-methylacetoacetyl-CoA, an intermediate of Ile catabolism, to acetyl-CoA and propionyl-CoA, which might also be one of the reasons why a higher TAG content is observed in the PCC mutant.
Figure 3.
Metabolite contents in wild-type (WT), two HIBCH overexpression lines (hibch-OE1 and hibch-OE3) and one PCC1 RNAi line (pcc1p) grown in f/2 (NaNO3 concentration was reduced to 500 µM) enriched artificial seawater media at day 2, 6 and 10. Error bars represent s.e. of three biological replicates.
Figure 4.
Transcript levels of genes encoding components involved in BCAA degradation in wild-type (WT, relative to 36 h), PCC RNAi silenced line (pcc1p, relative to WT) and HIBCH overexpression line (hibch-OE3, relative to WT). Transcriptional fold changes from triplicate technical replicates of duplicate cultures (n = 2). BCAT, branched-chain amino acid transaminase; BCKDH, branched-chain α-keto acid dehydrogenase; DHLTA, dihydrolipoyllysine-residue (2-methylpropanoyl) transferase; IVD, isovaleryl-CoA dehydrogenase; MCC, methylcrotonyl-CoA carboxylase; MGCHS, methylglutaconyl-CoA hydratase; HCL, hydroxymethylglutaryl-CoA lyase; MCD, 2-methylacyl-CoA dehydrogenase; ECHS, enoyl-CoA hydratase; HAD, 3-hydroxyacyl-CoA dehydrogenase; ACAT, acetyl-CoA C-acyltransferase; PCC, propionyl-CoA carboxylase; ACD, acyl-CoA dehydrogenase; HIBCH, 3-hydroxyisobutyryl-CoA hydrolase; HIBADH, 3-hydroxyisobutyrate dehydrogenase; MMSDH, methylmalonate-semialdehyde dehydrogenase.
(c). Overexpression of HIBCH increased Ile degradation and TAG accumulation
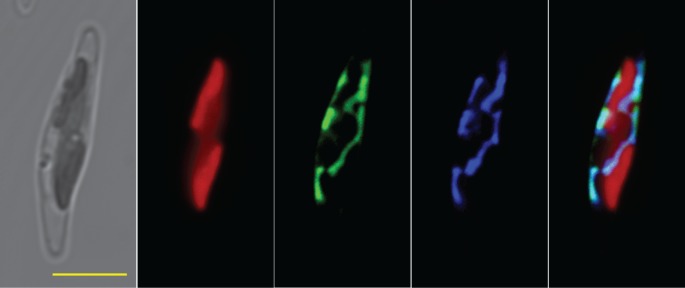
HIBCH catalyses the conversion of 3-hydroxyisobutyryl-CoA to 3-hydroxyisobutyrate involved in the Val catabolic pathway [27], and this mitochondrial enzyme is also reactive towards 3-hydroxypropionyl-CoA, giving it a dual role in a secondary pathway of propionate metabolism in humans [28,29]. In Arabidopsis, there are five putative genes coding HIBCH: two of them with the predicted localization in mitochondria and three in peroxisomes [10]. Mutation of CHY1, one of the peroxisomal HIBCH in Arabidopsis [30], resulted in a dramatically increased sensitivity to the toxic effects of excess propionate and isobutyrate but not of Val [10], which suggested that HIBCH is important for propionyl-CoA metabolism in plants. A hypothesized gene encoding HIBCH was found in P. tricornutum, and is 39% and 38% identical to a human HIBCH (HsHIBCH) and CHY1. HIBCH with different origins have conserved amino acids near the catalytic site of Gly-149 (in HsHIBCH) and Glu-169 (in HsHIBCH) (electronic supplementary material, figure S3) and are grouped into three distinct clades (electronic supplementary material, figure S4). HIBCH in five diatoms were clustered into the same group and clearly separated from the other organisms. Chlamydomonas reinhardti HIBCH (CrHIBCH), encoded by a single-copy gene, has a C-terminal peroxisome targeting signal and TargetP analysis predicted a mitochondrion location for P. tricornutum HIBCH. The fluorescence signal of HIBCH:GFP fusion proteins was around the plastid and showed a parallel staining with a MitoTracker Orange, which revealed that HIBCH was targeted to the mitochondria in P. tricornutum (figure 5). Different localizations of HIBCH in green algae and diatoms indicate that they have different evolutionary origins.
Figure 5.
Fluorescent microscope images of cells transformed with egfp fusions comprised full-length HIBCH. From left to right, panels show microscopical images of transmitted light, chlorophyll autofluorescence, GFP fluorescence, mitochondria stained by MitoTracker Orange and a merged image, scale bar represents 5 μm.
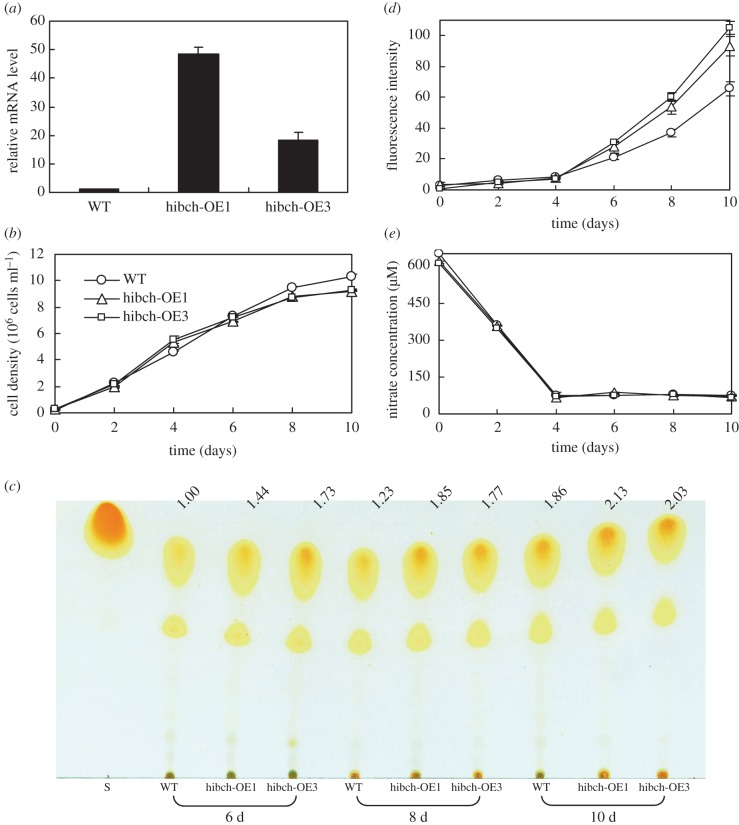
As mentioned earlier, the transcript level of HIBCH showed strong upregulation in the PCC1-silenced strain and was also upregulated from 48 to 108 h in wild-type (figure 4). Nitrate was depleted after 48 h, after which amino acid catabolism appeared to overtake anabolism and TAGs began to accumulate [7]. In order to understand the role of HIBCH in Ile degradation and how the degradation contributes to TAG accumulation in P. tricornutum, HIBCH overexpression strains were constructed. Wild-type cells and four independent transgenic lines harbouring full-length HIBCH cDNA driven by a FCP promoter were selected for gene expression analysis at day 8 of cell culture. HIBCH of two lines (denoted hibch-OE1 and hibch-OE3) showed a 48.3-fold and 18.4-fold increase, respectively, in transcript levels compared with wild-type cells (figure 6a). As shown in figure 3, all the 3 BCAA degradations were slightly enhanced (p < 0.05 on day 2, p > 0.05 on day 6 and 10) in the HIBCH overexpression strains. Although no significant difference was observed in the three BCAA contents at day 6 and 10, their contents decreased by 6–23% at day 2, suggesting that HIBCH played a key role in the BCAA degradation. According to the BCAA degradation pathway, overexpression of HIBCH promoted BCAA degradation directly or indirectly due to the interconversion among the three BCAAs existing in the photosynthetic organisms. Quantitative real-time PCR analysis showed that the transcript levels of most of the BCAA degradation-related genes were comparable or higher in strain hibch-OE3 at 60 h compared with wild-type, and many genes are further upregulated at 84 h (figure 4). For example, besides HIBCH which upregulated 297-fold, 2.5-fold, 3.2-fold and 6.1-fold upregulation were detected in DHLTA (BCAA degradation), MGCHS (Leu and Val degradation) and ACAT (Ile degradation) at 84 h in strain hibch-OE3. This is also one of the reasons why HIBCH overexpression enhanced all the three BCAA degradations. Downregulation of PCC in the overexpression strain indicated that metabolism of propionyl-CoA to methylmalonyl-CoA was inhibited.
Figure 6.
Effect of HIBCH gene overexpression on growth and neutral lipid accumulation. (a) Relative mRNA levels of HIBCH (error bars represent s.e. of triplicate technical replicates of duplicate cultures), (b) growth, (c) accumulation of TAGs detected by thin-layer chromatography (TLC), (d) accumulation of neutral lipid detected by Nile Red assay (fluorescence intensity normalized to cell number) and (e) nitrate utilization of wild-type (WT) and two overexpression lines (hibch-OE1 and hibch-OE3) grown in f/2 (NaNO3 concentration was reduced to 500 µM) enriched artificial seawater medium. mRNA levels were performed at day 8. The values above the TLC panel indicate the relative TAGs normalized to the WT at day 6, which was set as 1. Error bars in (b), (d) and (e) represent s.e. of three biological replicates.
No significant differences were observed in the growth between the HIBCH overexpression lines and wild-type until day 8 (figure 6b). A slightly lower cell density was obtained at day 10 but much higher TAG (figure 6c) and neutral lipid (figure 6d) were reached in the overexpression strains. In particular, neutral lipid increased by 45–63% and 42–60% at day 8 and day 10, respectively, in the two overexpression strains, compared with what was observed in wild-type cells. Increased supply carbon flux derived from the enhanced BCAA degradation resulted in increased TAG accumulation in the HIBCH overexpression strains. In addition, nitrate utilization showed no difference in the overexpression strains (figure 6e).
(d). Transcript level and metabolite profile analysis reveal Ile degradation pathway
Based on the phenotypes of PCC mutants and HIBCH overexpression lines, the Ile degradation pathway could be outlined in figure 1 and all genes involved in the pathway exhibited coordinated patterns of expression from 48 to 108 h, namely most of these genes showed upregulation (figure 4). Catabolism in the first three steps is common to all three BCAAs and genes involved in the steps were also strongly upregulated. The catabolism of Val takes place first through conversion to Leu and transcript levels of genes encoding enzymes involved in the conversion were upregulated during TAG accumulation in P. tricornutum [7]. All genes involved in Leu degradation were upregulated during TAG accumulation in this study. It was reported that BCAA degradations occur during TAG accumulation and provide carbon skeletons directed towards lipid synthesis [7,8,31].
According to PCA of metabolic profiles an obvious separation between PCC1 mutant and wild-type cells was detected both at day 2 and 10, and the separation between wild-type and HIBCH overexpression strains was only observed at day 10 (electronic supplementary material, figure S5). As mentioned earlier, BCAA contents differ to a greater or lesser degree, between transgenic lines and wild-type, and in particular, Ile is much higher in the PCC1 mutant at day 6. Although we did not analyse the protein levels of PCC1, accumulation of Ile in the PCC1 mutant is in agreement with the downregulation of PCC1. PCC1 silencing slowed down BCAA degradation, but did not result in BCAA accumulation in cells at day 10, and HIBCH overexpression promoted BCAA degradations. The phenotypes in these transgenic strains account for the fact that knockdown of PCC increases propionyl-CoA oxidation to acrylyl-CoA and eventually to acetyl-CoA. HIBCH, encoded by a single-copy gene in P. tricornutum, plays a key role in this oxidation pathway. In addition, serine, which can be metabolized to the intermediate of Ile degradation (S)-3-methyl-2-oxopentanoate [32], was also increased by 31% at day 6 in the PCC1 mutant, but decreased by 23–47% in the two HIBCH overexpression strains (electronic supplementary material, Data S3). Alanine (Ala), phenylalanine (Phe) and glutamate (Glu) were increased by 55%, 140% and 102%, respectively, in the PCC1 mutant at day 6, and accordingly these amino contents tended to decrease in HIBCH overexpression strains (figure 3 and electronic supplementary material, Data S3). 2-Oxoglutarate is the first substrate for Ala, Phe and BCAA catabolism, and can derive from Glu catalysed by glutamate dehydrogenase (GLDH) [33]. Increased 2-oxoglutarate (by 207%) at day 6 in the PCC1 mutant might result from the lowered amino acid degradation, thus decreased 2-oxoglutarate (by 20–62%) in HIBCH overexpression strains is also reasonable (figure 3). Besides 2-oxoglutarate, TCA cycle intermediates succinate, fumarate and malate also accumulated at day 6, indicating the cell metabolism change in the PCC1 mutant (figure 3).
Glycine (Gly) is the only proteinaceous amino acid that increased during nitrogen starvation and is a precursor of glutathione and glycine betaine, which play an important role in stress tolerance in plants [34–36]. Gly content in the PCC1 mutant was 23–144% higher from day 2 to 10 than that of wild-type (figure 3), and N,N-dimethylglycine and sarcosine, the intermediates of Gly synthetic metabolism, were also higher in the mutant (electronic supplementary material, Data S3). Increased Gly in cells might be a stress response to silencing of PCC1. Carnitine is a non-proteinaceous amino acid and is best known for its fundamental role in animal and fungal energy metabolism to import activated fatty acids into the mitochondrion and to feed the β-oxidation process [37,38]. This metabolite was not detected in the PCC1 mutant at day 2, which might indicate that cells sacrificed fatty acid β-oxidation to Ile degradation via a modified β-oxidation, while it recovered to levels comparable to those in wild-type at day 6 and 10 (electronic supplementary material, Data S3). In addition, glucose and the glycolysis intermediate glucose-6-phosphate accumulated at day 6 in the PCC1 mutant, and UDP-glucose involved in chrysolaminaran [39] was higher at day 2–6 (figure 3), suggesting that inhibition occurred for these metabolism pathways.
4. Conclusion
PCC has high transcript abundances in P. tricornutum and plays an important role in the metabolism of some amino acids and the fatty acids with an odd number of carbon atoms. Knockdown of PCC affects glycolysis, the TCA cycle, fatty acid oxidation and amino acid metabolism. As the most direct response, the metabolism of propionyl-CoA to methylmalonyl-CoA is inhibited and thus disturbs the catabolism of Ile through propionyl-CoA to methylmalonyl-CoA. However, PCC silencing will redirect propionyl-CoA to acetyl-CoA via a modified β-oxidation. Overexpression of HIBCH in P. tricornutum confirms that the activated β-oxidation accelerates the Ile degradation, thus enhancing carbon skeletons to the TCA cycle and giving rise to TAG accumulation under nitrogen limitation. Our study provides a good strategy to obtain high-lipid-yield transgenic diatoms for biofuel production by modifying the propionyl-CoA metabolism.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Mrs Fan Hu from the School of Foreign Languages at China University of Geosciences for proofreading the English.
Data accessibility
The data have been uploaded as the electronic supplementary material.
Authors' contributions
H.H. taking responsibility for the integrity of the work as a whole, conceived the research, designed and supervised experiments, and wrote the manuscript. H.H. and P.Y. analysed the data. P.Y., Y.J. and L.X. performed the experiments. G.Y. performed the statistical analysis of metabolites.
Competing interests
We have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (no. 41576144) and the Open Fund Project of State Key Laboratory of Freshwater Ecology and Biotechnology (no. 2015FB12).
References
- 1.Falkowski PG, Barber RT, Smetacek V. 1998. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206. ( 10.1126/science.281.5374.200) [DOI] [PubMed] [Google Scholar]
- 2.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 3.Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR. 2004. The evolution of modern eukaryotic phytoplankton. Science 305, 354–360. ( 10.1126/science.1103879) [DOI] [PubMed] [Google Scholar]
- 4.Montsant A, Jabbari K, Maheswari U, Bowler C. 2005. Comparative genomics of the pennate diatom Phaeodactylum tricornutum. Plant Physiol. 137, 500–513. ( 10.1104/pp.104.052829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen AE, et al. 2011. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 473, 203–207. ( 10.1038/nature10074) [DOI] [PubMed] [Google Scholar]
- 6.Bender SJ, Parker MS, Armbrust EV. 2012. Coupled effects of light and nitrogen source on the urea cycle and nitrogen metabolism over a diel cycle in the marine diatom Thalassiosira pseudonana. Protist 163, 232–251. ( 10.1016/j.protis.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 7.Ge F, Huang W, Chen Z, Zhang C, Xiong Q, Bowler C, Yang J, Xu J, Hu H. 2014. Methylcrotonyl-CoA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 26, 1681–1697. ( 10.1105/tpc.114.124982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitan O, et al. 2015. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Proc. Natl Acad. Sci. USA 112, 412–417. ( 10.1073/pnas.1419818112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder S. 2010. Branched-chain amino acid metabolism in Arabidopsis thaliana. Arabidopsis Book 8, e0137 ( 10.1199/tab.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas KA, Filley JR, Erb JM, Graybill ER, Hawes JW. 2007. Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J. Biol. Chem. 282, 24 980–24 989. ( 10.1074/jbc.M701028200) [DOI] [PubMed] [Google Scholar]
- 11.Guillard RRL. 1975. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals (eds WL Smith, MH Chanley), pp. 29–60. New York, NY: Springer. [Google Scholar]
- 12.Collos Y, Mornet F, Sciandra A, Waser N, Larson A, Harrison PJ. 1999. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol. 11, 179–184. ( 10.1023/A:1008046023487) [DOI] [Google Scholar]
- 13.Yu E, Zendejas F, Lane P, Gaucher S, Simmons B, Lane T. 2009. Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation. J. Appl. Phycol. 21, 669–681. ( 10.1007/s10811-008-9400-y) [DOI] [Google Scholar]
- 14.Bligh EG, Dyer WJ. 1959. A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. ( 10.1139/o59-099) [DOI] [PubMed] [Google Scholar]
- 15.Reiser S, Somerville C. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl-coenzyme A reductase. J. Bacteriol. 179, 2969–2975. ( 10.1128/jb.179.9.2969-2975.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riso V De, Raniello R, Maumus F, Rogato A, Bowler C, Falciatore A. 2009. Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res. 37, e96 ( 10.1093/nar/gkp448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Hu H. 2014. High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Marine Genomics 16, 63–66. ( 10.1016/j.margen.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 18.Maheswari U, et al. 2010. Digital expression profiling of novel diatom transcripts provides insight into their biological functions. Genome Biol. 11, R85 ( 10.1186/gb-2010-11-8-r85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siaut M, Heijde M, Mangogna M, Montsant A, Coesel S, Allen A, Manfredonia A, Falciatore A, Bowler C. 2007. Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene 406, 23–35. ( 10.1016/j.gene.2007.05.022) [DOI] [PubMed] [Google Scholar]
- 20.Ding G, Che P, Ilarslan H, Wurtele ES, Nikolau BJ. 2012. Genetic dissection of methylcrotonyl CoA carboxylase indicates a complex role for mitochondrial leucine catabolism during seed development and germination. Plant J. 70, 562–577. ( 10.1111/j.1365-313X.2011.04893.x) [DOI] [PubMed] [Google Scholar]
- 21.Blanc G, Etten JLV. 2010. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22, 2943–2955. ( 10.1105/tpc.110.076406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schönknecht G, et al. 2013. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339, 1207–1210. ( 10.1126/science.1231707) [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama T, et al. 2003. Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc. Natl Acad. Sci. USA 100, 8007–8012. ( 10.1073/pnas.0932694100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otzen C, Bardl B, Jacobsen ID, Nett M, Brock M. 2014. Candida albicans utilizes a modified β-oxidation pathway for the degradation of toxic propionyl-CoA. J. Biol. Chem. 289, 8151–8169. ( 10.1074/jbc.M113.517672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CS, Sadre-Bazzaz K, Shen Y, Deng B, Zhou ZH, Tong L. 2010. Crystal structure of the α6β6 holoenzyme of propionyl-coenzyme A carboxylase. Nature 466, 1001–1005. ( 10.1038/nature09302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagan ZS, Dronov AS, Kretovich VL. 1968. Some properties of valine-isoleucine- and valine-glutamate-aminotransferases of pea sprouts. [In Russian.] Doklady Akademii Nauk SSSR 179, 1236–1239. [Google Scholar]
- 27.Hawes JW, Jaskiewicz J, Shimomura Y, Huang B, Bunting J, Harper ET, Harris RA. 1996. Primary structure and tissue-specific expression of human β-hydroxyisobutyryl-coenzyme A hydrolase. J. Biol. Chem. 271, 26 430–26 434. ( 10.1074/jbc.271.42.26430) [DOI] [PubMed] [Google Scholar]
- 28.Loupatty FJ, et al. 2007. Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am. J. Hum. Genet. 80, 195–199. ( 10.1086/510725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters H, Ferdinandusse S, Ruiter JP, Wanders RJA, Boneh A, Pitt J. 2015. Metabolite studies in HIBCH and ECHS1 defects: implications for screening. Mol. Genet. Metab. 115, 168–173. ( 10.1016/j.ymgme.2015.06.008) [DOI] [PubMed] [Google Scholar]
- 30.Zolman BK, Monroe-Augustus M, Thompson B, Hawes JW, Krukenberg KA, Matsuda SPT, Bartel B. 2001. Chy1, an Arabidopsis mutant with impaired β-oxidation, is defective in a peroxisomal β-hydroxyisobutyryl-CoA hydrolase. J. Biol. Chem. 276, 31 037–31 046. ( 10.1074/jbc.M104679200) [DOI] [PubMed] [Google Scholar]
- 31.Hockin NL, Mock T, Mulholland F, Kopriva S, Malin G. 2012. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 158, 299–312. ( 10.1104/pp.111.184333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa H, Gomi T, Fujioka M. 2000. Serine hydroxymethyltransferase and threonine aldolase: are they identical? Int. J. Biochem. Cell Biol. 32, 289–301. ( 10.1016/S1357-2725(99)00113-2) [DOI] [PubMed] [Google Scholar]
- 33.Hochachka PW, Mustafa T. 1973. Invertebrate facultative anaerobiosis. Science 178, 1056–1060. ( 10.1126/science.178.4065.1056) [DOI] [PubMed] [Google Scholar]
- 34.Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. ( 10.1016/S1360-1385(02)02312-9) [DOI] [PubMed] [Google Scholar]
- 35.Banu MN, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, Murata Y. 2009. Proline and glycine betaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 166, 146–156. ( 10.1016/j.jplph.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 36.Badran EG, Abogadallah GM, Nada RM, Alla MMN. 2015. Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 252, 835–844. ( 10.1007/s00709-014-0720-2) [DOI] [PubMed] [Google Scholar]
- 37.Kerner J, Hoppel C. 2000. Fatty acid import into mitochondria. BBA-Mol. Cell Biol. Lipids 1486, 1–17. ( 10.1016/S1388-1981(00)00044-5) [DOI] [PubMed] [Google Scholar]
- 38.Rippa S, Zhao Y, Merlier F, Charrier A, Perrin Y. 2012. The carnitine biosynthetic pathway in Arabidopsis thaliana shares similar features with the pathway of mammals and fungi. Plant Physiol. Biochem. 60, 109–114. ( 10.1016/j.plaphy.2012.08.001) [DOI] [PubMed] [Google Scholar]
- 39.Kroth PG, et al. 2008. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 3, e1426 ( 10.1371/journal.pone.0001426) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data have been uploaded as the electronic supplementary material.