Abstract
Aims
To describe factors associated with prevalence or absence of microvascular and macrovascular complications in people with Type 1 diabetes of very long duration and to investigate the risk factors associated with the incidence of such complications.
Methods
We included individuals with Type 1 diabetes who had been entered in the Swedish National Diabetes Register between 2002 and 2004 (n = 18 450). First, risk factor distribution in people with diabetes duration of ≥ 50 years was compared between people with and without complications. Second, the incidence of complications during a 10‐year follow‐up period was studied in all individuals who had no complications at baseline.
Results
Among people with a diabetes duration of ≥ 50 years (n = 1023), 453 (44%) had macrovascular disease, 534 (52%) had microvascular disease and 319 (31%) did not have either of the diagnoses. Factors that differed significantly between people with and without macrovascular disease were gender, age, HbA1c, BMI, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, albuminuria, antihypertensive medication and lipid‐lowering medication. The same factors differed significantly between people with and without microvascular disease, with the exception of gender and HDL cholesterol. During the follow‐up period, 6.1% of the study cohort were diagnosed with macrovascular disease and 19.6% with microvascular disease. Incidence of macrovascular disease was significantly associated with HbA1c levels. Hazard ratios decreased with longer diabetes duration.
Conclusions
People with Type 1 diabetes who have survived ≥ 50 years without complications are significantly younger, and have significantly lower HbA1c levels, BMI and triglyceride levels than survivors with complications. HbA1c level is a predictor of macrovascular disease, independently of diabetes duration.
What's new?
Previous studies in long‐term survivors of Type 1 diabetes have mainly been cross‐sectional, relying on self‐reported data, and the results have been inconsistent.
This register‐based study of 18 450 people with Type 1 diabetes shows that those who have survived ≥ 50 years without complications are significantly younger and have significantly lower HbA1c levels, BMI and triglyceride levels than survivors with complications.
HbA1c is a predictor of macrovascular disease, independently of diabetes duration, even after 50 years’ duration of Type 1 diabetes.
It is important to identify factors that may protect individuals with Type 1 diabetes from major complications.
What's new?
Previous studies in long‐term survivors of Type 1 diabetes have mainly been cross‐sectional, relying on self‐reported data, and the results have been inconsistent.
This register‐based study of 18 450 people with Type 1 diabetes shows that those who have survived ≥ 50 years without complications are significantly younger and have significantly lower HbA1c levels, BMI and triglyceride levels than survivors with complications.
HbA1c is a predictor of macrovascular disease, independently of diabetes duration, even after 50 years’ duration of Type 1 diabetes.
It is important to identify factors that may protect individuals with Type 1 diabetes from major complications.
Introduction
Type 1 diabetes is associated with an increased risk of microvascular and macrovascular disease. People with Type 1 diabetes have a two to three times higher risk of death and a life expectancy that is shorter by more than a decade 1, 2. A follow‐up study in Swedish people with diabetes who were hospitalized between 1965 and 1983 found that the majority of deaths (62%) were attributed to circulatory disease 3. Some people with diabetes, however, never develop complications despite long duration of the disease.
Glycaemic control has been shown to be one of the strongest predictors of diabetes complications 4, 5, 6, 7, 8. Previous studies in people with very long duration of diabetes have suggested several possible protective factors against these complications 9, 10. The design of these studies has, however, been mainly cross‐sectional, relying on self‐reported data, and the results are inconsistent. The Golden Years Study reported relatively mediocre glycaemic control among long‐term survivors, whereas the Joslin 50‐year Medalist study found no association between HbA1c levels and diabetes complications 9, 10.
This register‐based, national cohort study aimed to describe the factors that are associated with prevalence or absence of microvascular and macrovascular complications in people with Type 1 diabetes of very long duration, as well as to investigate the factors that are associated with the incidence of such complications during a 10‐year follow‐up period.
Study population and methods
The present study was based on information from linking the Swedish National Diabetes Register (NDR), Hospital Discharge Register, Cause of Death Register and Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA). The Regional Ethical Review Board at the University of Gothenburg approved the study.
Databases
The NDR has been described previously 11. Briefly, the register was launched in 1996 as a tool for quality assurance in diabetes care, and contains information about risk factors, complications and treatment among people with diabetes aged ≥ 18 years. Trained nurses and physicians report data at least once a year either online or by electronic transmission of patient charts. Data are collected during appointments at specialist clinics and primary healthcare centres nationwide. All participants give their informed consent before inclusion in the NDR. Data on an estimated 90% of all adults with Type 1 diabetes in Sweden have been entered into the NDR 12.
The Hospital Discharge Register and Cause of Death Register are administered by the Swedish National Board of Health and Welfare. Since 1987, the Hospital Discharge Register has included data for all inpatient care. As of 2001, data from outpatient appointments with both private and public care providers have also been included. The data concern diagnoses, procedures and length of hospital stay.
The Cause of Death Register contains data for all Swedes, regardless of whether they died in Sweden or abroad.
Administered by Statistics Sweden, the LISA register contains information about marital status, educational level and disposable income for all Swedes.
Study population
In the present study we included all people with Type 1 diabetes entered into the NDR between 1 January 2002 and 31 December 2004, with a BMI > 18.5 kg/m2 (n = 18 450). Type 1 diabetes was defined on the basis of epidemiological data, i.e. treatment with insulin and diagnosis at the age of ≤ 30 years. This definition has been validated as accurate in 97% of the cases entered into the NDR 13.
Baseline examinations and definitions
The clinical characteristics reported to the NDR at baseline are age, gender, diabetes duration, HbA1c, weight, height, blood pressure, total cholesterol, HDL cholesterol, triglycerides, microalbuminuria, macroalbuminuria, smoking status and use of antihypertensive and lipid‐lowering medication. Smoking status is recorded as either yes or no. Marital status is either single, married/registered partner, divorced or widowed.
All laboratory analyses were performed at local facilities. HbA1c values are expressed in mmol/mol as specified by the International Federation of Clinical Chemistry and converted to National Glycohemoglobin Standardization Program [Diabetes Control and Complications Trial (DCCT) standard] values (%) 14. BMI was calculated as weight in kg/height in m2. LDL cholesterol concentration was based on Friedewald's formula 15. Microalbuminuria was defined as two positive results for three samples obtained within 1 year. A positive result was defined as an albumin/creatinine ratio of 3–30 mg per millimole (~30–300 mg/g) or a urinary albumin clearance of 20–200 μg/min (20–300 mg/l). Macroalbuminuria was defined as an albumin/creatinine ratio > 30 mg/mmol (~300 mg/g) or a urinary albumin clearance > 200 μg/min (> 300 mg/l).
Follow‐up and definition of endpoints
People who did not have a history of cardiovascular disease (CVD), microvascular disease or albuminuria (n = 12 334) were monitored from the date of registration until death, first diagnosis of CVD/microvascular disease or the end of the period (31 December 2012; Fig. 1). CVD was defined as a primary or contributory diagnosis of one of the following conditions or procedures: ischaemic heart disease; myocardial infarction; stroke; unstable angina; percutaneous coronary intervention; coronary artery bypass grafting; and peripheral vascular disease or amputation. Cases were retrieved by linking Swedish personal identity numbers with the Hospital Discharge Register and the Cause of Death Register. Detailed information about the International Classification of Diseases (ICD) codes used is presented in the Supporting Information (File S1).
Figure 1.
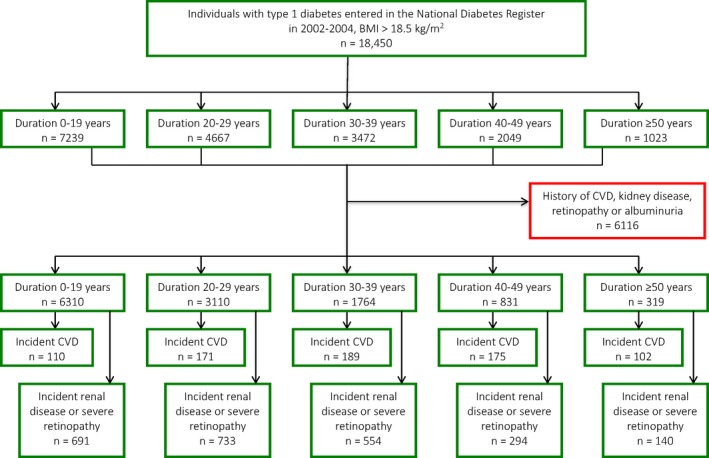
Study flowchart. CVD, cardiovascular disease.
Microvascular disease was defined as renal impairment and/or severe retinopathy. Renal impairment was defined as a primary or contributory diagnosis of one of the following diagnoses or conditions: acute renal failure; chronic renal failure; specified diabetic kidney disease; unspecified diabetic kidney disease; other kidney disease; dialysis; haemodialysis; peritoneal dialysis; kidney transplant; or renal dialysis and transplantation.
Severe retinopathy was defined as a primary or contributory diagnosis using ICD codes stated in the Supporting Information (File S1). In Sweden, diabetic retinopathy is always diagnosed by an ophthalmologist, which enables a high sensitivity and specificity.
Statistics
The sample was stratified into six diabetes duration groups: 0–19 years; 20–29 years; 30–39 years; 40–49 years; and ≥ 50 years. Between 6 and 37% of the people included in the study had missing values for at least one of the following variables: HbA1c; blood pressure; total cholesterol; lipoproteins; triglycerides; albuminuria; smoking status; marital status; educational level; or disposable income. Multiple imputation, generating 10 datasets, was used to avoid a reduction in sample size. Chi‐squared tests were used to compare people with and without previous CVD or microvascular disease. Cox proportional hazards regression was used to estimate hazard ratios for the association between various baseline variables and incidence of CVD and microvascular disease across the diabetes duration groups. The proportional hazards assumption was confirmed by plotting the negative log of the estimated survivor functions vs time. The survival analysis included only people who did not have a history of CVD, microvascular disease and albuminuria. The following variables were included in the models: age; gender; systolic blood pressure; BMI; total cholesterol; HDL cholesterol; triglycerides; antihypertensive medication; lipid‐lowering medication; educational level; marital status; and income. All analyses were performed in sas version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics for the diabetes duration groups
The risk factor distribution among the various groups of diabetes duration is shown in Table 1. Of those with a diabetes duration ≥ 50 years (n = 1023), 453 (44%) had a history of CVD and 534 (52%) had a history of kidney disease or severe retinopathy, while 319 (31%) did not have any of these diagnoses. Descriptive statistics of people with diabetes duration of ≥ 50 years, with and without previous CVD or microvascular disease, are shown in Tables 2 and 3. Factors that differed significantly in this duration group between those with and those without previous CVD were: gender; age; HbA1c; BMI; LDL cholesterol; HDL cholesterol; triglycerides; systolic blood pressure; antihypertensive medication; lipid‐lowering medication; and albuminuria. The same factors, with the exception of gender and HDL cholesterol, differed significantly in this duration group between people with and without previous renal impairment or severe retinopathy. Diastolic blood pressure also differed significantly between people with and without previous microvascular disease. Mean systolic blood pressure was significantly higher in people with previous microvascular disease, whereas it was significantly lower in people with previous CVD in this duration group.
Table 1.
Descriptive statistics of individuals with various diabetes durations
| Variable | 0–19 years (n = 7239) | 20–29 years (n = 4667) | 30–39 years (n = 3472) | 40–49 years (n = 2049) | ≥ 50 years (n = 1023) |
|---|---|---|---|---|---|
| Diabetes duration | 11.2 (5.5) | 24.3 (2.9) | 34.0 (2.9) | 43.9 (2.8) | 55.4 (5.0) |
| Male, n (%) | 4143 (57.2) | 2596 (55.6) | 1809 (52.1) | 1057 (51.6) | 524 (51.2) |
| Age, years | 29.3 (6.9) | 39.0 (8.3) | 48.2 (8.1) | 56.8 (7.4) | 65.9 (7.5) |
| Age at diagnosis, years | 18.1 (7.0) | 14.6 (7.7) | 14.2 (7.7) | 12.9 (7.1) | 10.5 (6.6) |
| BMI, kg/m2 | 25.3 (4.0) | 25.5 (3.6) | 25.4 (3.7) | 25.2 (3.5) | 25.3 (3.8) |
| HbA1c | |||||
| mmol/mol | 64 ± 16 | 66 ± 14 | 66 ± 13 | 64 ± 12 | 63 ± 12 |
| % | 8.0 ± 3.6 | 8.2 ± 3.4 | 8.2 ± 3.3 | 8.0 ± 3.3 | 7.9 ± 3.2 |
| LDL cholesterol, mmol/l | 2.7 (0.8) | 2.8 (0.8) | 2.8 (0.8) | 2.8 (0.8) | 2.8 (0.8) |
| HDL cholesterol, mmol/l | 1.5 (0.4) | 1.6 (0.5) | 1.7 (0.5) | 1.7 (0.5) | 1.7 (0.5) |
| Total cholesterol, mmol/l | 4.7 (1.0) | 4.9 (0.9) | 5.1 (0.9) | 5.1 (0.9) | 5.0 (0.9) |
| Triglycerides, mmol/l | 1.2 (1.0) | 1.2 (0.9) | 1.2 (0.8) | 1.1 (0.7) | 1.2 (0.8) |
| Systolic blood pressure, mmHg | 121.6 (12.8) | 127.8 (15.9) | 134.0 (16.9) | 139.9 (17.1) | 143.8 (18.5) |
| Diastolic blood pressure, mmHg | 73.5 (8.6) | 75.3 (8.8) | 74.5 (8.9) | 72.4 (8.7) | 70.9 (9.6) |
| Previous CVD, n (%) | 62 (0.9) | 238 (5.1) | 548 (15.8) | 568 (27.7) | 453 (44.3) |
| Previous kidney disease, n (%) | 348 (4.8) | 609 (13.0) | 749 (21.6) | 633 (30.9) | 459 (44.9) |
| Previous severe retinopathy, n (%) | 105 (1.5) | 475 (10.2) | 492 (14.2) | 357 (17.4) | 173 (16.9) |
| Previous kidney disease and severe retinopathy, n (%) | 26 (0.4) | 158 (3.4) | 169 (4.9) | 142 (6.9) | 98 (9.6) |
| Smoker, n (%) | 896 (13.2) | 633 (14.3) | 486 (14.8) | 241 (12.4) | 72 (7.4) |
| Lipid‐lowering medication, n (%) | 332 (4.8) | 767 (17.2) | 981 (29.7) | 773 (40.0) | 452 (46.6) |
| Albuminuria, n (%) | 589 (8.1) | 1041 (22.3) | 1094 (31.5) | 688 (33.6) | 393 (38.4) |
| Microalbuminuria, n (%) | 455 (7.3) | 637 (15.7) | 597 (19.4) | 370 (20.5) | 205 (22.6) |
| Macroalbuminuria, n (%) | 134 (2.2) | 404 (10.0) | 497 (16.1) | 318 (17.6) | 188 (20.7) |
| Married, n (%) | 1555 (21.6) | 1872 (40.2) | 1831 (53.1) | 1207 (59.7) | 628 (62.9) |
| Divorced, n (%) | 293 (4.1) | 511 (11.0) | 510 (14.8) | 337 (16.7) | 158 (15.8) |
| Widow/widower, n (%) | 5 (0.1) | 16 (0.3) | 55 (1.6) | 90 (4.5) | 97 (9.7) |
| High education level, n (%) | 2199 (30.7) | 1452 (31.3) | 979 (28.4) | 502 (24.9) | 149 (15.0) |
| Income: top quintile, n (%) | 1148 (15.9) | 1152 (24.8) | 829 (24.1) | 412 (20.4) | 125 (12.5) |
Data are presented as means (±sd) unless otherwise indicated.
Table 2.
Descriptive statistics of individuals with diabetes duration ≥ 50 years with and without previous cardiovascular disease
| Variable | Previous cardiovascular disease | P | |
|---|---|---|---|
| No (n = 570) | Yes (n = 453) | ||
| Diabetes duration | 54.9 (4.8) | 56.0 (5.3) | < 0.001 |
| Male sex, n (%) | 276 (48.4) | 248 (54.7) | 0.044 |
| Age, years | 65.0 (7.5) | 66.9 (7.4) | < 0.001 |
| Age at diagnosis, years | 10.1 (6.6) | 10.9 (6.6) | 0.059 |
| HbA1c | |||
| mmol/mol | 62 ± 12 | 64 ± 12 | < 0.001 |
| % | 7.8 ± 3.3 | 8.0 ± 3.2 | |
| BMI, kg/m2 | 25.0 (3.7) | 25.6 (4.0) | < 0.001 |
| LDL cholesterol, mmol/l | 2.9 (0.8) | 2.7 (0.8) | 0.001 |
| HDL cholesterol, mmol/l | 1.8 (0.5) | 1.6 (0.5) | < 0.001 |
| Total cholesterol, mmol/l | 5.2 (0.9) | 4.9 (0.9) | 0.567 |
| Triglycerides, mmol/l | 1.1 (0.6) | 1.4 (1.0) | < 0.001 |
| Systolic blood pressure, mm Hg | 144.5 (18.4) | 143.0 (18.7) | < 0.001 |
| Diastolic blood pressure, mm Hg | 70.9 (9.6) | 70.9 (9.5) | 0.055 |
| Previous kidney disease, n (%) | 89 (15.6) | 370 (81.7) | < 0.001 |
| Previous severe retinopathy, n (%) | 73 (12.8%) | 100 (22.1%) | < 0.001 |
| Smoker, n (%) | 43 (7.9) | 29 (6.7) | 0.489 |
| Antihypertensive medication, n (%) | 345 (61.5) | 375 (83.7) | < 0.001 |
| Lipid lowering medication, n (%) | 192 (35.7) | 260 (60.2) | < 0.001 |
| Albuminuria, n (%) | 180 (31.6) | 213 (47.0) | < 0.001 |
| Microalbuminuria, n (%) | 100 (19.5) | 105 (26.5) | 0.012 |
| Macroalbuminuria, n (%) | 80 (15.6) | 108 (27.3) | < 0.001 |
| Married, n (%) | 345 (61.4%) | 283 (64.8) | 0.274 |
| Divorced, n (%) | 88 (15.7) | 70 (16.0) | 0.877 |
| Widow/widower, n (%) | 57 (10.1) | 40 (9.2) | 0.600 |
| High education level | 91 (16.2) | 58 (13.4) | 0.210 |
| Income: top quintile, n (%) | 80 (14.2) | 45 (10.3) | 0.062 |
Data are presented as means (±sd) unless otherwise indicated.
Table 3.
Descriptive statistics of individuals with diabetes duration ≥ 50 years with and without previous microvascular disease
| Variable | Previous microvascular disease | P | |
|---|---|---|---|
| No (n = 489) | Yes (n = 534) | ||
| Diabetes duration, years | 54.9 (4.9) | 55.8 (5.1) | < 0.001 |
| Male, n (%) | 239 (48.9%) | 285 (53.4%) | 0.151 |
| Age, years | 65.1 (7.5) | 66.5 (7.6) | < 0.001 |
| Age at diagnosis, years | 10.3 (6.7) | 10.7 (6.5) | < 0.001 |
| HbA1c, mmol/mol (%) | 61 ± 11 (7.7 ± 3.2) | 65 ± 12 (8.1 ± 3.3) | < 0.001 |
| BMI, kg/m2 | 25.1 (3.5) | 25.5 (4.1) | < 0.001 |
| LDL cholesterol, mmol/l | 2.8 (0.8) | 2.7 (0.8) | 0.001 |
| HDL cholesterol, mmol/l | 1.8 (0.5) | 1.7 (0.5) | 0.302 |
| Total cholesterol, mmol/l | 5.1 (0.9) | 5.0 (0.9) | < 0.001 |
| Triglycerides, mmol/l | 1.1 (0.6) | 1.3 (0.9) | < 0.001 |
| Systolic blood pressure, mmHg | 143.2 (17.4) | 144.4 (19.4) | < 0.001 |
| Diastolic blood pressure, mmHg | 70.1 (9.6) | 71.6 (9.5) | < 0.001 |
| Previous cardiovascular disease, n (%) | 66 (13.5) | 387 (72.5) | < 0.001 |
| Previous kidney disease, n (%) | 459 (86.0) | ||
| Previous severe retinopathy, n (%) | 173 (32.4) | ||
| Smoker, n (%) | 40 (8.5) | 32 (6.3) | 0.198 |
| Antihypertensive medication, n (%) | 300 (62.1) | 420 (79.8) | < 0.001 |
| Lipid‐lowering medication, n (%) | 171 (36.5) | 281 (56.0) | < 0.001 |
| Albuminuria, n (%) | 125 (25.6) | 268 (50.2) | < 0.001 |
| Microalbuminuria, n (%) | 85 (19.6) | 120 (25.2) | 0.044 |
| Macroalbuminuria, n (%) | 40 (9.2) | 148 (31.1) | < 0.001 |
| Married, n (%) | 296 (61.7) | 332 (64.0) | 0.452 |
| Divorced, n (%) | 73 (15.2) | 85 (16.4) | 0.613 |
| Widow/widower, n (%) | 52 (10.8) | 45 (8.7) | 0.249 |
| High education level | 82 (17.1) | 67 (13.0) | 0.068 |
| Income: top quintile, n (%) | 68 (14.2) | 57 (11.0) | 0.129 |
Data are presented as means (±sd) unless otherwise indicated.
Incidence of cardiovascular disease
The descriptive statistics of those included in the survival analysis are shown in Table S1. During the mean follow‐up period of 9.4 years, 747 people (6.1%) were diagnosed with CVD (6.4 per 1000 person‐years). The relationships between various risk factors and incidence of CVD in the different diabetes duration groups are shown in Table S2. After adjustment for potential confounders, age and HbA1c were the only factors significantly associated with incidence of CVD in people with diabetes duration ≥ 50 years. Age and HbA1c were also the only factors significantly associated with incidence of CVD in all duration groups. The hazard ratios for HbA1c showed an inverse relationship with diabetes duration (Fig. 2).
Figure 2.
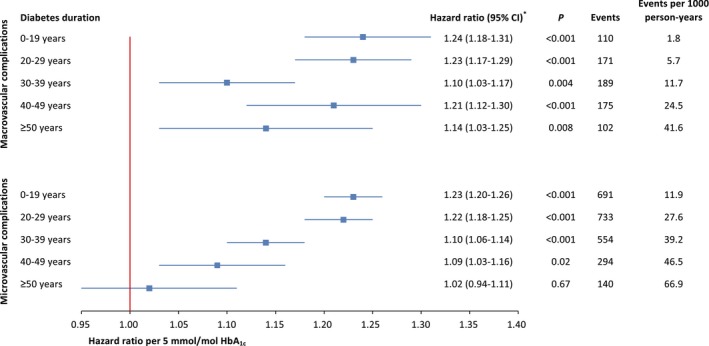
Hazard ratio per 5‐unit increase (mmol/mol) in HbA1c for microvascular and macrovascular complications. *Adjusted for age, gender, smoking status, systolic blood pressure, BMI, total cholesterol, HDL cholesterol, triglycerides, antihypertensive medication, lipid‐lowering medication, educational level, marital status and income. CI, confidence interval.
Incidence of microvascular disease
During the mean follow‐up period of 8.7 years, 2412 people (19.6%) were diagnosed with renal impairment or severe retinopathy (22.5 per 1000 person‐years). The relationships between various risk factors and incidence of microvascular disease in the different diabetes duration groups are shown in Table S3. Marital status was the only factor that was significantly associated with incidence of microvascular disease in people with a diabetes duration of ≥ 50 years. The hazard ratios for HbA1c showed a similar inverse relationship with diabetes duration, as in the case of CVD, although HbA1c levels were not significantly associated with incidence of microvascular disease in people with a diabetes duration of ≥ 50 years (Fig. 2).
Discussion
In this register‐based cohort study, we found that people who had survived ≥ 50 years with Type 1 diabetes without microvascular or macrovascular complications were significantly younger and had significantly lower HbA1c levels, BMI and triglyceride levels than those who did have a history of such complications. They were also found to have higher LDL cholesterol levels, less albuminuria and less use of antihypertensive or lipid‐lowering medication. In addition, we found that HbA1c levels were significantly associated with incidence of macrovascular complications, independently of duration of Type 1 diabetes. The risk estimates were inversely associated with duration of Type 1 diabetes, suggesting that HbA1c is less of a risk factor among people who have survived a very long diabetes duration.
A previous cross‐sectional study of 400 people with > 50 years duration of Type 1 diabetes (the Golden Years cohort in the UK) suggested that they were relatively well protected from microvascular and macrovascular disease, possibly as a result of elevated HDL cholesterol levels 9. The present study showed significantly higher levels of HDL cholesterol in corresponding people who did not have a history of CVD at baseline. A similar trend was observed in people who did not have a history of microvascular disease at baseline, although these differences were not statistically significant. Another cross‐sectional study of 351 people with > 50 years duration of Type 1 diabetes (the Joslin 50‐year Medalist study conducted in the USA) found lower HDL cholesterol levels in people who reported a history of CVD 10. The same study, however, did not report any differences in HDL cholesterol levels among people with a history of nephropathy, retinopathy or neuropathy. In addition, a survival analysis of the present cohort showed no independent association between HDL cholesterol and incidence of microvascular or macrovascular complications in any of the diabetes duration groups. People without previous CVD or microvascular disease were also found to have significantly higher LDL cholesterol levels in the present study. This might be attributable to the fact that lipid‐lowering medication was less common in these people than in those with a history of such complications and who were therefore undergoing secondary prevention measures.
In addition to having higher HDL cholesterol levels, long‐term diabetes survivors have also been reported to have normal body weight, normal blood pressure and low insulin dosages 16. Information on insulin dosage was not available in the present study, but people with diabetes duration ≥ 50 years without previous CVD or microvascular disease were found to have a significantly lower BMI than those with a history of such complications. People with a diabetes duration of ≥ 50 years without previous CVD had significantly higher systolic blood pressure than those with previous CVD in the present study; however, ~84% of people with a history of such complications reported the use of antihypertensive medication. The corresponding proportion of those without previous CVD was ~62%, which is more than twice as high as the figures reported in the Golden Years cohort 9. This is somewhat surprising given that the mean age among people without complications in the present cohort was only 64 years, as opposed to 69 years in the Golden Years study, and is probably attributable to recent changes in risk factor treatment practices. People without previous microvascular disease were found to have significantly lower systolic and diastolic blood pressure than those with a history of such disease. Systolic blood pressure was, however, not associated with incidence of microvascular or macrovascular complications in any of the diabetes duration subgroups after adjustment for potential confounding factors.
Studies have generated conflicting results about the importance of glycaemic control among long‐term survivors. The Joslin Medalist study reported a mean HbA1c of 53 mmol/mol (7%) at the time of examination, whereas the Golden Years study reported a non‐DCCT mean HbA1c of 7.6% (equivalent to 60 mmol/mol). The present study indicated even higher levels of HbA1c among people both without [61–62 mmol/mol (7.7–7.8%)] and with [64–65 mmol/mol (8.0–8.1%)] a history of complications. Although a single HbA1c measurement is indicative of glycaemic control only during the past 3 months, it has been shown that a random measurement of HbA1c is highly correlated with long‐term HbA1c 17, 18.
Studies based on the NDR have also shown that glycaemic control concomitant with a diagnosis of Type 1 diabetes in childhood or adolescence can predict glycaemic control in early adulthood 19. Individuals with a high mean HbA1c level 3–15 months after diagnosis have macroalbuminuria and retinopathy significantly more often in early adulthood 19. The development of complications is likely to be caused by the level of glycaemia over a period of many years (i.e. metabolic memory) against the backdrop of genetic disposition and other risk factors 20, 21, 22. The present study showed that HbA1c levels were associated with incidence of microvascular and macrovascular complications, but that predictability was inversely related to diabetes duration.
The relatively high baseline HbA1c in long‐term, complication‐free survivors indicates the presence of other protective factors. It has been suggested that circulating and endothelial progenitor cells are involved in protection from cardiovascular complications and nephropathy 23. It was also shown that certain advanced glycation endproducts were inversely associated with proliferative diabetic retinopathy in long‐term survivors, as opposed to previous studies that showed strong associations between advanced glycation endproducts and diabetes complications 18. Thus, long‐term survivors of diabetes appear to develop a compensatory mechanism against the hazardous effects of advanced glycation endproducts, but the exact dynamic has yet to be discovered 24. Given that longevity has been shown to be high among parents of long‐term survivors, genetic factors are hypothesized to play a significant role, and studies to find protective genetic factors are underway 9, 10.
Being married, divorced or widowed was, compared to being single, associated with a significantly lower incidence of microvascular disease among those with diabetes of ≥ 50 years duration. Previous studies from the NDR have shown a general protective effect of being married/cohabiting 25. One could speculate that this protective effect will also remain for a certain time after the marriage has ended, if the individual was married long enough (e.g. ~50 years); in other words, a legacy effect of being married.
The main strength of the present study is the large number of participants, including people from a nationwide diabetes register with a high participation rate and data from day‐to‐day clinical practice. Another strength is the prospective design with a mean follow‐up period of almost 10 years, which reduces the risk of reverse causation. Most previous studies on long‐term survivors of Type 1 diabetes have been cross‐sectional and included significant percentages of self‐reported data. The present study, by contrast, obtained events from the Hospital Discharge Register and Cause of Death Register. Validation studies of the Hospital Discharge Register have been shown to have high overall specificity 26. Overall, the registry study design differs significantly from previous studies of long‐term survivors, where people have generally been brought into a centre for evaluation specifically for the study. This makes comparisons with previous findings more complex; however, because the present study included data from daily clinical practice, the results are likely to have a high external validity.
The present study was limited to individuals who had survived long enough to be included in the NDR. The Hospital Discharge Register has been nationwide only since 1987, such that early diabetes complications may not have been entered for some people with very long diabetes duration. In addition, some cases may be treated only in primary care, which is not covered by the Hospital Discharge Register; however, almost all people with Type 1 diabetes receive treatment at specialist hospital clinics. Thus, the assumption is that most diabetes complications are reported. We did not have information on whether the myocardial infarctions were silent or not, but we do not believe that this lack of information would have resulted in any systematic bias of our findings. Because all diagnoses were settled during a hospital stay, most cases are assumed to be valid.
Cases with urinary tract infections or recent ketoacidosis were not excluded from albuminuria cases. The numbers of people with these two complications were assumed to be low and therefore unlikely to alter the results in this large sample. Residual confounding is probable despite the analysis having been adjusted for several biological, lifestyle and social/demographic factors.
In conclusion, the present nationwide study shows that among people who had survived ≥ 50 years with Type 1 diabetes, > 30% did not have microvascular or macrovascular complications. These individuals were younger and had lower HbA1c, BMI and triglyceride levels than survivors with complications. They also had higher LDL cholesterol levels, less albuminuria and less antihypertensive/lipid‐lowering medication. In addition, this study shows that HbA1c is a predictor of macrovascular disease, independently of duration of Type 1 diabetes. The results suggest that HbA1c is less of a risk factor in people with very long diabetes duration.
Funding sources
This study was supported by grants from the Strategic Research Area Epidemiology for Health (EpiHealth) at Lund University.
Competing interests
None declared.
Supporting information
File S1. Definition of endpoints including ICD‐codes.
Table S1. Descriptive statistics of individuals included in survival analysis.
Table S2. Hazard ratios for cardiovascular disease during follow‐up in relation to risk factors in various duration groups.
Table S3. Hazard ratios for microvascular disease during follow‐up in relation to risk factors in various duration groups.
Table S4. Number of events during follow‐up in men and women with different diabetes duration.
Acknowledgements
We would like to thank the regional NDR coordinators, participating nurses, physicians and other staff members who have contributed to the NDR. Most of all, we would like to thank the patients who support the NDR, both individually and collectively through the Swedish Diabetes Federation.
Diabet. Med. 34: 411–418 (2017)
References
- 1. Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H et al Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014; 371: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 2. Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N et al Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008‐2010. JAMA 2015; 313: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiderpass E, Gridley G, Nyren O, Pennello G, Landstrom AS, Ekbom A. Cause‐specific mortality in a cohort of patients with diabetes mellitus: a population‐based study in Sweden. J Clin Epidemiol 2001; 54: 802–809. [DOI] [PubMed] [Google Scholar]
- 4. Writing Team for the Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G . Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287: 2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Writing Team for the Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003; 290: 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY et al Intensive diabetes therapy and carotid intima‐media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348: 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY et al The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006; 55: 3556–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bain SC, Gill GV, Dyer PH, Jones AF, Murphy M, Jones KE et al Characteristics of Type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003; 20: 808–811. [DOI] [PubMed] [Google Scholar]
- 10. Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J et al Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50‐year medalist study. Diabetes Care 2007; 30: 1995–1997. [DOI] [PubMed] [Google Scholar]
- 11. Gudbjornsdottir S, Cederholm J, Nilsson PM, Eliasson B. Steering Committee of the Swedish National Diabetes R. The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care 2003; 26: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 12. Swedish National Diabetes Register . 20 years of successful improvements. Available at https://www.ndr.nu/pdfs/20%20years%20of%20successful%20improvements_lowres_singelpage.pdf 2016.
- 13. Eeg‐Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S et al Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 2010; 33: 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I et al IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method‐comparison study. Clin Chem 2004; 50: 166–174. [DOI] [PubMed] [Google Scholar]
- 15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 16. Gale EA. How to survive diabetes. Diabetologia 2009; 52: 559–567. [DOI] [PubMed] [Google Scholar]
- 17. Tarnow L, Kjeld T, Knudsen E, Major‐Pedersen A, Parving HH. Lack of synergism between long‐term poor glycaemic control and three gene polymorphisms of the renin angiotensin system on risk of developing diabetic nephropathy in type I diabetic patients. Diabetologia 2000; 43: 794–799. [DOI] [PubMed] [Google Scholar]
- 18. Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR et al Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50‐year medalist study. Diabetes Care 2011; 34: 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuelsson U, Steineck I, Gudbjornsdottir S. A high mean‐HbA1c value 3‐15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood–a pilot study using two nation‐wide population based quality registries. Pediatr Diabetes 2014; 15: 229–235. [DOI] [PubMed] [Google Scholar]
- 20. The relationship of glycemic exposure . (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995; 44: 968–983. [PubMed] [Google Scholar]
- 21. Lind M, Oden A, Fahlen M, Eliasson B. The shape of the metabolic memory of HbA1c: re‐analysing the DCCT with respect to time‐dependent effects. Diabetologia 2010; 53: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 22. Brownlee M. The pathobiology of diabetic complications – A unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez SL, Gong JH, Chen L, Wu IH, Sun JK, Keenan HA et al Characterization of circulating and endothelial progenitor cells in patients with extreme‐duration type 1 diabetes. Diabetes Care 2014; 37: 2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinik A. The question is, my dear watson, why did the dog not bark?: the joslin 50‐year medalist study. Diabetes Care 2011; 34: 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rawshani A, Svensson AM, Rosengren A, Eliasson B, Gudbjornsdottir S. Impact of Socioeconomic Status on Cardiovascular Disease and Mortality in 24,947 Individuals With Type 1 Diabetes. Diabetes Care 2015; 38: 1518–1527. [DOI] [PubMed] [Google Scholar]
- 26. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Definition of endpoints including ICD‐codes.
Table S1. Descriptive statistics of individuals included in survival analysis.
Table S2. Hazard ratios for cardiovascular disease during follow‐up in relation to risk factors in various duration groups.
Table S3. Hazard ratios for microvascular disease during follow‐up in relation to risk factors in various duration groups.
Table S4. Number of events during follow‐up in men and women with different diabetes duration.


