Abstract
Background
Canine atopic dermatitis (CAD) is an inflammatory and pruritic allergic skin disease caused by interactions between genetic and environmental factors. Previously, a genome‐wide significant risk locus on canine chromosome 27 for CAD was identified in German shepherd dogs (GSDs) and Plakophilin‐2 (PKP2) was defined as the top candidate gene. PKP2 constitutes a crucial component of desmosomes and also is important in signalling, metabolic and transcriptional activities.
Objectives
The main objective was to evaluate the role of PKP2 in CAD by investigating PKP2 expression and desmosome structure in nonlesional skin from CAD‐affected (carrying the top GWAS SNP risk allele) and healthy GSDs. We also aimed at defining the cell types in the skin that express PKP2 and its intracellular location.
Animals/Methods
Skin biopsies were collected from nine CAD‐affected and five control GSDs. The biopsies were frozen for immunofluorescence and fixed for electron microscopy immunolabelling and morphology.
Results
We observed the novel finding of PKP2 expression in dendritic cells and T cells in dog skin. Moreover, we detected that PKP2 was more evenly expressed within keratinocytes compared to its desmosomal binding‐partner plakoglobin. PKP2 protein was located in the nucleus and on keratin filaments attached to desmosomes. No difference in PKP2 abundance between CAD cases and controls was observed.
Conclusion
Plakophilin‐2 protein in dog skin is expressed in both epithelial and immune cells; based on its subcellular location its functional role is implicated in both nuclear and structural processes.
Résumé
Contexte
La dermatite atopique canine (CAD) est une dermatose allergique prurigineuse et inflammatoire due à des interactions entre les facteurs génétiques et environnementaux. Précédemment, un locus de risque significatif sur le chromosome 27 de chien du génome sauvage du Berger Allemand (GSD) a été identifié et la Plakophilin‐2 (PKP2) a été défini comme principal gène candidat. PKP2 constitue un composant crucial des desmosomes et est également important dans les activités signal, métabolique et transcription.
Objectifs
L'objectif principal était d’évaluer le rôle de PKP2 dans la CAD par étude de l'expression de PKP2 et de la structure desmosomique dans la peau non lésionnelle de GSDs atteint de CAD (porteurs de l’’allèle GWAS SNP majeur de risque) et des GSD sains. Nous voulons aussi définir le type cellulaire qui exprime PKP2 et sa localisation intracellulaire.
Sujets/Méthodes
Des biopsies cutanées ont été prélevées pour neuf GSDs atteints de CAD et cinq contrôles sains. Les biopsies ont été congelées pour immunofluorescence et fixées pour morphologie et immunomarquage par microscopie électronique.
Résultats
Nous observons l'expression de PKP2 dans les cellules dendritiques et les cellules T dans la peau de chien. En outre, nous détectons que PKP2 a été plus intensément exprimé par les kératinocytes comparé à la plakoglobine. La protéine PKP2 a été localisée dans le noyau et sur des filaments de kératine attachés aux desmosomes. Aucune différence de quantité de PKP2 entre les cas atopiques et contrôles n'a été observée.
Conclusion
La protéine Plakophiline‐2 dans la peau de chien est exprimée à la fois par les cellules épithéliales et par les cellules immunitaires et, basé sur sa localisation subcellulaire, son rôle fonctionnel est impliqué à la fois dans les processus nucléaires et structurels.
Resumen
Introducción
La dermatitis atópica canina (CAD) es una enfermedad inflamatoria y pruriginosa alérgica de la piel causada por interacciones entre factores genéticos y ambientales. Anteriormente, se identificó un locus de riesgo significativo en el genoma del cromosoma 27 canino para CAD en perros de Pastor Alemán (GSD) y placofilina‐2 (PKP2) se definió como el gen candidato más posible. PKP2 constituye un componente crucial de los desmosomas y también es importante actividades de señal, metabólicas y transcripcionales.
Objetivos
El objetivo principal fue evaluar el papel de la PKP2 en CAD mediante la investigación de la expresión de PKP2 y la estructura desmosómica en la piel no afectada de perros de raza GSD con CAD (portadores del alelo GWAS SNP de alto riesgo) y sanos. También se intentó definir los tipos de células en la piel que expresan PKP2 y su ubicación intracelular.
Animales/Métodos
Se recolectaron biopsias cutáneas de nueve perros afectados por CAD y cinco controles de raza GSD. Las biopsias se congelaron para realizar inmunofluorescencia y se fijaron para inmunomarcaje y estudio morfológico mediante microscopía electrónica.
Resultados
Se observó por vez primera la expresión de PKP2 en células dendríticas y células T en la piel del perro. Por otra parte, detectamos que PKP2 fue más uniformemente expresada dentro de queratinocitos en comparación con su proteína de unión en desmosomas, placoglobina. La proteína PKP2 se localizó en el núcleo y en filamentos de queratina unidos a desmosomas. No se observó diferencia en la abundancia de PKP2 entre los casos afectados por CAD y los controles.
Conclusión
La proteína placofilina‐2 en la piel de perro se expresa tanto en las células epiteliales como inmunitarias, y basados en su ubicación subcelular su papel funcional estaría implicado en procesos nucleares y estructurales.
Zusammenfassung
Hintergrund
Die atopische Dermatitis des Hundes (CAD) ist eine entzündliche und juckende allergische Hauterkrankung, die durch Wechselwirkungen von genetischen und Umweltfaktoren verursacht wird. Bisher wurde ein Genom‐weiter signifikanter Risikolokus am caninen Chromosom 27 bei Deutschen Schäferhunden (GSs) für CAD identifiziert und Plakophilin‐2 (PKP2) wurde als Top Kandidatengen definiert. PKP2 stellt eine wesentliche Komponente der Desmosomen dar und ist auch wichtig bei der Nachrichtenübermittlung, dem Stoffwechsel und bei der Transkription.
Ziele
Das Hauptziel war eine Evaluierung der Rolle von PKP2 bei der CAD mittels Untersuchung der PKP2 Exprimierung und der Desmosomenstruktur in nichtveränderter Haut von Schäferhunden mit CAD (Träger des Top GWAS SNP Risikoallels) und gesunden Schäferhunden. Wir versuchten auch die Zelltypen in der Haut, die PKP2 exprimieren zu definieren und ihre intrazelluläre Lokalisation zu bestimmen.
Tiere/Methoden
Es wurden Hautbiopsien von neun Schäferhunden mit CAD und fünf Schäferhunden als Kontrolle genommen. Die Biopsien wurden für eine Immunfluoreszenzuntersuchung eingefroren und fixiert für die Immunmarkierung und Morphologie‐Analyse mittels Elektronenmikroskop.
Ergebnisse
Als neue Beobachtung fanden wir die Exprimierung von PKP2 in denritischen Zellen und in T Zellen der Hundehaut. Zusätzlich fanden wir, dass PKP2 im Vergleich zum desmosomalen Bindungspartner Plakoglobin innerhalb der Keratinozyten gleichmäßiger exprimiert wurde. PKP2 Protein war im Kern lokalisiert und durch Keratinfilamente an den Desmosomen fixiert. Es wurde kein Unterschied im Ausmaßs des PKP2 zwischen den CAD Fällen und den Kontrollen beobachtet.
Schlussfolgerung
Plakophilin‐2 Protein in der Hundehaut wird sowohl in epithelialen wie auch in Immunzellen exprimiert und aufgrund seiner subzellulären Lokalisation wird seine funktionelle Rolle sowohl in Vorgängen im Kern wie auch in der Struktur vermutet.
要約
背景
犬アトピー性皮膚炎(CAD)は、遺伝的要因と環境的要因との相互作用によって引き起こされる炎症性および掻痒性アレルギー性皮膚疾患である。これまで、ジャーマンシェパードドッグ(GSD)のCADゲノムワイド解析により、その重要な危険遺伝子座としてイヌ第27染色体が同定され、最重要候補遺伝子としてPlakophilin‐2(PKP2)が同定された。PKP2は、デスモソームの重要な構成要素であり、シグナル伝達、代謝および転写活性においても重要である。
目的
主要な目的は、CADに罹患しているGSD(GWASにおける最重要SNP危険対立遺伝子を保有する)の非正常皮膚および健常なGSDのPKP2発現およびデスモソーム構造を調べることにより、CADにおけるPKP2の役割を評価することであった。我々はまた、皮膚におけるPKP2を発現する細胞の種類およびその細胞内での位置を同定することを目的とした。
供与動物/方法
CAD罹患した9頭のGSDおよび5頭のコントロールGSDから皮膚生検を採取した。 生検材料を免疫蛍光検査のために凍結させ、電顕免疫標識検査および形態検査のために固定した。
結果
我々は、犬の皮膚における樹状細胞およびT細胞におけるPKP2発現を新たに発見した。さらに、PKP2は、デスモソームの結合相手であるプラコグロビンと比較して、ケラチノサイト内でより均一に発現していた。PKP2タンパク質は核内およびデスモソームに付着したケラチンフィラメント上に局在していた。CAD症例とコントロールとの間にPKP2発現量に差異は認められなかった。
結論
イヌの皮膚において、プラコフィリン‐2タンパク質は、上皮および免疫細胞の両方に発現していた。また、細胞内の発現位置により、その機能的役割は、核および構造形成過程の両方に関与すると考えられる。
摘要
背景
犬异位性皮炎(CAD)由遗传和环境因素的相互作用而引起,是一种炎性和瘙痒性过敏皮肤病。已有研究证实,在患异位性皮炎德国牧羊犬(GSDs)的第27号染色体上,存在一组重要风险基因,桥粒斑菲素蛋白2(PKP2)被认为是最高风险的候选基因。PKP2是构成桥粒的关键组分,在信号传导,代谢和转录活性中也十分重要。
目的
主要目的是研究发生CAD(携带GWAS SNP风险等位基因的顶部)和健康GSDs的无病灶性皮肤中,PKP2的表达和细胞桥粒结构,来评估PKP2在CAD中的作用。同时,我们期望能确定在皮肤中表达PKP2的细胞类型,及其在细胞内的位置。
动物/方法
从9只CAD和5只健康对照的德国牧羊犬上收集皮肤活检样品。将活检样品冷冻用免疫荧光检测,固定后用于电子显微镜免疫标记检测和形态学观察。
结果
我们首次发现,在犬皮肤的树突状细胞和T细胞中存在PKP2的表达。结果显示,与桥粒斑珠蛋白相比,PKP2在角质细胞内能更均匀地表达。PKP2蛋白位于细胞核内和附着于桥粒的角蛋白丝上。PKP2的丰度在CAD病例组与对照组之间没有差异。
结论
犬皮肤中的PKP2蛋白在上皮细胞和免疫细胞中均有表达,根据其亚细胞定位推测,其功能可能涉及细胞核和细胞结构的构建过程。
Resumo
Contexto
A dermatite atópica canina (DAC) é uma dermatopatia alérgica inflamatória e pruriginosa causada por interações entre fatores genéticos e ambientais. Anteriormente, um loco no cromossomo canino 27 foi identificado por métodos genômicos como um risco significativo para DAC em pastores alemães (PAs), e o gene placofilina‐2 (PKP2) foi definido como o principal candidato. PKP2 é um componente crucial dos desmossomos e também é importante nas atividades metabólicas, transcricionais e de sinalização.
Objetivos
O principal objetivo foi avaliar o a função da PKP2 na DAC a partir da investigação na expressão de PKP2 e da estrutura dos desmossomos na pele alesional de cães com DAC (portadores do alelo de risco GWAS SNP) e PAs saudáveis.
Animais/métodos
Biópsias cutâneas foram coletadas de nove cães atópicos e cinco PAs saudáveis (controle). Os fragmentos foram congelados para imunofluorescência e fixados para imunomarcação e análise de morfologia em microscópio eletrônico.
Resultados
Observou‐se a expressão de PKP2 em células dendríticas e células T em cães, um achado inédito. Além disto, detectou‐se que PKP2 foi expressada mais uniformemente dentro de queratinócitos quando comparado com a placoglobina, seu complemento de ligação desmossômico. A proteína PKP2 foi localizada no núcleo e nos filamentos de queratina ligados aos desmossomos. Não foi observada diferença na abundância de PKP2 entre cães atópicos e cães controle.
Conclusão
A placofilina‐2 é um proteína expressada tanto em células epiteliais quanto do sistema imunológico, e, baseado em sua localização subcelular, a sua função está relacionada tanto a processos nucleares quanto estruturais.
Introduction
In a genome‐wide association study (GWAS) we identified a locus on canine chromosome 27 (CFA 27) associated with canine atopic dermatitis (CAD) in German shepherd dogs (GSDs).1 The strongest genetic association was detected near the PKP2 gene encoding the protein plakophilin‐2. Plakophilin proteins are crucial for the proper assembly of desmosomes,2 which are intercellular mechanical junctions that contribute to strength and integrity in tissues, such as the myocardium and the epidermis, that exhibit mechanical stress.3 Plakophilins, which belong to the armadillo protein family, bind to plakoglobins and are essential for recruiting desmoplakins to the desmosomal plaque, thereby providing an important linkage to the stabilizing and stress‐bearing intermediate filaments inside the cell.4 Plakophilins are also known to be involved in multiple signalling and metabolic processes, and in transcriptional activities.5 Fine‐mapping of the risk locus in GSDs identified genetic variants located in tissue‐specific enhancers (e.g. epithelial‐specific enhancers) and suggested the possibility of alterations in PKP2 gene expression in CAD‐affected GSDs.6 This suggests that PKP2 expression may be altered in the skin of GSDs carrying the risk variants compared to dogs without the risk variants. Expression patterns of the adhesion molecules corneodesmosin, desmoglein‐1, desmocollin‐1, claudin‐1 and E‐cadherin were compared in dog skin with acute CAD lesions and healthy skin from the same dogs, and the most striking differences were found for corneodesmosin and claudin‐1.7 This implies that differential expression of desmosomal proteins are involved in the pathogenesis of atopic dermatitis (AD) in dogs and that PKP2 could also contribute to the pathogenesis of CAD.
In this present study, we collected skin biopsies from nine GSDs with CAD, either homozygous or heterozygous for the risk allele at the top GWAS‐single nucleotide polymorphism (SNP), and five healthy control GSDs homozygous for the control allele. These samples were submitted to studies using immunofluorescence and electron microscopy (EM). We aimed at investigating the role of PKP2 in dog skin and evaluated the overall PKP2 expression in dog skin by defining which cell types and where in the cell PKP2 is localized. We also examined dog skin morphology with the focus on desmosomes and searched for potential differences between CAD cases and GSD controls in terms of the intensity of PKP2 expression and desmosome structure, with regards to previous GWAS results.1
Materials and methods
Sampling and ethics statement
Skin biopsies (6 mm in diameter) were collected from 14 GSDs: nine diagnosed with CAD and five healthy controls (see the inclusion criteria below and Table 1) and also from one greyhound (unaffected by CAD and euthanized due to unrelated reasons). The GSDs were part of a previous study of CAD.1 The genotype at the top‐associated GWAS SNP defined the dogs as either homozygous risk, heterozygous risk or homozygous control. From each dog we collected three biopsies of skin from the dorsal trunk and three skin biopsies from the axilla area. The biopsies were collected from nonlesional axilla and dorsal skin to represent typical atopy affected and nonaffected skin locations, respectively. The CAD cases were under, or had recently been receiving, treatment and the skin was therefore defined as nonlesional. Owner consent was sought and the study was approved by the Swedish Animal Ethical Committee (no. C138/12) and the Swedish Animal Welfare Agency (no. 31‐1711/10).
Table 1.
Details of the dogs included in the study of PKP2 expression in the skin
| Dog ID | Sex | CAD | Genotype top GWAS SNP1 | Represented in figure |
|---|---|---|---|---|
| 1 | Female | Case | Homozygote risk | 1b–d, S1a, S1c, S3b–d |
| 2 | Male | Case | Homozygote risk | |
| 3 | Male | Case | Homozygote risk | |
| 4 | Female | Case | Heterozygote risk | S1b, S1d |
| 5 | Female | Case | Heterozygote risk | |
| 6 | Male | Case | Heterozygote risk | |
| 7 | Female | Case | Heterozygote risk | |
| 8 | Male | Case | Heterozygote risk | 1a |
| 9 | Male | Case | Heterozygote risk | |
| 10 | Male | Control | Homozygote control | 2b |
| 11 | Female | Control | Homozygote control | 3a, 3b, S6a, S6B |
| 12 | Male | Control | Homozygote control | S2 |
| 13 | Male | Control | Homozygote control | 2a, S3a, S4a–c |
| 14 | Female | Control | Homozygote control |
CAD canine atopic dermatitis, GWAS genome‐wide association study, SNP single nucleotide polymorphism.
CAD phenotype characterization
The CAD cases were between 6 and 11 years old at the time of sampling and fulfilled established criteria,8 with clinical signs compatible with CAD and positive reactions on allergen‐specific IgE test (intradermal or IgE serology test), either with or without concurrent cutaneous adverse food reactions (CAFR). Clinical diagnoses were established by first ruling out other causes of pruritus such as ectoparasite infestation, staphylococcal pyoderma and Malassezia dermatitis. Moreover, hypoallergenic diet trials (of at least 8 weeks duration followed by a challenge period) were conducted in order to evaluate the potential contribution of CAFR to the clinical signs. A CAD diagnosis was concluded in dogs not adequately controlled on hypoallergenic diet and with positive reactions on intradermal or IgE serology tests.
All healthy control dogs were between 9 and 11 years of age, and had never suffered from pruritus, repeated otic inflammation or skin lesions compatible with CAD, neither prior to nor at the time of sampling. The information was based on owner questionnaire and clinical examination.
Skin biopsy samples
Two of the skin biopsies were split in half by a cut perpendicular to the skin surface. Three pieces per location were used in this study and were fixed as follows: (i) embedded in OCT cryomount (Histolab; Gothenburg, Sweden) in a plastic mould and frozen in an isopentane bath, cooled with dry ice, (ii) fixed in 4% paraformaldehyde with 0.2% glutaraldehyde for EM‐immunolabelling, and (iii) fixed in 2.5% glutaraldehyde for EM. The fourth piece, fixed in 4% paraformaldehyde and embedded in paraffin, and the third whole biopsy, treated with RNA‐later, were not included in this study.
Immunofluorescence microscopy
Five or 30 μm thick sections were cut from frozen skin blocks and mounted on Super frost plus glass slides (Gerhard Menzel GmbH; Braunschweig, Germany). Immunofluorescence for 5 μm sections was carried out as follows: the slides were thawed at room temperature (RT) for 30 minutes, fixed in pre‐cooled acetone for 5 minutes at 4°C and air‐dried at RT for 30 minutes. The sections were washed three times in phosphate buffered saline (PBS, pH 7.4) and then blocked in Background sniper blocking solution (Biocare Medical; Concord, CA, USA) for 10–15 minutes at RT. For double staining, a mix of two primary antibodies diluted in PBS with 1% bovine serum albumin (BSA, Sigma‐Aldrich; St Louis, MO, USA) was added to the sections and incubated at 4°C overnight. Antibodies and specific dilutions are reported in Table S1, briefly slides were stained for CD3, CD4, CD11c, Junction plakoglobin (JUP), MHC‐II and Plakophilin‐2. Slides were washed three times with PBS. A mix of two fluorescently labelled secondary antibodies (Table S2) specific for the primary antibodies was diluted in PBS with 1% BSA added and incubated for 30 minutes at RT. The slides were washed three times in PBS. The sections were then stained with DAPI (4′,6‐diamidino‐2‐phenylindole) for 5 minutes followed by washing four times in PBS. The sections were mounted in Fluoromount G anti‐fade media (Southern Biotech; Birmingham, AL, USA) and covered with a cover glass fastened with nail polish and stored at 4°C until analysed by using a LSM 700 Confocal Laser Scanning microscope (Zeiss; Oberkochen, Germany) and LSM software Zen black (Zeiss).
For triple staining, four additional steps were added to the protocol above. After the step where secondary antibodies had been washed off, a third primary antibody was added diluted in PBS with 1% BSA and incubated for 1 h at RT or at 4°C overnight. The slides were washed three times in PBS. Fluorescently labelled secondary antibody, specific for the primary antibody, diluted in PBS with 1% BSA, was incubated for 30 minutes at RT and the slides were washed three times in PBS.
For the staining of 30 μm sections, slight modifications of the triple‐staining protocol above were applied. The acetone fixation step was increased to 7 minutes. After the initial wash, a permeabilization step was added where the sections were incubated for 10 minutes in PBS with 0.2% Triton X‐100 (Sigma‐Aldrich) at RT followed by washing three times in PBS. All subsequent washing steps were increased to four washes and the two last washes lasted for 10 minutes. The primary antibody incubations were performed overnight at 4°C or for 3–4 h at RT. All secondary antibodies were incubated for 2 h at RT. The final wash in PBS after the DAPI staining was performed five times.
Evaluation of the intensity of PKP2 expression in dendritic cells using immunofluorescence
The intensity of PKP2 protein expression in dendritic cells was compared between cases homozygous for the top GWAS SNP 27:19,140,8371 (n = 3), heterozygote cases (n = 6) and controls homozygous for the control allele at the top GWAS SNP (n = 5) in skin triple‐stained with antibodies specific for PKP2, MHC II and CD11c. Dendritic cells were defined as cells with clear dendritic cell shape (i.e. presence of dendrites) that also expressed CD11c and MHC II, or cells with moderate to high expression of CD11c and MHC II. For each sample, four z‐stacks of 92.4 × 92.4 μm spanning a 30 μm section were analysed and the 10 dendritic cells with the strongest PKP2 staining were chosen for further measurements. Using Fiji software,9 each cell was outlined by a polygon selection encompassing only this cell and using a triangle‐threshold within this selection, the area of the PKP2 expression could be defined and the intensity of PKP2 expression measured in each 0.6 μm slice. PKP2 protein is expressed throughout the cell; hence the area of PKP2 expression equals the area of the cell. The intensity mean per slice × area of the cell was calculated for each slice and the total intensity of all slices divided by volume of the cell (area of each slice × 0.6 μm) gave a measurement defined as intensity/μm.3
Immuno‐electron microscopy for PKP2
Dog skin samples fixed in 4% paraformaldehyde with 0.2% glutaraldehyde were embedded in Lowicryl® K4M (Polysciences; Warrington, PA, USA). Immunolabelling was performed as follows: ultra‐thin, 50 nm sections were blocked in 1% BSA (Sigma‐Aldrich) in PBS for 20 minutes, incubated with primary antibody against PKP2, as shown in the Western blot method given below (Sigma‐Aldrich) in 1:10 dilution in TBS (Tris‐buffered saline) pH 7.4 overnight at 20°C. The sections were washed four times in TBS pH 7.4 and incubated with goat anti‐rabbit antibody conjugated with 20 nm gold particles (115‐205‐144, Jackson Immunoresearch; West Grove, PA, USA) diluted 1:100 in TBS pH 8.3 for 2 h. Next, the sections were washed four times in TBS pH 7.4 and rapidly rinsed in distilled water (dH2O). Thereafter, they were incubated in 5% uranyl acetate for 10 minutes, jet‐washed in dH2O followed by lead citrate 1–2 minutes. After this, the sections were jet‐washed in dH2O and air‐dried. In the negative controls, the primary antibody was omitted. Sections were examined at 80 kV in a Tecnai G2 transmission electron microscope (FEI Company; Eindhoven, Netherlands). Figures were acquired using an ORIUS™ SC200 CCD camera (Gatan Inc.; Pleasanton, CA, USA) using the Gatan Digital Micrograph software.
Transmission electron microscopy
For transmission electron microscopy (TEM), the tissue sample (half of a whole biopsy sample q.v.) was fixed in 2.5% glutaraldehyde within minutes after collection and after maximum 1 h in RT stored at 4°C at least overnight until further processed. A part of the biopsy was cut off and used for embedding for TEM. Samples were rinsed twice with 0.1 M sodium caccodylate buffer for 10 min prior to 1 h incubation in 1% osmium tetroxide (TAAB Laboratories; Aldermaston, UK) in 0.1 M sodium caccodylate buffer. After a further rinse in 0.1 M sodium caccodylate buffer, samples were dehydrated in graded ethanol (70–99.9%) for a total of 2 h followed by 10 minutes incubation in propylene oxide. The tissue samples were then placed in a mixture of Agar resin (Agar 100 resin kit, Agar Scientific Ltd; Stansted, UK) and propylene oxide (1:1) for 1 h, followed by two changes of 100% resin, the first for 2–4 h and the last overnight. Subsequently, samples were embedded in capsules in newly prepared agar resin and left for 1 h and then polymerized at 60°C for 48 h. Ultrathin sections (50–60 nm) were contrasted in 5% uranyl acetate and lead citrate for 10 and 2 minutes, respectively. Sections were examined and figures were acquired as described in the immuno‐electron microscopy section.
Skin samples from the axilla region of CAD affected GSDs, heterozygote (n = 6) and homozygote (n = 3) for the risk allele at the GWAS SNP 27:19,140,837,1 were compared with samples from five healthy GSDs homozygous for the control allele. The length of all desmosomes in three keratinocytes per sample, at similar locations in the epidermis, were evaluated. The percentage of desmosomes that appeared longer than average (i.e. with a length of more than 0.4 μm), was calculated in three cells per sample. The number of desmosomes with what appeared to be unstructured or unevenly distributed binding of filaments to the desmosome was counted and compared to the total number of desmosomes calculated in the three cells for each dog. The number of desmosomes relative to the length of the cell membrane per image was also evaluated in three cells per dog by estimating the number of images that had visibly fewer desmosomes than the average distribution of desmosomes. The amount of keratin filaments in the cytoplasm in each image covering three cells was estimated as either low, medium, moderately high or high, and the proportion of images representing the levels was calculated for each sample.
Western blot analysis
For protein extraction, canine skin from the axilla region from the greyhound and canine left ventricle cardiac muscle from a great dane dog (supplied by Ingrid Ljungvall and colleagues) were used. Frozen OCT cryomount was removed from two skin tissue specimens by dissolving the OCT cryomount in PBS pH 7.2. Skin tissues were pooled before homogenization. Homogenization was performed using Precellys Evolution (Bertin Technologies; Montigny le Bretonneux, France) with Precellys CK14 2 mL tubes. Tissues were homogenized 2 × 30 s at 6,500 rpm in a shaker (Precellys 24, Bertin Corp., MD, USA) in 0.5 mL (cardiac muscle) or 0.3 mL (skin) PBS pH 7.2 containing 2% n‐Octylglucoside (Santa Cruz Biotechnology Inc.; Dallas, TX, USA) and 1% Triton X‐100 (Sigma‐Aldrich) with one Pierce protease and phosphatase inhibitor mini tablet (Thermo Scientific; Rockford, IL, USA) dissolved in 10 mL of buffer immediately before use. Homogenates were centrifuged at 17,000 × g for 30 minutes at 4°C and supernatant protein concentration was determined using Bradford method (Bio‐Rad protein assay, Bio‐Rad Laboratories AB; Solna, Sweden) with BSA (Merck KGaA; Darmstadt, Germany) as standard. Homogenates were stored at −20°C until analysis.
Protein extracts from canine skin and heart (left ventricle) tissues (100 μg/lane) were separated on 12% SDS‐polyacrylamide gels (ClearPAGE precast gels, C.B.S. Scientific Company Inc.; Del Mar, CA, USA) and transferred to polyvinylidene difluoride membranes (Immobilon‐FL, Millipore Corporation; Bellerica, MA, USA) at 0.24 ampere for 1 h using protein transfer system (Bio‐Rad Laboratories AB). The membranes were blocked in TBST (TBS with 0.1% Tween 20) plus 5% nonfat milk power for 1 h at RT and then incubated with rabbit anti‐human PKP2 antibody (Sigma‐Aldrich; Stockholm, Sweden) or mouse anti‐human JUP (junctional plakoglobin) antibody (Sigma Aldrich) at 1:200 dilution in TBST plus 5% nonfat milk power at 4°C O/N. After washing three times with TBST the membranes were then incubated with anti‐rabbit respective anti‐mouse IgG, horseradish peroxidase linked secondary antibodies (GE Healthcare; Little Chalfont Buckinghamshire, UK) at 1:5,000 dilution in TBST plus 5% nonfat milk power for 2 h at RT, washed three times in TBST and detected by the enhanced chemiluminescence immunodetection system (ECL prime western blotting detection reagent, GE Healthcare). The images were captured by using Chemidoc touch imaging system (Bio‐Rad Laboratories AB).
Statistical analysis
The Mann–Whitney t‐test was used for statistical analysis. When comparing multiple groups the One‐way ANOVA Kruskal–Wallis test with Dunn's post hoc test was used. P‐values lower than 0.05 were considered significant. All analyses were performed using Graphpad Prism™ 4.0 software (GraphPad Software Inc.; La Jolla, CA, USA).
Results
Immunofluorescence to study PKP2 expression in dog skin
In order to detect and evaluate PKP2 protein expression in dog skin, the sample sections were stained with an anti‐PKP2 antibody. We initially evaluated dorsal and axilla skin from one control dog (greyhound) for optimization of permeabilization, incubation times and dilutions of all antibodies (including double and triple staining). We detected PKP2 expression in keratinocytes as well as T cells and dendritic cells and concluded that no differences could be detected between axilla and dorsal skin. Because skin from the axilla represents the part of the dog's body typically affected by lesions in CAD it was regarded as the most relevant tissue for detection of potential differences between CAD‐case and control dog skin. The following studies were therefore carried out in axilla skin from the 14 sampled GSDs.
Plakophilin‐2 was expressed in keratinocytes in both CAD dogs and control GSDs. In some of the keratinocytes, the PKP2 staining was somewhat stronger close to the plasma membrane but we did not observe an expression gradient throughout the keratinocyte layers (Figure S1a). The observed staining was specific for the keratinocytes as shown by the lack of staining in the negative control. There was nonspecific staining of the stratum corneum caused by the secondary antibody, but this was clearly not affecting the staining in the keratinocytes or the other cells in the skin (Figure S1b). At a higher magnification an increase in expression of PKP2 closer to the plasma membrane was more evident. However, the difference in strength compared to the rest of the cytoplasm was small (Figure S1c). Next, the location of PKP2 proteins was compared to the location of plakoglobins in axilla skin. Plakoglobin is one of PKP2's binding partners within the desmosomes. The double staining with anti‐PKP2 and anti‐plakoglobin antibodies showed that plakoglobin staining was restricted to the keratinocytes in the epidermis, whereas PKP2 staining was seen also in other cells of the epidermis and dermis (Figure 1a). Within the keratinocytes in the epidermis, PKP2 expression was more homogenous in the cytoplasm compared to plakoglobin, and the location near the plasma membrane was more evident and distinct for plakoglobin compared to PKP2 (Figure 1b–d). The same pattern was seen in skin samples from all dogs (n = 14).
Figure 1.
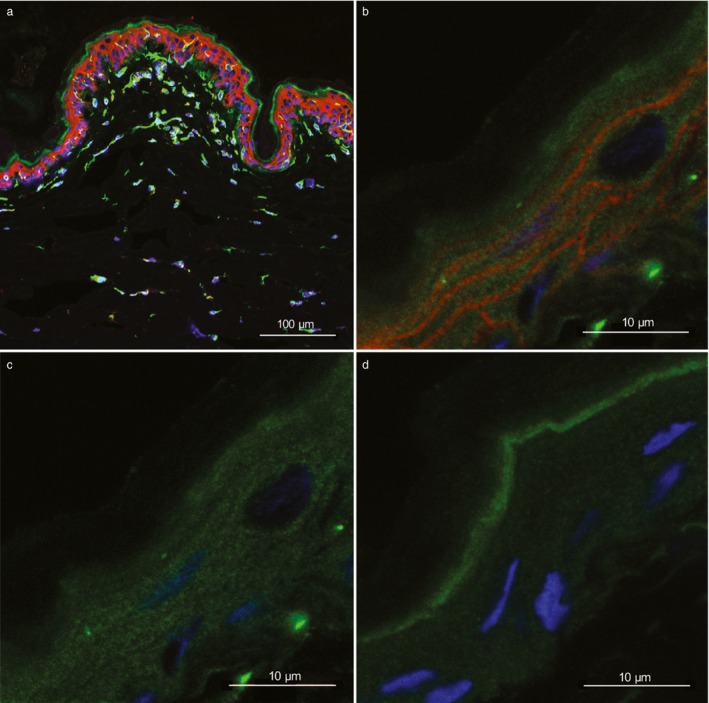
Frozen axilla skin, sections from German shepherd dogs with atopic dermatitis from one heterozygote (a, ID = 8) and one homozygote (b–d, ID = 1) for the top genome‐wide association study (GWAS) single nucleotide polymorphism (SNP) risk allele.1 (a, b) Immunostaining for PKP2 (green), plakoglobin (red) and 4′,6‐diamidino‐2‐phenylindole (DAPI) staining (blue) show both cytoplasmic and cell membrane expression of both PKP2 and plakoglobin in keratinocytes of the epidermis. In addition, PKP2 expression is present in other cell types in both epidermis and dermis. (c) The same picture as (b) with red and blue colours omitted, thus showing only PKP2 expression. (d) A negative control with only secondary antibodies showing nonspecific (green fluorescence) binding to stratum corneum. The protein expression of PKP2 in the keratinocyte cytoplasm is more homogenous compared with plakoglobin, while the expression at the cell border is more evident and distinct for plakoglobin. Magnification is ×20 (a), and ×63 with ×3.1 zoom (b–d). The scale bars are 100 μm (a) and 10 μm (b–d).
We detected PKP2 expression also in other cells in the axilla skin. Some of these cells displayed even higher expression intensity than keratinocytes, including cells that appeared to be dendritic cells or melanocytes, and endothelial cells judging by the cell shapes and locations (Figure S2), as well as cells that could possibly be T cells due to their location close to vessels. To further examine some of these cell types (other than keratinocytes) that expressed PKP2, we performed triple staining with antibodies against the T‐cell markers CD4 and CD3, and the dendritic cell markers CD11c and MHC II. Both T cells (Figure 2a and Figure S3) and dendritic cells (Figure 2b and Figure S4) showed strong PKP2 expression in both the cytoplasm and in the nuclei. We did not note any PKP2‐positive cells with dendrites in the epidermis that were not positive for CD11c/MHC II; however, we cannot rule out possible PKP2 expression in melanocytes because we did not use any melanocyte‐specific makers.
Figure 2.
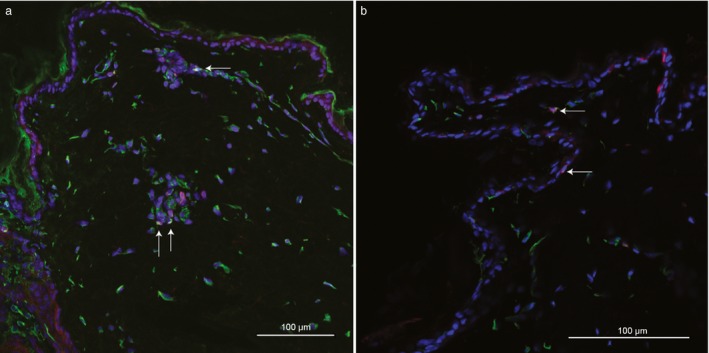
Frozen axilla skin sections from a control German shepherd dog (ID = 13) homozygous for the control allele at the top genome‐wide association study (GWAS) single nucleotide polymorphism (SNP).1 (a) Immunostaining for PKP2 (green), CD3 (red), CD4 (purple) and DAPI staining (blue) show that PKP2 is expressed in T cells in dog skin. (b) Immunostaining for PKP2 (green), CD11c (red), MHC II (purple) and DAPI staining (blue) show that PKP2 protein is expressed in CD11c+ and MHC II + dendritic cells in control dog skin (ID = 10). Triple stained cells are indicated with arrows. The epidermis is present both in the top and bottom of the picture, only a few keratinocytes thick. Magnification is ×20 in (a) and ×63 in (b). Scale bars are 100 μm (a) and 20 μm (b).
Intensity of PKP2 expression in skin dendritic cells
In order to further investigate the expression of PKP2 in dendritic cells and to compare the expression pattern between CAD cases and controls, we calculated the intensity of PKP2 expression in the ten dendritic cells with the highest intensity per skin sample. This was performed on axilla skin samples from all 14 dogs. The cells were chosen from four z‐stacks triple stained for PKP2, CD11c and MHC II. The average intensity per μm3 for the different groups were as follows: homozygous cases 16.3 (ranging from 10.4 to 26.5), heterozygous cases 18.9 (12.7–23.7) and controls 14.8 (11.3–20.5; Figure S5). No significant differences between group averages were detected using Mann–Whitney t‐test (two tailed with 95% confidence intervals), for cases versus controls (P = 0.44), for homozygous cases versus controls (P = 0.79) or for heterozygous cases versus controls (P = 0.18).
PKP2 expression in dog skin using immuno‐electron microscopy
Next, we aimed at further defining the subcellular location of PKP2 in epidermal tissue from dog skin. For this purpose, ultrathin sections from one healthy control GSD were immunolabelled with anti‐PKP2 antibodies and analysed using immuno‐electron microscopy. Gold particles, indicating binding of anti‐PKP2 antibodies, were detected in the vicinity of desmosomes on keratin filaments attached to desmosomes (Figure 3a) and in the majority of the nuclei (Figure 3b). The negative control, with omission of the primary antibody, only showed very few gold particles in the section. A few of these gold particles were located in these nuclei (Figure S6a) but all other nuclei were negative for staining. No gold particles were located in or near the desmosomes in the negative control (Figure S6b).
Figure 3.
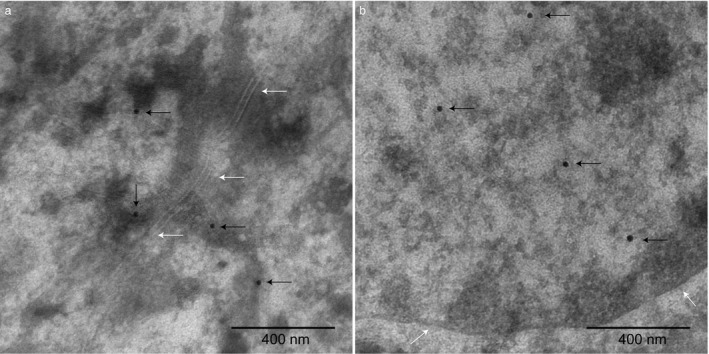
Electron microscopy immunohistochemistry with gold particles (20 nm) indicate immunoreactivity to PKP2 in the epidermis in axilla skin from a control German shepherd dog (ID = 11) homozygous for the control allele at the top genome‐wide association study (GWAS) single nucleotide polymorphism (SNP).1 (a) Black arrows indicate gold particles binding to PKP2 in the vicinity of desmosomes on keratin filaments attached to desmosomes (desmosomes are marked with white arrows). (b) Black arrows indicate gold particles binding to PKP2 in the nucleus (the nucleus border is marked out with white arrows). The magnification is ×43,000 and scale bars 400 nm.
Skin morphology – electron microscopy
In order to detect possible differences in skin morphology between cases and controls, we evaluated the skin samples using TEM. Considering desmosome length and number of desmosomes in relation to cell membrane length, we could not detect any significant differences between the groups. To assess if a defect in binding of keratin filaments to the desmosomes could result in an increased risk for developing CAD, we also analysed the levels of visible keratin filaments in the cytoplasm and the number of desmosomes where the binding of keratin filaments appeared to be uneven or unstructured. In this analysis, we found no significant differences between CAD cases (neither separated into either heterozygote or homozygote cases or considered as one case group) and controls using Kruskal–Wallis one‐way ANOVA (Figure S7a–g).
Western blot analysis
Because the anti‐PKP2 and anti‐JUP antibodies were developed for human proteins, we confirmed specific binding to canine PKP2 and JUP by performing Western blot analyses using extracts from canine skin and heart tissues (left ventricle). The results for anti‐PKP2 and anti‐JUP antibodies showed specific binding to target proteins of the expected sizes (PKP2 93 kDa and JUP 82 kDa) for both these two canine proteins thus validating the across‐species specificity of both antibodies (Figure S8).
Discussion
The use of EM immunolabelling of ultrathin skin sections confirmed PKP2 located in the nuclei and on keratin filaments in close vicinity to desmosomes. Previously, EM analyses of human skin using immunolabelling with anti‐PKP2 antibodies, showed binding near the cytoplasmic side of the desmosomal plaque in stratified epithelial tissue.10 This suggests that the subcellular expression of PKP2 in keratinocytes is similar in both human and dog skin. Previous studies in dogs have shown that there is a gradient in expression of PKP1, with stronger expression in the upper layers of the skin compared to the layers closer to dermis.11 This is in concordance with the expression in human skin. The same human study also showed that PKP2 expression was higher in the lower layers and weaker in the top layers of human skin whereas PKP3 was expressed more evenly in all skin layers.12 We could not observe any gradient in expression of PKP2 in the studied dog skin samples using IF. However, the epidermis of the axilla skin was very thin, only 1–3 keratinocytes thick. The expression pattern of PKP2 differed from its binding‐partner plakoglobin, which was more restricted to cell membranes. This indicates a role for plakoglobin more limited to desmosomes in contrast to the multiple functions implicated for PKP2.
We confirmed that CD11c+ and MHC II+ cells (i.e. dendritic cells) as well as CD4+ and CD3+ cells (i.e. T cells) in dog skin express PKP2. Expression of PKP2 in T cells and dendritic cells in skin is a novel finding. Although, according to BioGPS (www.biogps.org) mRNA expression of PKP2 has been detected in human T cells and CD33+ myeloid cells in the blood (e.g. myeloid dendritic cells) but not in epidermal dendritic cells.13 Interestingly, in skin samples from the Human Protein Atlas (www.proteinatlas.org), anti‐PKP2 antibodies showed reactivity to several non‐keratinocyte cells that stained with a higher intensity than the keratinocytes in the samples presented. This is very similar to the staining pattern that we observed in dog skin. On the Protein Atlas website, there is no report of observed staining with the anti‐PKP2 antibody in Langerhans cells (LCs). However, we studied the CD11c+ (ITGAX)‐staining from the Protein Atlas site and detected positive staining of cells looking very similar to the strongest PKP2‐stained cells (i.e. defined here as dendritic cells) in the skin samples from our study. This suggests that PKP2 is expressed in dendritic cells also in human skin. LCs are specialized dendritic cells in the skin that are activated by perturbation of the stratum corneum. The activated LCs extend their dendrites through the tight junction barrier located in the stratum spinosum of the skin and thereby efficiently capture allergens, which they present on their MHC molecules to the T cells.14 This initiates an immune response that can result in AD. Thus, the strong expression by the CAD‐candidate gene PKP2 by both T cells and LCs may be of high relevance for CAD development.
We did not detect any significant differences in PKP2 protein expression between the CAD cases and controls used in our study. This is in concordance with mRNA sequence data from axilla skin biopsies from nine of the dogs used in the present study. In that data there were no PKP2 expression differences between CAD cases and controls. Instead, preliminary analyses of mRNA expression data suggest differences related to treatment effects (Tengvall et al., unpublished data). Thus, possibly PKP2 gene and protein expression differences between CAD cases and controls may be masked by treatment, or relate to other functions by this protein, potentially even in other tissues. It may also be due to other genes being affected by the genetic risk variants. Nevertheless, both mRNA and protein expression data are of strong value to further evaluate the potential role of PKP2 in the pathogenesis of CAD. The reason for not detecting any differences may be due to the lack of untreated lesional skin from CAD cases. All the CAD dogs were in remission by various treatment protocols and skin biopsies were collected from nonlesional skin. Protein expression in the skin may also be affected by post‐translational mechanisms in relation to skin inflammation in lesional CAD skin compared to medically treated nonlesional CAD skin. An altered skin structure may thus only be detectable when assessing skin morphology in lesional CAD skin. In a previous study of CAD in various dog breeds (cases, n = 20, controls n = 17), PKP2 mRNA expression in skin was significantly upregulated in CAD skin compared to controls.15 A difference was detected in nonlesional skin versus control dog skin (P = 0.03) but was more pronounced in lesional skin versus skin from healthy control dogs (P = 0.001). These data support the role of PKP2 in CAD skin and that the effect is induced during lesional stages of CAD.
The lack of detected differences between CAD cases and controls with respect to desmosome structure may have been influenced by the fact that TEM captures only a limited fraction of the total skin sample and thereby a very low number of desmosomes was actually analysed. Differences could potentially be related to the fraction of altered desmosomes per area rather than all desmosomes in CAD skin being different from control skin. The evaluation was also subjective although obvious structural alterations should have been observed with this approach. Ongoing post‐translational compensatory mechanisms may also contribute to the lack of visible differences between desmosomes of the skin from CAD‐affected and healthy controls. Truncating mutations located within PKP2 are known to cause heart disease due to defected heart desmosomes.16 In PKP2 knock‐out mice the effect on heart desmosomes were so severe that the homozygous embryos died at mid‐gestation, but no alterations in other PKP2‐expressing epithelia was detected in these animals.17 This was likely due to compensatory effects of other PKP isoforms expressed in many cell types except for the heart (which express only PKP2).18 Likewise, in the human skin fragility syndrome, caused by complete or partial absence of PKP1, it was shown that other PKPs were upregulated, which may partially have compensated for the loss of PKP1 in unaffected skin areas.19
Apart from the important role as desmosomal cell adhesion proteins, plakophilins have been implicated in processes such as cell signalling, cytoskeleton organization and protein biosynthesis. For example, PKP2 binds to β‐catenin in the cytoplasm and overexpression of PKP2 has been suggested to reduce the pool of β‐catenin available for E‐cadherin binding, which may thereby affect cell adhesion.18 PKP2 is also known to be present in the nuclei and involved in gene transcription activities.10 It forms complexes with RNA polymerase III subunits and is generally present in the nuclei of many cell types. Interestingly, in stratified epithelia PKP2 is excluded from desmosomes but accumulated in the nuclei of the keratinocytes.18 We confirmed PKP2 expression in the nuclei of keratinocytes, dendritic cells and T cells. However, with the current methods used, we could not evaluate the functional role of PKP2 in the nuclei or compare CAD cases with controls regarding PKP2 expression in the nuclei.
We were unable to define any differences between CAD cases and controls with respect to PKP2 protein expression pattern and intensity nor desmosomal morphology. Perhaps, an altered PKP2 expression associated with the defined genetic variants affects other pathways, mechanisms and/or cell types, undetectable by the currently used materials and methods. The strong expression of PKP2 in LCs and T cells may imply that an altered expression of PKP2 in these cell types may correlate to atopic disease. Or possibly, nuclear processes of stratified epithelial cells may be affected rather than structural properties of the basal epithelial layers. Moreover, intestine integrity and allergen uptake is of high relevance in atopic skin manifestations20, 21 and a rather high mRNA and protein expression of PKP2 has been detected in the digestive tract (www.proteinatlas.org). Thus, this may be a target tissue for this risk locus in CAD development as GSDs are known to be particularly prone to gastrointestinal problems and are also affected by cutaneous adverse food reactions. An altered regulation of PKP2 mRNA expression in the intestinal epithelial cells may lead to changes in PKP2 protein levels that potentially could disturb desmosome stability and subsequently increase allergen penetrance and uptake through the intestine.
In conclusion, PKP2 expression was detected in keratinocytes, dendritic cells and T cells in the epidermis and dermis, and was more evenly expressed within keratinocytes compared to its desmosomal binding‐partner plakoglobin. The expression of PKP2 in T cells and dendritic cells in skin is likely to be of relevance for CAD development and has previously not been reported. PKP2 protein was located in the nucleus as well as on keratin filaments attached to desmosomes. No difference in PKP2 abundance between CAD cases and controls was observed. We report a wide expression pattern of PKP2 in dog skin that is in accordance with previous reports suggesting that this protein contributes to multiple functions within the cell including both structural and transcriptional activities.
Supporting information
Table S1. Primary antibodies used for indirect immunofluorescence.
Table S2. Secondary antibodies for immunofluorescence.
Figure S1. PKP2 expression in keratinocytes.
Figure S2. PKP2 expression in dendritic cells and endothelial cells in dog skin.
Figure S3. PKP2 expression in T cells.
Figure S4. PKP2 expression in dendritic cells.
Figure S5. Intensity of PKP2 expression in dendritic cells.
Figure S6. Negative controls for electron microscopy immunohistochemistry.
Figure S7. Assessment of desmosomes and keratin filaments in keratinocytes.
Figure S8. Western blot confirming antibody specificity to canine proteins.
Acknowledgements
We would like to thank all owners of the dogs that participated in this study as well as the veterinarians Rebecka Frey, Carina Kubacki, Ylva Sjöström and Susanne Åhman who helped with the collection of biopsies at different veterinary clinics in Sweden. Help with immuno‐electron microscopy including preparation of samples, preparation of samples for morphology evaluation and expert guidance in electron microscopy, as well as guidance and use of the confocal microscopy equipment, was provided by the staff at the BioVis facility at SciLifeLab, Uppsala University, Uppsala, Sweden. We thank Anna Svensson at the Department of Clinical Sciences, at the Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden, for protein extraction service and Susanne Gustafsson at the Canine Biobank (SLU and Uppsala University) for sample management. We also would like to thank Ingrid Ljungvall, Jens Häggström and Åsa Ohlsson Andersson for supplying us with canine heart tissue for the western blot analyses.
Kerstin Lindblad‐Toh and Göran Andersson contributed equally to this work.
Conflicts of interest: No conflicts of interest have been declared.
Funding: This work was financially supported by the grants to KLT: Swedish Research Council, http://www.vr.se/, grant number 521‐2012‐2826, Swedish Research Council FORMAS, http://www.formas.se/, grant number: 221‐2009‐1689 and the European Research Council ERC, http://erc.europa.eu/, starting grant agreement: 310203; and a grant to BAL from the Swedish Research Council 524‐2012‐7053.
References
- 1. Tengvall K, Kierczak M, Bergvall K et al. Genome‐wide analysis in German shepherd dogs reveals association of a locus on CFA 27 with atopic dermatitis. PLoS Genet 2013; 9: e1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen X, Bonne S, Hatzfeld M et al. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta‐catenin signaling. J Biol Chem 2002; 277: 10,512–10,522. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen TB, Nissen PH, Palmfeldt J et al. Truncating plakophilin‐2 mutations in arrhythmogenic cardiomyopathy are associated with protein haploinsufficiency in both myocardium and epidermis. Circ Cardiovasc Genet 2014; 7: 230–240. [DOI] [PubMed] [Google Scholar]
- 4. Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol 2007; 127: 2,499–2,515. [DOI] [PubMed] [Google Scholar]
- 5. Bass‐Zubek AE, Godsel LM, Delmar M et al. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol 2009; 21: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tengvall K, Kozyrev S, Kierczak M et al. Multiple regulatory variants located in cell type‐specific enhancers within the PKP2 locus form major risk and protective haplotypes for canine atopic dermatitis in German shepherd dogs. BMC Genet 2016; 17: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olivry T, Dunston SM. Expression patterns of superficial epidermal adhesion molecules in an experimental dog model of acute atopic dermatitis skin lesions. Vet Dermatol 2015; 26: 53–56, e‐17–18. [DOI] [PubMed] [Google Scholar]
- 8. Favrot C, Steffan J, Seewald W et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol 2010; 21: 23–31. [DOI] [PubMed] [Google Scholar]
- 9. Schindelin J, Arganda‐Carreras I, Frise E et al. Fiji: an open‐source platform for biological‐image analysis. Nat Methods 2012; 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 1996; 135: 1,009–1,025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bizikova P, Linder KE, Olivry T. Immunomapping of desmosomal and nondesmosomal adhesion molecules in healthy canine footpad, haired skin and buccal mucosal epithelia: comparison with canine pemphigus foliaceus serum immunoglobulin G staining patterns. Vet Dermatol 2011; 22: 132–142. [DOI] [PubMed] [Google Scholar]
- 12. Desai BV, Harmon RM, Green KJ. Desmosomes at a glance. J Cell Sci 2009; 122: 4,401–4,407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su AI, Wiltshire T, Batalov S et al. A gene atlas of the mouse and human protein‐encoding transcriptomes. Proc Natl Acad Sci USA 2004; 101: 6,062–6,067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol 2005; 17: 262–272. [DOI] [PubMed] [Google Scholar]
- 15. Wood SH, Clements DN, Ollier WE et al. Gene expression in canine atopic dermatitis and correlation with clinical severity scores. J Dermatol Sci 2009; 55: 27–33. [DOI] [PubMed] [Google Scholar]
- 16. Joshi‐Mukherjee R, Coombs W, Musa H et al. Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (ARVC)‐related plakophilin‐2 (PKP2) mutations. Heart Rhythm 2008; 5: 1,715–1,723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grossmann KS, Grund C, Huelsken J et al. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 2004; 167: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neuber S, Muhmer M, Wratten D et al. The desmosomal plaque proteins of the plakophilin family. Dermatol Res Pract 2010; 2010: 101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMillan JR, Haftek M, Akiyama M et al. Alterations in desmosome size and number coincide with the loss of keratinocyte cohesion in skin with homozygous and heterozygous defects in the desmosomal protein plakophilin 1. J Invest Dermatol 2003; 121: 96–103. [DOI] [PubMed] [Google Scholar]
- 20. Marsella R, Samuelson D. Unravelling the skin barrier: a new paradigm for atopic dermatitis and house dust mites. Vet Dermatol 2009; 20: 533–540. [DOI] [PubMed] [Google Scholar]
- 21. Marsella R, Samuelson D, Doerr K. Transmission electron microscopy studies in an experimental model of canine atopic dermatitis. Vet Dermatol 2010; 21: 81–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primary antibodies used for indirect immunofluorescence.
Table S2. Secondary antibodies for immunofluorescence.
Figure S1. PKP2 expression in keratinocytes.
Figure S2. PKP2 expression in dendritic cells and endothelial cells in dog skin.
Figure S3. PKP2 expression in T cells.
Figure S4. PKP2 expression in dendritic cells.
Figure S5. Intensity of PKP2 expression in dendritic cells.
Figure S6. Negative controls for electron microscopy immunohistochemistry.
Figure S7. Assessment of desmosomes and keratin filaments in keratinocytes.
Figure S8. Western blot confirming antibody specificity to canine proteins.


