Abstract
The large volume of adult living donor liver transplantations (ALDLTs) at our center affords a unique opportunity to examine the impact of acute‐on‐chronic liver failure (ACLF) among high–Model for End‐Stage Liver Disease MELD score patients. From February 1998 to March 2010, 1958 cirrhotic recipients were analyzed to study the relationship between MELD scores and ALDLT outcomes. A total of 327 high‐MELD score recipients were categorized into ACLF and non‐ACLF groups, and their outcomes were compared. The 5‐year graft and patient survival in the high‐MELD group were 75.2% and 76.4%, respectively, which were significantly worse than the low and intermediate MELD groups. The presence of ACLF associated with higher MELD scores appeared to be the dominant factor responsible for the inferior results of patients with MELD score of 30–34 points. The 5‐year graft survivals in the ACLF group was 70.5% and in the non‐ACLF group it was 81.0% (p = 0.035). Therefore, ALDLT should be performed as soon as possible in high‐MELD score patients prior to ACLF development. Moreover, ACLF patients should be separately categorized when analyzing the outcomes of ALDLT. ALDLT for ACLF patients should not be discouraged because favorable outcomes can be expected through timely ALDLT and comprehensive management.
Keywords: clinical research/practice, liver transplantation/hepatology, liver transplantation: living donor, liver disease
Short abstract
While adult living donor liver transplantation should be performed as soon as possible before acute‐on‐chronic liver failure develops, it should not be discouraged for patients with acute‐on‐chronic liver failure since timely transplantation and comprehensive management can bring a favorable outcome.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- ALDLT
adult living donor liver transplantation
- DDLT
deceased donor liver transplantation
- GRWR
graft‐to‐recipient weight ratio
- HCC
hepatocellular carcinoma
- HV
hepatic vein
- ICU
intensive care unit
- INR
international normalized ratio
- LDLT
living donor liver transplantation
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- RBC
red blood cell
- UNOS
United Network for Organ Sharing
Introduction
Adult living donor liver transplantation (ALDLT) for high–Model for End‐Stage Liver Disease (MELD) scores patients has been controversial due to concerns about poor outcomes. In 2002, the New York State Committee on Quality Improvement in Living Liver Donation recommended that living donor liver transplantation (LDLT) for high‐MELD score patients with more than 25 points should be prohibited 1. However, ALDLT for high‐MELD score patients has been performed in East Asia, including Korea, Japan, Taiwan, and Hong Kong because it is the only solution to save recipients due to a scarcity of deceased donors. However, high‐MELD score patients with over 30 points are more likely to require intensive care unit (ICU) stay, ventilator support, renal replacement therapy, and vasopressor treatment before transplantation. In addition, intraoperative transfusion requirements and vasopressors use are also significantly higher in high‐MELD score patients than low‐MELD score patients 2. Preoperative high‐MELD score patients were associated with postoperative graft failure following LDLT 3, and post‐LDLT survival was significantly different according to United Network for Organ Sharing (UNOS) status despite no difference in the MELD score 4. In contrast, high MELD scores did not impact graft and patient survival in reports from a few well‐known large LDLT centers 5, 6, 7.
One possibility for these conflicting reports is related to the number of acute‐on‐chronic liver failure (ACLF) patients, characterized by decompensation from an underlying chronic liver disease associated with organ failure that carries a high short‐term mortality.
At our institution, many ALDLTs for ACLF patients have been performed due to the scarcity of deceased organ donation in Korea. We have not applied a different liver transplantation (LT) indication between LDLT and deceased donor liver transplantation (DDLT) in contrast to the UNOS recommendation for LDLT in 2002. The reported LDLT outcomes from a few well‐known LDLT centers describing no survival differences between low‐ and high‐MELD score patients at the time of transplantation are unconvincing results compared to ours, which are the largest experiences in the world. In this preliminary study, we investigated the relationship between the recipient MELD scores and ALDLT outcomes of 1958 recipients with cirrhosis (confirmed by explanted liver histology). We excluded 111 fulminant hepatic failure patients from a total of 2069 primary ALDLTs performed at Asan Medical Center in Seoul, Korea between February 1998 and March 2010. We analyzed 327 recipients with a high MELD score (≥30) after categorizing them according to the consensus definition of ACLF by the World Congress of Gastroenterology 8, 9.
Materials and Methods
We retrospectively analyzed our prospective database containing all ALDLTs performed on chronic liver disease patients at our institution since February 1998. Fulminant liver failure patients without cirrhotic liver pathology on explanted liver and retransplantation were excluded. All patients were followed up regularly by the same team of surgeons. No patients were lost to follow‐up. The last census date for this study was June 30, 2015. This study was approved by the Institutional Review Board of our institution. All living donors were voluntary and altruistic. A total of 70% of all the donors were male with a median donor age of 30 years (range, 18–62). The type of liver graft varied to satisfy the metabolic demands of the cirrhotic recipients, including the right hemiliver (n = 1519, 77.6%), left hemiliver (n = 159, 8.1%), and dual grafts using two left hemilivers or a combination of the right and left hemiliver (n = 280, 14.3%). Venous drainage was reconstructed to avoid graft dysfunction related to venous congestion in 95.7% of the cases (n = 1874). In 4.3% of the cases (n = 84), the right lobe graft was not reconstructed due to the negligible size of middle hepatic vein (HV) tributaries with minimal congestion. We have applied strict donor selection criteria, particularly regarding the right hemiliver donation in consideration of donor safety. The minimally accepted expected remnant liver volume should be individualized from 30% to 35% according to the donor age and the degree of steatosis. Healthy volunteer donors aged up to 55 years and with less than 30% steatosis were possible candidates for right hemiliver donation 10. Currently, the degree of steatosis in the donor liver is primarily evaluated by nonenhanced plain computed tomography, and a preoperative liver biopsy is performed selectively 11. All ACLF patients, except those with full‐blown sepsis, were registered for ALDLT. Preoperatively, we attempted to satisfy the 1% expected graft‐to‐recipient weight ratio (GRWR) if possible, using a living liver graft with minimal steatosis from a young donor. Otherwise, dual‐graft LDLTs in consideration of donors' liver volume, hepatic steatosis, and age were often performed to meet the more stringent conditions compared to those for non‐ACLF patients 12. However, under inevitable situations considering the available donors, ALDLT was also performed for ACLF patients when an available liver graft was expected to have the minimum 0.8% GRWR and minimal steatosis from a young donor. Detailed descriptions of our institutional donor and recipient evaluation process, surgical techniques, and outcomes are published in other reports 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23.
ACLF versus non‐ACLF
The MELD score was calculated purely based on the liver disease severity at the time of the transplant. As a preliminary study before the comparison of ACLF versus non‐ACLF using our collected database, all 1958 ALDLT patients with cirrhosis displayed on the explanted liver pathology were stratified into three groups according to the MELD score: low MELD group, ≤19; intermediate MELD group, 20–29; high MELD group, ≥30; and the overall survival rates were compared among the MELD groups.
High–MELD score patients (≥30) were categorized into the ACLF and non‐ACLF group according to the consensus definition of the World Congress of Gastroenterology in 2014 8, 9. We used the cut‐off levels of The Chronic Liver Failure Consortium organ failure score described by Bernal et al and Jalan et al 8, 9 to categorize specific organ failure. A score of 3 is the definition of organ failure for each system, except for the kidney, for which a score of 2 or more is the definition. However, in the case of coagulation failure, we consider it to be one of the findings of liver failure itself and it was excluded for conditions of extrahepatic organ failure (Table S1). The total bilirubin and creatinine levels were based on μmol/L in the table and we used the cut‐off levels after a conversion to mg/dL due to the different units of those levels at our institution. The cut‐off levels are greater than 12.1 and 1.98 mg/dL, respectively. Hence, the liver failure was defined by the total bilirubin (>12.1 mg/dL) and/or international normalized ratio (≥2.5).
Assessment of transplant outcomes
The surgical outcomes were measured by the intraoperative time and transfusion amount, postoperative peak level of biochemical markers depicting hepatocyte injury and liver function (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and total bilirubin), the length of ICU and hospital stay, posttransplant complications (e.g. medical and technical complications), and the necessity of a reoperation. Regarding the posttransplant complications, when one of the recipients exhibited multiple complications, the most important and severe Clavien‐Dindo complication grade 24 affecting the recipient's outcome was recorded for simplification of the analysis. The long‐term transplant outcome was measured by the actuarial graft and patient survival at 1, 3, and 5 years.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 21 program. All values are expressed as the means ± standard deviation. The categorical variables were compared with a Fisher's exact test, and the continuous variables were compared with a Student's t‐test. We used the Kaplan–Meier method with a log‐rank test for the analysis of graft and patient survival. The variables reaching statistical significance via the univariate analysis were then included in the multivariate analysis. In the multivariate analyses, we used the Cox proportional hazards regression method with a stepwise procedure to determine the survival rates when a p‐value was ≤0.1 in the univariate analysis. A p‐value ≤0.05 was considered to be significant.
Results
Outcome of ALDLT according to the MELD scores
The MELD scores at the time of the transplant ranged from 6 to 57 (mean, 19.6 ± 9.5). A total of 1149 (58.9%) and 488 recipients (24.7%) belonged to the low‐ and intermediate‐MELD group, respectively, while 327 patients (16.4%) had a high MELD score (≥30). The detailed distribution of the MELD score is depicted in Figure 1. The low‐MELD group (≤19) graft and patient survival rates at 1, 3, and 5 years were 92.7%, 87.6%, and 86.2%, and 93.3%, 88.1%, and 86.7%, respectively. The intermediate‐MELD group (20–29) also had similar graft and patient survival rate at 1, 3, and 5 years as follows: 90.2%, 85.5%, and 85.1%, and 91.0%, 86.5%, and 86.1%, respectively. However, in the high MELD group (≥30), the graft and patient survival rates at 1, 3, and 5 years were significantly worse than the low and intermediate MELD: 82.9%, 76.7%, and 75.2% (p = 0.002), and 84.7%, 77.7%, and 76.4% (p = 0.003), respectively (Figure S1).
Figure 1.
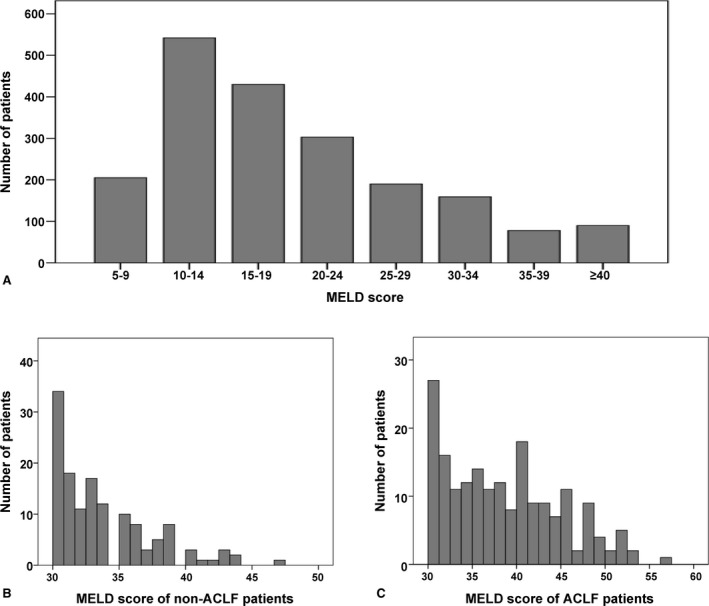
Distribution of the MELD score. (A) Indicates the 1958 adult living donor recipients, (B) indicates the high‐MELD score non‐ACLF patients, and (C) indicates the high‐MELD score ACLF patients. MELD, Model for End‐Stage Liver Disease; ACLF, acute‐on‐chronic liver failure.
Demographics of the high‐MELD (≥30) score recipients
The mean recipient age was 46.7 ± 8.8 years, with 64.8% of all recipients being male. Among the recipients, hepatitis B virus (HBV) (79.5%) was the most common etiology of primary liver disease, followed by alcohol (8.6%). Hepatocellular carcinoma (HCC) was present in 10.7% of the recipients. The mean total bilirubin, creatinine, international normalized ratio (INR), and MELD score at the time of transplantation were 30.5 ± 13.5 mg/dL, 2.0 ± 1.8 mg/dL, 3.2 ± 1.5, and 36.4 ± 6.2, respectively. Pretransplant hepatic encephalopathy grade 3 and 4 (32.7%), hepatorenal syndrome with >2.0 mg/dL (32.7%), hemodialysis (24.8%), ventilator support (20.8%), vasopressor (11.3%), and need for ICU stay (39.8%) were common features. The types of liver graft for ALDLT were the right hemiliver (71.3%), left hemiliver (10.1%), and dual grafts (18.7%). The mean weight of the liver graft was 691 ± 142 g with a mean GRWR of 1.0% ± 0.21%. The operation time from the skin incision to closure was 926 ± 201 min and the amount of red blood cell (RBC) transfusion was 19.9 ± 15.8 units (Table 1).
Table 1.
Clinical features of the high‐MELD score recipients in adult living donor liver transplantation
| Total (n = 327) | Non‐ACLF (n = 137) | ACLF (n = 190) | p | |
|---|---|---|---|---|
| Age (years) | 46.7 ± 8.8 | 45.8 ± 8.6 | 47.2 ± 8.9 | 0.173 |
| Male | 212 (64.8%) | 87 (63.5%) | 125 (65.8%) | 0.440 |
| Etiology, HBV | 260 (79.5%) | 110 (80.3%) | 150 (78.9%) | 0.773 |
| Alcoholic | 28 (8.6%) | 11 (8.0%) | 17 (8.9%) | |
| HCV | 8 (2.4%) | 1 (0.7%) | 7 (3.7%) | |
| AIH | 6 (1.8%) | 3 (2.2%) | 3 (1.6%) | |
| Wilson's | 6 (1.8%) | 3 (2.2%) | 3 (1.6%) | |
| SBC | 5 ((1.5%) | 3 (2.2%) | 2 (1.1%) | |
| PBC | 4 (1.2%) | 2 (1.5%) | 2 (1.1%) | |
| Others | 10 (3.1%) | 4 (2.9%) | 4 (3.2%) | |
| HCC | 35 (10.7%) | 11 (8.0%) | 24 (12.6%) | 0.125 |
| MELD score | 36.4 ± 6.2 | 33.7 ± 3.8 | 38.4 ± 6.9 | <0.001 |
| Total bilirubin | 30.5 ± 13.5 mg/dL | 31.0 ± 10.4 mg/dL | 30.1 ± 15.3 mg/dL | 0.529 |
| Creatinine | 2.0 ± 1.8 mg/dL | 1.1 ± 0.4 mg/dL | 2.6 ± 2.2 mg/dL | <0.001 |
| INR | 3.2 ± 1.5 | 3.3 ± 1.2 | 3.1 ± 1.7 | 0.226 |
| HEP grade 3, 4 | 107 (32.7%) | 0 (0%) | 107 (56.6%) | <0.001 |
| HRS | 130 (39.8%) | 0 (0%) | 130 (68.8%) | <0.001 |
| Hemodialysis | 81 (24.8%) | 0 (0%) | 81 (42.9%) | <0.001 |
| Ventilator | 68 (20.8%) | 0 (0%) | 68 (36.0%) | <0.001 |
| Vasopressor | 37 (11.3%) | 0 (0%) | 37 (19.5%) | <0.001 |
| ICU‐bound | 130 (39.8%) | 3 (2.2%) | 127 (67.2%) | <0.001 |
| Graft type, RL | 233 (71.3%) | 91 (65.9%) | 142 (75.1%) | 0.028 |
| LL | 33 (10.1%) | 21 (15.2%) | 12 (6.3%) | |
| Dual | 61 (18.7%) | 26 (18.8%) | 35 (18.5%) | |
| Graft weight | 691 ± 142 g | 671 ± 148 g | 705 ± 135 g | 0.054 |
| GRWR | 1.0 ± 0.21% | 1.0 ± 0.20% | 1.0 ± 0.23% | 0.231 |
| Operation time | 926 ± 201 min | 935 ± 161 min | 919 ± 226 min | 0.580 |
| Red blood cells | 19.9 ± 15.8 U | 17.2 ± 9.8 U | 21.9 ± 19.0 U | 0.014 |
| Platelet | 14.8 ± 9.1 U | 14.9 ± 7.4 U | 14.6 ± 10.3 U | 0.771 |
| Fresh‐frozen plasma | 25.8 ± 17.2 U | 24.4 ± 12.3 U | 26.9 ± 20.1 U | 0.761 |
| Cold ischemia | 77.9 ± 28.3 min | 78.0 ± 28.3 min | 77.4 ± 28.1 min | 0.673 |
| Warm ischemia | 45.0 ± 15.6 min | 44.9 ± 15.7 min | 45.5 ± 15.4 min | 0.382 |
HBV, hepatitis B virus; HCV, hepatitis C virus; AIH, autoimmune hepatitis; ACLF, acute‐on‐chronic liver failure; SBC, secondary biliary cirrhosis; PBC, primary biliary cirrhosis; MELD, Model for End‐Stage Liver Disease; INR, international normalized ratio; HEP, hepatic encephalopathy; HRS, hepatorenal syndrome; ICU, intensive care unit; HCC, hepatocellular carcinoma; GRWR, graft‐to‐recipient weight ratio; U, units; RL, right hemiliver; LL, left hemiliver; Dual, dual graft with two left livers or right and left liver
High‐MELD score recipients were categorized as 137 (41.9%) non‐ACLF and 190 (58.1%) ACLF patients, in which the ACLF group exhibited a significantly higher value for the MELD score, creatinine, INR and amount of RBC transfusion, as well as a higher frequency of hepatic encephalopathy 3 and 4, hepatorenal syndrome, preoperative intensive care unit–bound, use of hemodialysis, ventilator, and right hemiliver graft.
We subdivided the ACLF patients into ACLF‐1, 2, and 3 according to the number of extrahepatic organ failures. ACLF‐1 (n = 96) denotes one extrahepatic organ failure; ACLF‐2 (n = 43) indicates a double extrahepatic organ failure; and ACLF‐3 (n = 51) means that three or more organs have failed. More detailed data concerning the combination of extrahepatic organ failures are depicted in Figure 2.
Figure 2.
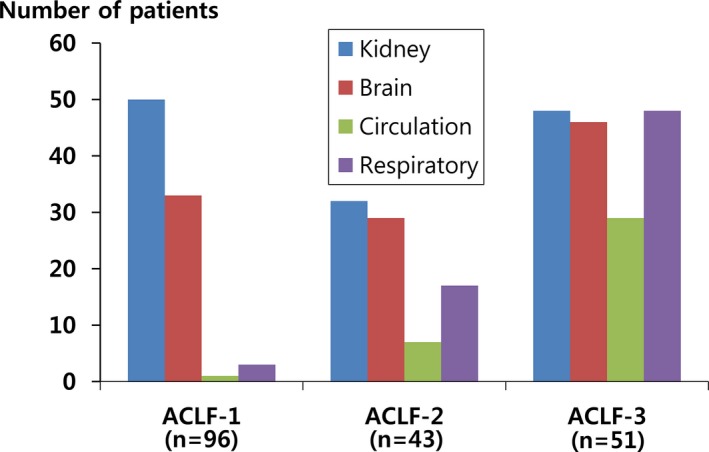
Types of extrahepatic organ failure by ACLF grade. ACLF, acute‐on‐chronic liver failure.
Impact of the pretransplant status on graft survival in high‐MELD score recipients
We analyzed the impact of pretransplant variables on the posttransplant graft survival. In the univariate analysis, age ≤59 years, male sex, GRWR 0.8–0.99%, non‐ACLF group, and the absence of HCC, hepatorenal syndrome, preoperative ICU‐stay, and vasopressor use were associated with significantly improved 1‐, 3‐, and 5‐year graft survival rates. However, in multivariate analysis, male sex, absence of HCC, and the non‐ACLF group revealed significantly improved survival rate with a hazard ratio of 1.84, 1.795, and 1.61, respectively (Table 2).
Table 2.
Posttransplant graft survival according to the pretransplant variables
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| 1‐YSR (%) | 3‐YSR (%) | 5‐YSR (%) | p | HR | 95% CI | p | |
| Age | |||||||
| ≤59 years | 82.9 | 77.7 | 76.4 | 0.005 | 1.92 | 0.97–3.79 | 0.060 |
| ≥60 years | 70.6 | 52.9 | 47.1 | ||||
| Sex | |||||||
| Male | 84.6 | 79.2 | 77.6 | 0.005 | 1.84 | 1.19–2.84 | 0.006 |
| Female | 75.3 | 66.7 | 65.4 | ||||
| Etiology | |||||||
| HBV | 83.5 | 77.7 | 76.2 | 0.251 | |||
| Non‐HBV | 76.1 | 70.0 | 70.0 | ||||
| HCC | |||||||
| None | 82.9 | 78.1 | 76.7 | 0.018 | 1.795 | 1.04–3.11 | 0.037 |
| Yes | 77.1 | 62.3 | 59.3 | ||||
| ACLF | |||||||
| None | 89.8 | 82.5 | 81.0 | 0.035 | 1.61 | 1.03–2.50 | 0.035 |
| Yes | 76.8 | 72.1 | 70.5 | ||||
| Graft | |||||||
| RL | 83.7 | 76.4 | 75.5 | 0.324 | |||
| LL | 75.8 | 72.7 | 66.7 | ||||
| Dual | 82.0 | 77.0 | 77.0 | ||||
| GRWR (%) | |||||||
| <0.8 | 81.8 | 66.7 | 63.6 | 0.010 | 1.06 | 0.77–1.47 | 0.729 |
| 0.8–0.99 | 89.5 | 85.1 | 84.2 | ||||
| ≥1.0 | 78.3 | 72.2 | 71.1 | ||||
| HRS | |||||||
| None | 85.3 | 80.2 | 79.2 | 0.042 | 0.80 | 0.41–1.58 | 0.523 |
| Yes | 77.7 | 69.9 | 68.4 | ||||
| HD | |||||||
| None | 84.6 | 78.0 | 76.4 | 0.204 | |||
| Yes | 75.3 | 70.3 | 70.3 | ||||
| HEP | |||||||
| Grade 0, 1, 2 | 85.4 | 77.6 | 76.3 | 0.457 | |||
| Grade 3, 4 | 75.9 | 74.1 | 72.2 | ||||
| Ventilator | |||||||
| None | 84.2 | 76.8 | 75.6 | 0.405 | |||
| Yes | 75.0 | 73.5 | 72.1 | ||||
| ICU stay | |||||||
| None | 87.3 | 80.7 | 79.2 | 0.017 | 0.76 | 0.42–1.39 | 0.376 |
| Yes | 74.6 | 70.0 | 68.4 | ||||
| Vasopressor | |||||||
| None | 84.5 | 78.3 | 76.5 | 0.021 | 1.95 | 1.12–3.40 | 0.19 |
| Yes | 64.9 | 62.2 | 62.2 | ||||
Univariate analysis using the Kaplan–Meier method with log rank test, multivariate analysis using Cox proportional hazards regression method with a stepwise procedure. 1‐,3‐,5‐YSR, 1, 3, and 5‐year graft survival rate; HR, hazard ratio; CI, confidence interval; p, p‐value; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ACLF, acute‐on‐chronic liver failure; RL, right hemiliver; LL, left hemiliver; Dual, dual‐graft with two left livers or right and left liver; GRWR, graft‐to‐recipient weight ratio; HRS, hepatorenal syndrome; HD, hemodialysis; HEP, hepatic encephalopathy; ICU, intensive care unit.
Impact of ACLF on the short‐term outcome
The peak AST and ALT within 48 h of reperfusion were not different between the ACLF and non‐ACLF group. The ACLF group stayed longer in the ICU during the posttransplant period than the non‐ACLF group (15.2 ± 16.2 days vs. 9.5 ± 10.3 days, p = 0.008); however, the total hospital days, including ICU stay after ALDLT, was not different between the two groups (ACLF 48.2 ± 32.2 days vs. non‐ACLF 41.9 ± 31.9 days, p = 0.156).
The frequency of total complications was similar between the ACLF and non‐ACLF groups (74.7% vs. 70.8%, p = 0.446). However, the ACLF group had a higher grade of complications categorized via the Clavien‐Dindo classification (p = 0.018) 24, as well as a higher hospital mortality (15.8% vs. 6.6%, p = 0.011). Reoperation and retransplantation were performed more frequently in the ACLF group, but this did not reach significance (p = 0.064 and 0.313, respectively) (Table S2).
Common technical complications of the ACLF group included bleeding from the operation fields requiring embolization or reoperation (29.6%), HV stenosis (21.0%), biliary complications including stricture and leakage (20.0%), hepatic artery complications including stenosis, dissection, thrombosis, and pseudoaneurysms (10.8%), and wound complications (7.4%). In the 30 cases of hospital mortality in the ACLF group, the cause of death was predominantly due to medical complications, such as pneumonia and acute rejection (23, 76.7%) rather than technical complications (7, 23.3%). Concerning technical complications, hepatic artery complications were the most common cause of hospital mortality.
Impact of ACLF on graft and patient survival
When we analyzed the survival between the ACLF and non‐ACLF groups, the posttransplant graft survival at 1, 3, and 5 years were significantly worse in the ACLF group (76.8%, 72.1%, and 70.5%, respectively) compared to the non‐ACLF group (89.8%, 82.5%, and 81.0%, respectively) (p = 0.035), however, the patient survival at 1, 3, and 5 years were not statistically different between the ACLF group (79.5%, 73.6%, and 72.1%, respectively) and the non‐ACLF group (90.5%, 83.2%, and 81.8%, respectively) (p = 0.063) (Figure 3). Among the ACLF‐1, ‐2, and ‐3 patients according to the number of extrahepatic organ failures, the 1‐, 3‐, and 5‐year graft survival rates were 81.3%, 76.0%, and 75.0% for ACLF‐1; 72.3%, 70.2%, and 68.0% for ACLF‐2; and 76.0%, 71.5%, and 71.5%, for ACLF‐3, respectively (p = 0.296). ACLF‐2 and ‐3 tended to exhibit a worse survival than ACLF‐1, but this did not reach statistical significance.
Figure 3.
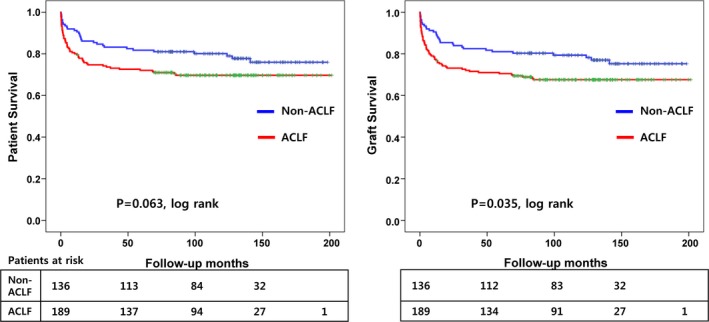
Survival differences between ACLF and non‐ACLF recipients following ALDLT. ACLF, acute‐on‐chronic liver failure; ALDLT, adult living donor liver transplantation.
Prognostic interrelationship between ACLF and the MELD score
To clarify the prognostic interrelationship between ACLF and the MELD score among high‐MELD score patients, we performed an additional analysis after subgrouping the high‐MELD score patients into a lower group (n = 169) indicating a MELD score of between 30 and 34 points, and a higher group (n = 158) indicating a MELD score of ≥35 points. The ACLF patients comprised 45 patients (32.8%) in the lower group and 124 patients (65.3%) in the higher group. In the higher group, the 1‐, 3‐, and 5‐year graft survival between the non‐ACLF and ACLF groups were 86.7%, 80.0%, and 75.6%, and 76.6%, 71.0%, and 69.4%, respectively (p = 0.289). In the lower group, the 1‐, 3‐, and 5‐year graft survival between the non‐ACLF and ACLF groups were 91.3%, 83.7%, and 83.7%, and 77.3%, 74.2%, and 72.6%, respectively (p = 0.088). In the multivariate analysis, the higher group did not exhibit any significant prognostic variables; however, the lower group was significantly affected by the presence of ACLF (p = 0.01) (Table 3).
Table 3.
Multivariate analysis of graft survival in the high‐MELD score patients depending on each subgroup
| Variable | p‐value | Hazard ratio | 95% CI |
|---|---|---|---|
| Low MELD group (30–34 points) | |||
| Age, (≥60 years) | 0.76 | 0.83 | 0.25–2.80 |
| Sex (female) | 0.20 | 1.34 | 0.86–2.07 |
| HCC (present) | 0.74 | 0.89 | 0.45–1.78 |
| ACLF (present) | 0.01 | 2.46 | 1.28–4.72 |
| GRWR (0.8–0.99%) | 0.70 | 1.15 | 0.56–2.38 |
| GRWR (≥1%) | 0.70 | 1.15 | 0.56–2.36 |
| HRS (yes) | 0.89 | 1.05 | 0.54–2.02 |
| ICU‐bound (yes) | 0.36 | 0.76 | 0.42–1.36 |
| Vasopressor (yes) | 0.17 | 0.54 | 0.22–1.31 |
| High MELD group (≥35 points) | |||
| Age (≥60 years) | 0.22 | 2.03 | 0.66–6.23 |
| Sex (female) | 0.97 | 1.01 | 0.61–1.66 |
| HCC (present) | 0.31 | 0.65 | 0.29–1.48 |
| ACLF (present) | 0.93 | 0.97 | 0.48–1.95 |
| GRWR (0.8–0.99%) | 0.69 | 0.86 | 0.40–1.83 |
| GRWR (≥1%) | 0.87 | 1.06 | 0.51–2.21 |
| HRS (yes) | 0.56 | 1.19 | 0.66–2.13 |
| ICU‐bound (yes) | 0.98 | 0.99 | 0.59–1.66 |
| Vasopressor (yes) | 0.42 | 1.31 | 0.68–2.52 |
MELD, Model for End‐Stage Liver Disease; CI, confidence interval; HCC, hepatocellular carcinoma; ACLF, acute‐on‐chronic liver failure; GRWR, graft‐to‐recipient weight ratio; HRS, hepatorenal syndrome; ICU, intensive care unit.
Discussion
Since the implementation of the MELD system by the UNOS in February 2002, there has been an ongoing debate regarding the posttransplant outcome of high‐MELD score patients in the series of DDLT 25, 26, 27, 28. In 2005, Habib et al reported a conclusive outcome that the pretransplant MELD score was inversely correlated with posttransplant survival based on a larger study with a longer follow‐up 29. In contrast, the prevailing opinion from a few well‐known LDLT centers is that there is no correlation between the pretransplant MELD score and posttransplant survival despite some conflicting results 3, 4, 5, 6, 7. In light of our clinical experiences based on the largest ALDLT series in the world, the prevailing opinions conflict with ours and we performed the present study to determine the effect of MELD scores on the survival outcome of patients in a large single‐center series of ALDLT. Furthermore, we aimed to investigate the reasons for the conflicting results of the reported LDLT series by an analysis of perioperative data and survival outcomes between ACLF and non‐ACLF groups of high‐MELD score recipients.
In our series, the high MELD (≥30) group exhibited a significantly lower graft and patient survival rate than the low and intermediate MELD groups. This might be a natural outcome because a high MELD score reflects the severity of the recipients' pretransplant illness. However, we achieved a minimum of 10% superior graft and patient survival in comparison with the DDLT series in the high MELD group 27, 28, 29. Timely ALDLT for the urgent high‐MELD score patients at our institution played a substantial role for achieving a better outcome in the scarcity of deceased donor livers in Korea 30. The selection of donors displaying a good quality of liver graft with less steatosis and a median age of 30 years, as well as the sustained efforts to satisfy ≥1% GRWR using various graft types (e.g. single‐graft ALDLT with right or left hemi‐liver, or dual‐graft ALDLT with two left hemi‐livers or right and left hemiliver) made it possible to achieve unique and excellent survival outcomes for high‐MELD score recipients 12, 18. Innovations in the operative techniques and intensive perioperative care by the dedicated team members also resulted in a favorable outcome 10, 31. In addition, the predominant etiology related to HBV (79.5%) might have an important role for achieving a better survival rate 6 because recipients with an HBV etiology had superior survival rates compared to the non‐HBV etiology despite no statistical significance.
We have performed multiple ALDLTs for high‐MELD score patients, and the presence of ACLF might be an important prognostic factor associated with the patient outcome. The definition was first established by the Asian Pacific Association for the study of liver disease (APASL) in 2009 32, in which it was defined as “acute hepatic insult manifesting as jaundice (serum bilirubin >5 mg/dL) and coagulopathy (INR >1.5), complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.” The European and American Association for the study of liver disease (EASL) also proposed a different definition in 2011 33, which defined it as an “acute deterioration of pre‐existing, chronic liver disease, usually related to a precipitating event and associated with increased mortality at 3 months due to multi‐system organ failure.” The differences were resolved as part of a consensus meeting organized under the auspices of the World Congress of Gastroenterology, and a new definition was created as a distinct clinical entity in 2014 9. Using this consensus definition of ACLF, we were able to categorize high‐MELD score cirrhotic recipients who had undergone ALDLT into ACLF and non‐ACLF groups, and analyze the clinical data. To our knowledge, this study is the first report demonstrating the impact of ACLF on the post‐ALDLT outcome using a newly established consensus definition.
The ACLF group in our series comprised 51.8% high‐MELD score recipients and the preoperative clinical data revealed a more severe clinical status than in the non‐ACLF group. Moreover, the ACLF group required a larger amount of intraoperative RBC transfusion and had worse short‐term outcomes compared to the non‐ACLF group. Considering the higher preoperative disease severity of ACLF, these are reasonable results. In the multivariate analysis, unexpectedly good outcomes in the men was related to a higher frequency of HBV‐related liver cirrhosis (82.9%), which is associated with a better outcome after LT than other etiologies 6 in comparison to the female recipients (69.1%) (p = 0.008); this was also related to relatively less severe sarcopenia, which has a poorer outcome 34, 35. Although the presence of HCC unfavorably affected patient survival, the distribution and stage of the HCC patients was similar between ACLF and non‐ACLF groups and we did not exclude HCC patients for the survival analysis between the two groups. Although hepatorenal syndrome, preoperative ICU stay, and vasopressor use were not significant prognostic variables in the multivariate analysis, the clinical importance was reflected by ACLF, which was a significantly worse prognostic factor in the multivariate analysis.
In contrast to the superior graft survival rate in the non‐ACLF group, the patient survival rate was not significantly better because four out of seven patients in the ACLF group survived for a long period after retransplantation, despite the uncommon frequency; this offset the difference in the patient survival between the ACLF and non‐ACLF groups.
Regarding the postoperative complications in ACLF patients, technical complications related to hospital mortality decreased further in the later period, but hepatic artery complications, which typically resulted from trauma to the arterial wall during hilar dissection under operative fields involving copious bleeding, were still important causes of hospital‐related mortality. During the study period from February 1998 to March 2010, 21% of the high‐MELD score patients underwent HV stenting following ALDLT due to suspicion of HV stenosis. During the later study period, the rate of HV stenting was decreased to approximately 9% following the introduction of HV plasty into the recipient side and further decreased less than 2% following the routine application of HV plasty in both the liver graft and recipient side in 2010 36, 37. The portal vein (PV) complication rates were low at approximately 2%, which was related to the introduction of an intraoperative cine‐portogram since 2003 for recipients experiencing preoperative complications such as PV thrombosis and/or stenosis 38. When indicated (despite PV thrombectomy and/or plasty), PV stent placement is performed 39.
Regarding the prognostic interrelationship between the ACLF and MELD score, the presence of ACLF was not a significant prognostic factor in the higher MELD group (≥35 points) but was a significant prognostic factor in the lower MELD group (30–34 points) after ALDLT in the multivariate analysis. In addition, when we excluded 190 ALCF recipients from total 1958 ALDLT recipients during the study period, the posttransplant graft and patient survival at 1, 3, and 5 years in the high‐MELD (≥30 points) group did not differ from the graft and patient survival of the low‐ and intermediate‐MELD groups (Figure S2). These findings are in line with the prevailing opinions of a few well‐known LDLT centers 5, 6, 7. The conflicting worse outcome after ALDLT for the high‐MELD score patients in our series could be explained by the high percentage of ACLF patients (58.1%) in the high‐MELD (≥ 30 points) group compared to a few well‐known LDLT centers.
In conclusion, timely ALDLT using various graft types with good quality to satisfy the ≥1% GRWR for high‐MELD score patients when there is a scarcity of deceased donor livers guaranteed excellent short‐term and long‐term survival rates. Considering the prognostic significance of the presence of ACLF in high‐MELD score patients, particularly in the lower‐grade group (30–34 points), ALDLT should be performed as soon as possible in high‐MELD score patients prior to the development of ACLF. Moreover, ACLF patients should be categorized separately when analyzing the outcomes of ALDLT. In addition, ALDLT for ACLF patients should no longer be discouraged because favorable outcomes can be expected through performing timely ALDLT and comprehensive management.
Author Contributions
Lee S‐G, Moon D‐B, Park GC, Song G‐W, Ha T‐Y: Study conception and design; Moon D‐B, Kang W‐H, Jung D‐H, Song G‐W, Park G‐C, Kim W‐J, Cho H‐D, Jwa E‐K: Acquisition of data; Moon D‐B, Lee S‐G, Jung D‐H, Kim W‐J, Cho H‐D, Jwa E‐K, Kim H‐J: Analysis and interpretation of data; Moon D‐B, Lee S‐G, Ha T‐Y, Jung D‐H, Song G‐W, Kim H‐J: Drafting of manuscript; Moon D‐B, Lee S‐G, Ha T‐Y, Park G‐C, Song G‐W, Jung D‐H, Kim H‐J: Critical revision; Lee S‐G, Moon D‐B, Song G‐W, Jung D‐H: Final approval of the version to be published; Moon D‐B, Lee S‐G, Kang W‐H, Park G‐C: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Figure S1: Overall survival following ALDLT according to MELD score.
Figure S2: Overall survival following ALDLT according to MELD score when excluding ACLF patients from the high‐MELD score recipients.
Table S1: Modified version of the Chronic Liver Failure Consortium organ failure score.
Table S2: Postoperative data according to the acute‐on‐chronic liver failure following adult living donor liver transplantation.
Moon D‐B, Lee S‐G, Kang W‐H, Song G‐W, Jung D‐H, Park G‐C, Cho H‐D, Jwa E‐K, Kim W‐J, Ha T‐Y & Kim H‐J. Adult Living Donor Liver Transplantation for Acute‐on‐Chronic Liver Failure in High–Model for End‐Stage Liver Disease Score Patients. Am J Transplant 2017; 17: 1833–1842
References
- 1. New York State Department of Health, Committee on Quality Improvement in Living Liver Donation. 2002. [cited 2016 Dec 21]. Available from: http://www.health.state.ny.us.
- 2. Xia VW, Du B, Braunfeld M, et al. Preoperative characteristics and intraoperative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transpl 2006; 12: 614–620. [DOI] [PubMed] [Google Scholar]
- 3. Marubashi S, Dono K, Asaoka T, et al. Risk factors for graft dysfunction after adult‐to‐adult living donor liver transplantation. Transplant Proc 2006; 38: 1407–1410. [DOI] [PubMed] [Google Scholar]
- 4. Akyildiz M, Karasu Z, Arikan C, et al. Impact of pretransplant MELD score on posttransplant outcome in living donor liver transplantation. Transplant Proc 2004; 36: 1442–1444. [DOI] [PubMed] [Google Scholar]
- 5. Selzner M, Kashfi A, Cattral MS, et al. Live donor liver transplantation in high MELD score recipients. Ann Surg 2010; 251: 153–157. [DOI] [PubMed] [Google Scholar]
- 6. Yi NJ, Suh KS, Lee HW, et al. Improved outcome of adult recipients with a high model for end‐stage liver disease score and a small‐for‐size graft. Liver Transpl 2009; 15: 496–503. [DOI] [PubMed] [Google Scholar]
- 7. Chan AC, Fan ST, Lo CM, et al. Liver transplantation for acute‐on‐chronic liver failure. Hepatol Int 2009; 3: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute‐on‐chronic liver failure. Lancet 2015; 386: 1576–1587. [DOI] [PubMed] [Google Scholar]
- 9. Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an improved definition of acute‐on‐chronic liver failure. Gastroenterology 2014; 147: 4–10. [DOI] [PubMed] [Google Scholar]
- 10. Lee SG. A complete treatment of adult living donor liver transplantation: A review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015; 15: 17–38. [DOI] [PubMed] [Google Scholar]
- 11. Moon SK, Park YH, Moon DB, et al. How to perform selective liver biopsy in living liver donors using plain computed tomography. Transplantation 2016; 100: 2398–2403. [DOI] [PubMed] [Google Scholar]
- 12. Song GW, Lee SG, Moon DB, et al. Dual‐graft adult living donor liver transplantation: An innovative surgical procedure for live liver donor pool expansion. Ann Surg 2016. May 17. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Hwang S, Lee SG, Sung KB, et al. Long‐term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl 2006; 12: 831–838. [DOI] [PubMed] [Google Scholar]
- 14. Hwang S, Lee SG, Ha TY, et al. Simplified standardized technique for living donor liver transplantation using left liver graft plus caudate lobe. Liver Transpl 2004; 10: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 15. Hwang S, Lee SG, Lee YJ, et al. Donor selection for procurement of right posterior segment graft in living donor liver transplantation. Liver Transpl 2004; 10: 1150–1155. [DOI] [PubMed] [Google Scholar]
- 16. Gyu Lee S, Min Park K, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002; 74: 54–59. [DOI] [PubMed] [Google Scholar]
- 17. Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: How to make living donations safe. Liver Transpl 2006; 12: 920–927. [DOI] [PubMed] [Google Scholar]
- 18. Lee S, Hwang S, Park K, et al. An adult‐to‐adult living donor liver transplant using dual left lobe grafts. Surgery 2001; 129: 647–650. [DOI] [PubMed] [Google Scholar]
- 19. Hwang S, Lee SG, Ahn CS, et al. Outflow vein reconstruction of extended right lobe graft using quilt venoplasty technique. Liver Transpl 2006; 12: 156–158. [DOI] [PubMed] [Google Scholar]
- 20. Ha TY, Hwang S, Ahn CS, et al. Role of hand‐assisted laparoscopic surgery in living‐donor right liver harvest. Transplant Proc 2013; 45: 2997–2999. [DOI] [PubMed] [Google Scholar]
- 21. Park JI, Kim KH, Lee SG. Laparoscopic living donor hepatectomy: A review of current status. J Hepatobiliary Pancreat Sci 2015; 22: 779–788. [DOI] [PubMed] [Google Scholar]
- 22. Park CS, Jung BH, Hwang S, et al. External biliary drainage in living donor liver transplantation using duct‐to‐duct anastomosis. Transplant Proc 2014; 46: 678–681. [DOI] [PubMed] [Google Scholar]
- 23. Lee SG, Moon DB, Namgoong JM. Living donor liver transplantation procedure and surgical technique In: Allan DK, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA, editors. Textbook of organ transplantation. Oxford: Wiley Blackwell, 2014. [Google Scholar]
- 24. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown RS Jr, Kumar KS, Russo MW, et al. Model for end‐stage liver disease and Child‐Turcotte‐Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transpl 2002; 8: 278–284. [DOI] [PubMed] [Google Scholar]
- 26. Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: Utility of the model for end‐stage liver disease and a newly derived discrimination function. Transplantation 2004; 77: 99–106. [DOI] [PubMed] [Google Scholar]
- 27. Onaca NN, Levy MF, Sanchez EQ, et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl 2003; 9: 117–123. [DOI] [PubMed] [Google Scholar]
- 28. Jacob M, Copley LP, Lewsey JD, et al. Pretransplant MELD score and post liver transplantation survival in the UK and Ireland. Liver Transpl 2004; 10: 903–907. [DOI] [PubMed] [Google Scholar]
- 29. Habib S, Berk B, Chang CC, et al. MELD and prediction of post‐liver transplantation survival. Liver Transpl 2006; 12: 440–447. [DOI] [PubMed] [Google Scholar]
- 30. Moon DB, Lee SG. Liver transplantation. Gut Liv 2009; 3: 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moon DB, Lee SG, Hwang S, et al. More than 300 consecutive living donor liver transplants a year at a single center. Transplant Proc 2013; 45: 1942–1947. [DOI] [PubMed] [Google Scholar]
- 32. Sarin SK, Kumar A, Almeida JA, et al. Acute‐on‐chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3: 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olson JC, Kamath PS. Acute‐on‐chronic liver failure: Concept, natural history, and prognosis. Curr Opin Crit Care 2011; 17: 165–169. [DOI] [PubMed] [Google Scholar]
- 34. Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 2012; 18: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 35. Hamaguchi Y, Kaido T, Okumura S, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014; 20: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 36. Hwang S, Ahn CS, Kim KH, et al. Standardization of modified right lobe grafts to minimize vascular outflow complications for adult living donor liver transplantation. Transplant Proc 2012; 44: 457–459. [DOI] [PubMed] [Google Scholar]
- 37. Hwang S, Ha TY, Ahn CS, et al. Hemodynamics‐compliant reconstruction of the right hepatic vein for adult living donor liver transplantation with a right liver graft. Liver Transpl 2012; 18: 858–866. [DOI] [PubMed] [Google Scholar]
- 38. Moon DB, Lee SG, Ahn C, et al. Application of intraoperative cine‐portogram to detect spontaneous portosystemic collaterals missed by intraoperative doppler exam in adult living donor liver transplantation. Liver Transpl 2007; 13: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 39. Moon DB, Lee SG, Ahn CS, et al. Section 6. Management of extensive nontumorous portal vein thrombosis in adult living donor liver transplantation. Transplantation 2014; 97(Suppl 8): S23–S30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Overall survival following ALDLT according to MELD score.
Figure S2: Overall survival following ALDLT according to MELD score when excluding ACLF patients from the high‐MELD score recipients.
Table S1: Modified version of the Chronic Liver Failure Consortium organ failure score.
Table S2: Postoperative data according to the acute‐on‐chronic liver failure following adult living donor liver transplantation.


