Abstract
Objectives
The European Centre for Disease Prevention and Control (ECDC) supports countries to monitor progress in their response to the HIV epidemic. In line with these monitoring responsibilities, we assess how, and to what extent, the continuum of care is being measured across countries.
Methods
The ECDC sent out questionnaires to 55 countries in Europe and Central Asia in 2014. Nominated country representatives were questioned on how they defined and measured six elements of the continuum. We present our results using three previously described frameworks [breakpoints; Joint United Nations Programme on HIV/AIDS (UNAIDS) 90‐90‐90 targets; diagnosis and treatment quadrant].
Results
Forty countries provided data for at least one element of the continuum. Countries reported most frequently on the number of people diagnosed with HIV infection (37; 93%), and on the number in receipt of antiretroviral therapy (ART) (35; 88%). There was little consensus across countries in their approach to defining linkage to, and retention in, care. The most common breakpoint (>19% reduction between two adjacent elements) related to the estimated number of people living with HIV who were diagnosed (18 of 23; 78%).
Conclusions
We present continuum data from multiple countries that provide both a snapshot of care provision and a baseline against which changes over time in care provision across Europe and Central Asia may be measured. To better inform HIV testing and treatment programmes, standard data collection approaches and definitions across the HIV continuum of care are needed. If countries wish to ensure an unbroken HIV continuum of care, people living with HIV need to be diagnosed promptly, and ART needs to be offered to all those diagnosed.
Keywords: antiretroviral therapy, breakpoints, cascade, Central Asia, continuity of patient care, Europe
Introduction
Interest has grown in the HIV continuum of care since the Centers for Disease Control and Prevention (CDC) highlighted the spectrum of engagement in HIV care in the USA 1, 2, 3, 4. The continuum of care emphasizes the importance of continuity of good‐quality and accessible HIV services so that people living with HIV experience long‐term viral suppression. This has long‐term health benefits for the individual receiving treatment and general public health benefits as community‐level viral suppression reduces onward viral transmission 5, 6, 7, 8, 9.
Several authors have reported HIV care continuum data from European countries including Belgium 10, Denmark and Sweden 11, France 12, Ireland 13, the Netherlands 14, Russia 15, Spain 16 and the UK 17. However, issues have been raised about the utility of the HIV continuum of care, particularly relating to cross‐country comparisons. Across countries there is no consensus as to which elements should be included, which elements should be compared, and how these elements should be defined. Also, importantly, to date no analytic framework has been agreed that allows cross‐country comparison of continuum data. Potential frameworks include breakpoints 18, 90‐90‐90 targets 19, and quadrants 20.
In 2014, Raymond and colleagues presented continuum data from several countries and analysed these data using the concept of breakpoints in the continuum, points where there is a large drop between successive elements18. This concept was previously used to comment on US continuum data 21, and has been referred to as “leaks” 15, 22. Raymond and colleagues quantified the drop needed between elements to constitute a breakpoint as at least 19 percentage points 18.
An alternative analytic framework for continuum of care data is the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90‐90‐90 targets 19. The targets are that, by 2020, 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained ART, and 90% of all people receiving ART will have viral suppression 19. These targets relate to four elements of the HIV care continuum: the estimated number of people living with HIV; the number who know their HIV status; the number currently on ART; the number with viral suppression.
Using “cut‐points” of 60%, Kelly and Wilson divide countries into quadrants based on observed data on the number of people living with HIV who are diagnosed and the number diagnosed on ART 20. Quadrant 1 includes countries with high diagnosis and treatment rates; quadrant 2 includes countries with high diagnosis rates but low treatment rates; quadrant 3 includes countries with low diagnosis and treatment rates; quadrant 4 includes countries with low diagnosis rates but high treatment rates.
In 2004, European and Central Asian countries adopted the Dublin Declaration concerning the region's response to HIV 23. Since 2010, the European Centre for Disease Prevention and Control (ECDC) has supported countries to monitor progress in implementing this declaration. There have been three reporting rounds, in 2010, 2012 and 2014, for the region's 55 countries 24. In line with these monitoring responsibilities, we assess how, and to what extent, the HIV continuum of care is measured across Europe and Central Asia. We also present baseline analysis of continuum data and consider whether the three frameworks discussed above assist the analysis and interpretation of continuum data from multiple countries.
Method
A question on the HIV continuum of care was included in the questionnaire used to measure implementation of the Dublin Declaration in 2014. The question asked for data on six continuum elements: the number of people 1 living with HIV; 2 diagnosed with HIV infection 3;linked to care 4; retained in care 5; on ART 6; who have an undetectable viral load. In December 2013, the questionnaire was circulated in electronic (PDF) format to nominated representatives in 55 countries. It was requested that responses be forwarded to ECDC by the end of March 2014. Data validation consisted of raising data queries with country representatives directly and sharing draft analytical outputs for comment.
Country representatives were asked to describe their methods for defining and measuring continuum elements. Data sources were categorized as cumulative population‐based data, annual population‐based data, and cohort studies. In our results, we summarize the data sources used and, for each element, present examples of definitions/approaches to measurement (chosen based on being the most frequently reported, being a unique example, or being an example that could be considered for wider uptake across countries).
For countries reporting two or more elements, analysis was conducted to identify and describe progression along the care pathway. To facilitate this analysis, three frameworks were considered: breakpoints 18, global targets19, and quadrant assignment 20. A country was considered to have a breakpoint if there was a reduction of more than 19% between two adjacent elements (there are potentially five breakpoints in a six‐point continuum). A country was considered to be meeting one or more of the 90‐90‐90 targets if the percentage of people in one element (numerator) was 90% or more of the number of people in the preceding element (denominator). Countries were classified into a quadrant depending on the percentage of people estimated to be living with HIV who had been diagnosed and the percentage of those diagnosed who were on treatment.
For comparative purposes, in some of our analyses we stratify countries according to whether they were a European Union (EU)/European Economic Area (EEA) country or not. We include Switzerland in the EU/EEA group. Results are presented as among countries for which relevant information was available.
Results
Data availability
Of the 55 countries contacted, 40 (73%) provided quantitative data for at least one element of the HIV continuum. Of the 32 EU/EEA (plus Switzerland) countries contacted, 28 (88%) provided quantitative data for at least one element, and 12 (52%) of the 23 non‐EU/EEA countries contacted provided data. Thirty‐two (58%) of all countries contacted provided data for at least four elements and 13 (24%) provided data for all six elements. The 13 countries were Armenia, Austria, Azerbaijan, Bulgaria, Denmark, Georgia, Luxembourg, the Netherlands, Romania, Serbia, Spain, Switzerland and the United Kingdom.
Among the 40 countries providing quantitative data, the elements most frequently reported were the number of people diagnosed (37 countries; 93%) and the number of people on ART (35 countries; 88%) (see Fig. 1). Of these 40 countries, 31 (77%) based their response on cumulative population‐based data, six (15%) on annual population‐based data, and three (8%) on cohort data.
Figure 1.
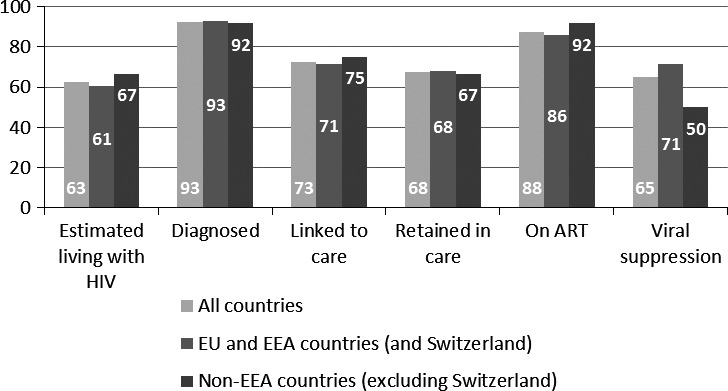
Percentage of European and Central Asian countries reporting quantitative data for different elements of the HIV continuum of care (n = 40). ART, antiretroviral therapy; EEA, European Economic Area; EU, European Union.
Defining continuum of care elements
Countries use different definitions of continuum elements. Although some countries do report the UNAIDS Spectrum tool estimates of numbers of people living with HIV, many countries reported concerns about the relevance of these estimates for a region with concentrated and low‐level HIV epidemics. Luxembourg generated survey‐based estimates showing the percentage of people living with HIV who knew their status. Germany reported that the number of people living with HIV is estimated based on symptoms and CD4 count at the time of HIV diagnosis.
Diagnosed with HIV
Seventy‐five per cent (30 of 40) of countries define this element as the cumulative number ever diagnosed. The cumulative figure may or may not exclude persons known to have died. In the United Kingdom, where access to HIV care is open and free, the annual number of people seen for HIV care was considered a proxy of the total number of people living with diagnosed HIV.
Linked to care
Possibly reflecting diverse health systems across the region, there was little consensus across countries in how to define linkage to care in terms of both population and time period. In some countries, registration was considered evidence of linkage to care. In some countries, a diagnosed person was considered to be linked to care only if they attended a particular type of health facility. A specific laboratory test was considered as evidence of linkage to care in some countries. In the United Kingdom, a person was considered linked to care when there was evidence of a CD4 count test result within 3 months of diagnosis. In Spain, the time period was 6 months and in Serbia it was 12 months.
Retained in care
In some countries, this was defined as having at least one clinical visit per year; in others, it was defined as being in care for a certain amount of time after linkage to care (for example, 1 year), and in some countries those retained in care were considered a subset of those on ART. In one country, the term was interpreted as in‐patient care.
On treatment and undetectable viral load
The number of persons in receipt of ART was defined as the number ever started on treatment or the number of those on treatment at the end of the year or when last seen. The cut‐off level at which the virus was considered undetectable varied across countries. Of the 25 countries reporting data, 11 (44%) reported the threshold they used for viral suppression. A further three countries who did not report data also reported the threshold they used. Of the 14 countries reporting thresholds for viral suppression, eight (57%) used <50 HIV‐1 RNA copies/mL. However, countries reported a wide range of thresholds from <20 to <500 copies/mL. Five countries reported rates of viral suppression using at least two thresholds.
Frameworks for analysing and interpreting continuum data
Breakpoints in the continuum
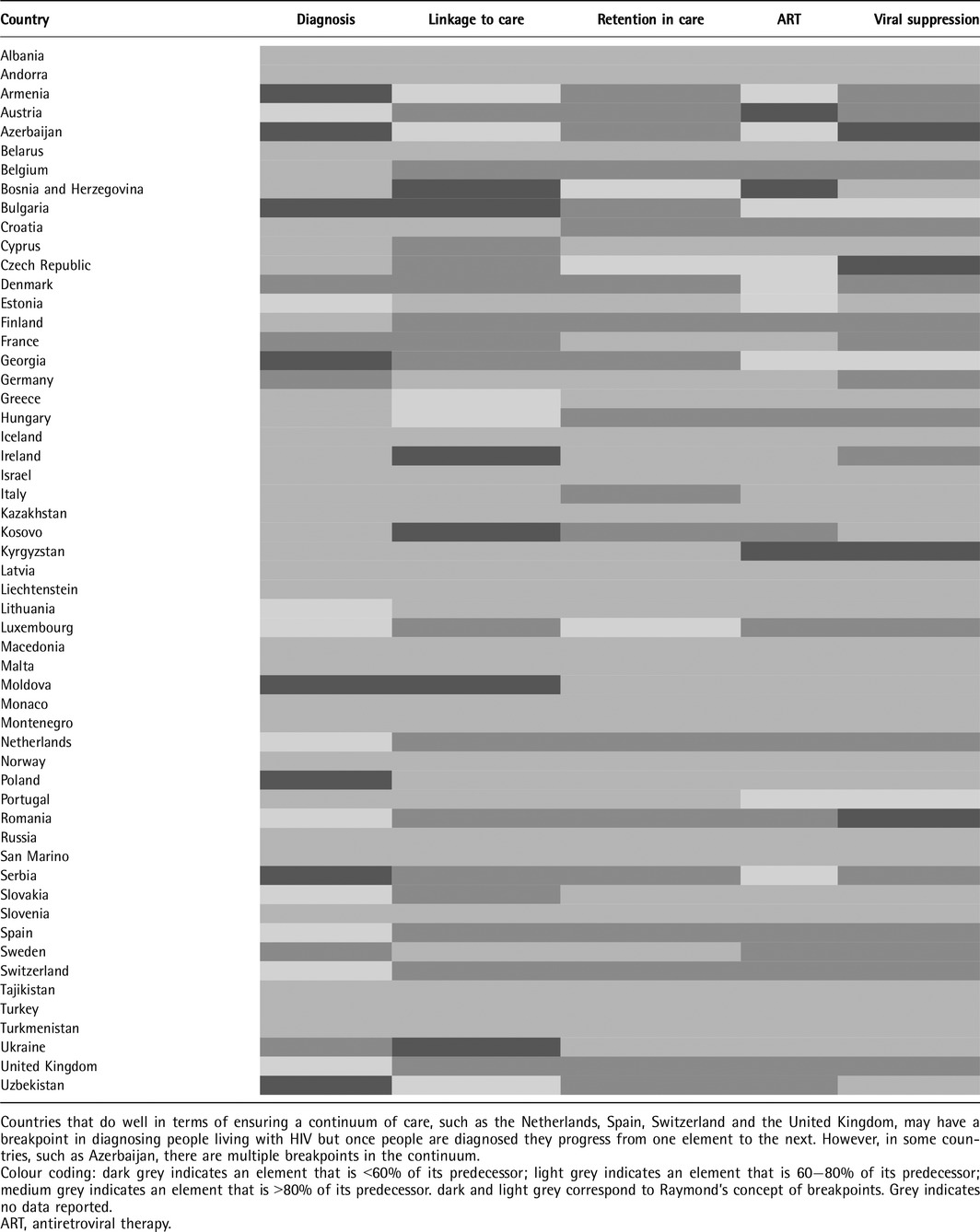
Countries most frequently reported a breakpoint between the estimated number of people living with HIV and those diagnosed. Of the 23 countries reporting on these two adjacent elements, 18 (78%) reported a breakpoint > 19%. Counties least commonly reported a breakpoint between linkage to and retention in care. Of the 22 countries providing data, only three (14%) reported a breakpoint >19% between these two adjacent elements. Table 1 presents breakpoints in the HIV continuum in Europe and Central Asia by country.
Table 1.
Breakpoints in the HIV continuum in Europe and Central Asia by country
UNAIDS 90‐90‐90 targets
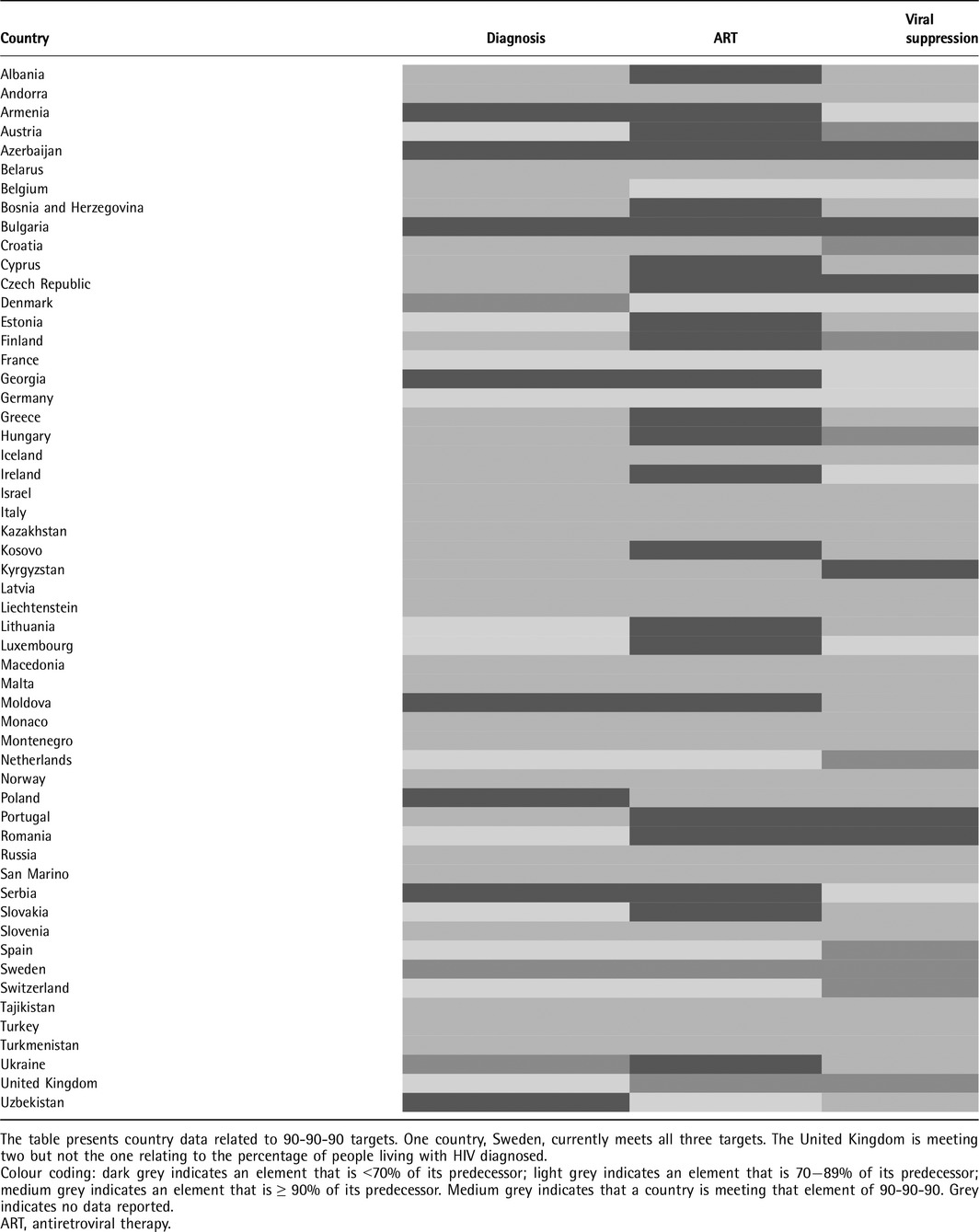
Table 2 presents country data related to 90‐90‐90 targets. Sweden reported that they met all three targets, with the UK reporting that they met two targets. Of 23 countries providing data, three (13%) reported that > 90% of those estimated to be living with HIV had been diagnosed. Of 33 countries providing data, two (6%) reported that > 90% of those diagnosed with HIV were on ART, and nine of 24 (38%) countries reported that > 90% of those on ART were virally suppressed.
Table 2.
How are European and Central Asian countries performing against 90‐90‐90?
Quadrants based on numbers of people living with HIV diagnosed and treated
Figure 2 presents countries as assigned to quadrants depending on the percentage of people estimated to be living with HIV who have been diagnosed and the percentage of those diagnosed on treatment. The figure shows the majority of western European countries, as well as the EU/EEA average, to be in quadrant 1 (≥60% on both axes). In particular, Sweden stands out with 91% reportedly being diagnosed and 92% of these being reported to be on ART. Alongside five countries (Armenia, Azerbaijan, Bulgaria, Georgia and Moldova), the non‐EU/EEA average is shown to be placed in quadrant 3 where both criteria are <60%.
Figure 2.
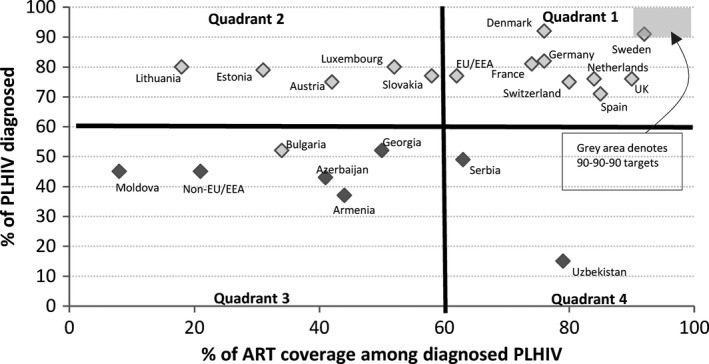
How are European and Central Asian countries performing in terms of ensuring people living with HIV (PLHIV) are diagnosed and treated? Quadrant 1: countries with high diagnosis and treatment rates. Quadrant 2: countries with high diagnosis rates but low treatment rates. Quadrant 3: countries with low diagnosis and treatment rates. Quadrant 4: countries with low diagnosis rates but high treatment rates. Quadrants were defined following the definition of Kelly and Wilson 20. Colour coding: light grey indicates European Union (EU) and European Economic Area (EEA) countries plus Switzerland; dark grey indicates non‐EU/EEA countries less Switzerland. The grey box denotes the countries within quadrant 1 that are meeting the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90‐90‐90 targets. ART, antiretroviral therapy.
Four‐element continuum
As reported above, the elements with least agreement across countries on how to define them were linkage to, and retention in, care. Based on this finding, we present a framework for analysing and interpreting continuum data based on the remaining four elements (living with HIV; diagnosed with HIV; on ART; undetectable viral load).
Sixteen countries reported data on these four elements. Figure 3 lists the 16 countries and presents cumulative figures for them by each of the four elements. In the 16 countries, 76% of people living with HIV had been diagnosed, 78% of those diagnosed were on treatment, and 88% of those on treatment were virally suppressed. Of all people living with HIV, 53% were virally suppressed. Figure 3 shows levels of continuity of care in non‐EU/EEA countries to be lower than those in EU/EEA countries.
Figure 3.
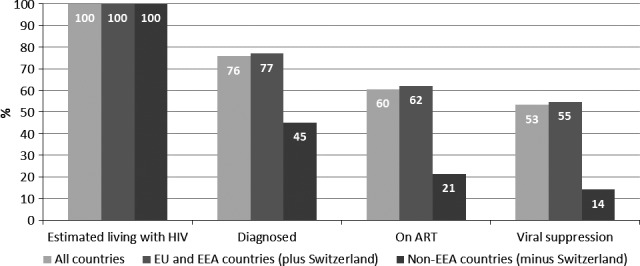
Continuum of care in 16 countries of Europe and Central Asia. European Union (EU) and European Economic Area (EEA) countries included: Austria, Bulgaria, Denmark, France, Germany, Luxembourg, the Netherlands, Romania, Spain, Sweden and the United Kingdom; non‐EEA countries included: Armenia, Azerbaijan, Georgia and Serbia. ART, antiretroviral therapy.
Discussion
The HIV continuum of care may be applied as a public health tool to measure the effectiveness of health systems to monitor the percentage of those diagnosed among people living with HIV and to ensure those diagnosed are on treatment for their own personal benefit and the public health benefit of reducing onward transmission. In this paper, we present evidence of how the different elements of the HIV continuum of care are defined and measured in Europe and Central Asia.
We present both a snapshot of care provision and a baseline against which changes over time in care provision across Europe and Central Asia may be measured. We also present data according to different frameworks for analysing and interpreting the continuum of care.
Our analysis clearly identifies variation in the methods applied to describe and measure elements, and in the availability of data. Most countries base their figures on population‐based data, with a few using cohort studies. The accuracy of population‐based data will vary given that they assimilate data from different sources which may represent different collection methods and different time periods. Cohort studies avoid some of the limitations of population‐based data but often fail to provide accurate national data/estimates on the number diagnosed as, in most cases, it is not known how representative the data are for the country as a whole. Combining cohort‐based and population‐based approaches may allow triangulation of data from different sources.
We report that countries were more likely to have data on the number of people diagnosed with HIV infection and the number of people on ART than for other continuum elements. Consequently, the ECDC recently developed a tool for countries to use to estimate the number of people living with HIV 25. In terms of standardization and data availability, the most problematic elements were linked to, and retained in, care. It may be difficult to obtain a Europe‐wide definition of these elements given the wide variations in health systems.
It should prove possible to introduce standard definitions for the remaining four elements. Based on the responses to our questionnaire, we recommend that the number of people diagnosed be defined as the cumulative number of people diagnosed with HIV infection less those known to have died, and, where possible, the number known to have left the country. The number on treatment should be defined as those known to be on treatment at the end of the calendar year (potentially assessed based on date last seen for care), and 200 copies/mL should be used as the standard threshold for viral suppression.
Variability in data availability, quality, sources and measurement makes it difficult to compare results across countries and between adjacent elements and ensures that there are limitations to our analyses. Missing data demand that caution should be exercised when interpreting our results. For example, it is likely that, as a result of missing data on one or more elements, the true number of breakpoints in the continuum will be higher than we report. All figures were self‐reported by countries and, although some validation of data was conducted, figures have not been fully, independently verified and are dependent on the reliability of country systems.
As definitions, data sources and health systems differ across countries, caution is required when comparing data between countries and in aggregating data across countries. Even where similar methods have been applied across countries to measure an element, the accuracy of the outputs may vary. For example, we show that many countries rely on the UNAIDS Spectrum of people living with HIV to report on this first element. It is highly likely that the accuracy of these estimates will vary between countries depending on the quality of data sources utilized and national HIV prevalence. Countries reporting to the ECDC have aired concern about the relevance of UNAIDS estimates in countries with concentrated and low‐level HIV epidemics.
In assessing whether the various frameworks assist the analysis and interpretation of multiple country continuum data, we also came across limitations. In relation to the concept of breakpoints in the continuum, we applied a figure of > 19% as reported by Raymond and colleagues18. This figure is somewhat arbitrary. Also somewhat arbitrary is the quadrant threshold of 60% of people living with HIV being diagnosed and ≥ 60% of those diagnosed being on treatment. We recommend further analysis where different breakpoints are applied between elements and where a higher threshold is applied across the quadrants.
Regarding the strengths of the different frameworks, we believe both the breakpoint and quadrant frameworks provide a useful approach to identifying where problems occur and where resources should be focused to improve a country's response to its HIV epidemic. Across countries, the most important breakpoint relates to ensuring that people living with HIV are diagnosed. To maximize the benefits of earlier detection (by improving a person's health and life expectancy through treatment) and to minimize the risk of onward transmission (through ART and behavioural change), it is essential that the percentage of people living with undiagnosed HIV who are diagnosed is increased in all countries. In relation to countries in Europe and Central Asia, we believe that the quadrant framework highlights the most important elements of the continuum, namely diagnosis and treatment, and presents a clear and concise visual way of comparing and contrasting country data.
Based on our analysis, we also recommend the use of two additional frameworks for analysing continuum data. The UNAIDS 90‐90‐90 targets provide a useful framework for analysing a country's continuum of care and in providing objectives to which countries can aspire. We present evidence for a number of countries meeting one or more of these targets.
Having highlighted little consensus across countries in their approach to defining linkage to, and retention in, care, we finally recommend the use of a four‐element continuum (living with HIV; diagnosed with HIV; on ART; undetectable viral load). This framework focuses on those elements for which introducing standard definitions is possible and which link to the UNAIDS 90‐90‐90 targets. As monitoring of quality of care is important, we recommend that separate measures be used to monitor the remaining two quality‐of‐care indicators as secondary outcomes.
Conclusions
To better inform HIV testing and treatment programmes across countries in Europe and Central Asia, standard definitions of the elements in the HIV continuum of care are needed. Many countries of the region have data on the continuum and are able to report against its elements. If countries wish to ensure an unbroken HIV continuum of care, people living with HIV need to be diagnosed promptly, and ART needs to be offered to all those diagnosed regardless of CD4 count in accordance with updated World Health Organization (WHO) guidelines 26. It is essential that countries continue to focus on strengthening all aspects of the continuum of care to achieve higher rates of viral suppression, to improve care quality for those living with HIV, and to decrease HIV transmission risk at the population level.
Disclaimer
Reference to Kosovo is without prejudice to positions on status, and is in line with UNSC 1244 and the ICJ Opinion on the Kosovo Declaration of Independence.
Author Contributions
KA, RD and DH worked as consultants in collecting and analysing Dublin data under the direction and guidance of TN. RD, BR, KR, KA, DH, AP and TN were all involved in developing the questions related to the continuum of care for the questionnaire. KR, BR and VD were all involved in submitting country data for the process. An initial draft report was prepared by RD, CV and TN. This was reviewed and revised by BR, VD, KR, AA, AP, CV and LT. The final version was prepared by RD, CV, BR and TN. All authors reviewed and approved the final draft.
Acknowledgements
ECDC would like to acknowledge the support and guidance provided by members of the Dublin Declaration advisory group. Members of the advisory group include Jasmina Pavlic (Croatia), Kristi Rüütel (Estonia), Ines Perea, Gesa Kupfer (Germany), Vasileia Konte (Greece), Derval Igoe (Ireland), Lella Cosmaro (Italy), Silke David (Netherlands), Arild Johan Myrberg (Norway), Mioara Pedrescu (Romania), Sladjana Baros (Serbia), Danica Stanekova (Slovakia), Olivia Castillo (Spain), Maria Axelsson (Sweden), Luciano Ruggia (Switzerland), Olga Varetska (Ukraine), Brian Rice (UK), Anna Zakowicz, Raminta Stuikyte (EU Civil Society Forum), Matthias Schuppe (European Commission), Klaudia Palczak, Dagmar Hedrich (EMCDDA), Taavi Erkkola (UNAIDS) and Annemarie Steengard (WHO Regional Office for Europe). ECDC would also like to thank the following country focal points for providing data on the HIV continuum of care through the Dublin Declaration questionnaire in March 2014: Roland Bani (Albania), Montse Gessé (Andorra), Samvel Grigoryan (Armenia), Jean‐Paul Klein (Austria), Esmira Almammadova (Azerbaijan), Inna Karabakh (Belarus), Andre Sasse, Dominique Van Beckhoven (Belgium), Šerifa Godinjak (Bosnia and Herzegovina), Tonka Varleva (Bulgaria), Jasmina Pavlic (Croatia), Ioannis Demetriades (Cyprus), Veronika Šikolová (Czech Republic), Jan Fouchard (Denmark), Kristi Rüütel, Liilia Lõhmus, Anna‐Liisa Pääsukene (Estonia), Henrikki Brummer‐Korvenkontio (Finland), Bernard Faliu (France), Tamar Kikvidze (Georgia), Gesa Kupfer, Ulrich Marcus, Christine Hoepfner, Ursula von Rueden (Germany), Vasileia Konte, Chryssoula Botsi, Jenny Kremastinou, Theodoros Papadimitriou (Greece), Katalin Szalay (Hungary), Guðrún Sigmundsdóttir (Iceland), Derval Igoe (Ireland), Daniel Chemtob (Israel), Maria Grazia Pompa, Anna Caraglia, Barbara Suligoi, Laura Camoni, Stefania D'Amato, Anna Maria Luzi, Anna Colucci, Marco Floridia, Alessandra Cerioli, Lella Cosmaro, Massimo Oldrini, Laura Rancilio, Maria Stagnitta, Michele Breveglieri, Margherita Errico (Italy), Irina Ivanovna Petrenko (Kazakhstan), Laura Shehu, Pashk Buzhala, Bajram Maxhuni (Kosovo*), Dzhainagul Baiyzbekova (Kyrgyzstan), Šarlote Konova (Latvia), Irma Caplinskiene (Lithuania), Pierre Weicherding (Luxembourg), Jackie Maistre Melillo (Malta), Violeta Teutu (Moldova), Aleksandra Marjanovic (Montenegro), Silke David (Netherlands), Nina Søimer Andresen, Adélie Dorseuil, Arild Johan Myrberg (Norway), Iwona Wawer, Piotr Wysocki, Beata Zawada (Poland), Antonio Diniz and Teresa de Melo (Portugal), Mariana Mardarescu (Romania), Danijela Simic, Sladjana Baros (Serbia), Danica Staneková and Peter. Truska (Slovakia), Irena Klavs (Slovenia), Mercedes Díez (Spain), Maria Axelsson (Sweden), Luciano Ruggia (Switzerland), Muratboky Beknazarov (Tajikistan), Nurcan Ersöz (Turkey), Kay Orton and Valerie Delpech (United Kingdom), Igor Kuzin (Ukraine) and Zulfiya Abdurakhimova (Uzbekistan). ECDC would like to thank the operational contact points for HIV surveillance from EU/EEA Member States and the national HIV/AIDS surveillance focal points from other countries of the WHO European Region for making available HIV/AIDS surveillance data. ECDC would like to thank EMCDDA and UNAIDS for harmonizing their monitoring systems with ECDC and for making available country‐reported data for the purposes of monitoring the Dublin Declaration. ECDC would also like to thank the WHO Regional Office for Europe for jointly coordinating HIV surveillance in the WHO European Region.
Conflicts of interest: The authors have no conflicts of interest to report.
Financial disclosure: Funding for this work was provided by ECDC.
References
- 1. Cohen SM, Van Handel MM, Branson BM et al Vital Signs: HIV prevention through care and treatment — United States. MMWR 2011; 60(47): 1618–1623. [PubMed] [Google Scholar]
- 2. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test‐and‐treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52(6): 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall HI, Frazier EL, Rhodes P et al Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173 (14): 1337–1344. [DOI] [PubMed] [Google Scholar]
- 4. Thaker HK, Snow MH. HIV viral suppression in the era of antiretroviral therapy. Postgrad Med J 2003; 79(927): 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO . Global update on HIV treatment 2013: results, impact and opportunities [Internet]. 2013. [cited 2016 Aug 4]. Available from: http://www.unaids.org/sites/default/files/media_asset/20130630_treatment_report_en_0.pdf
- 6. Salminen M, Pharris A, Van De Laar M, Smith C. Evaluating HIV treatment as prevention in the European context [Internet]. 2012. [cited 2016 Aug 29]. Available from: http://ecdc.europa.eu/en/publications/publications/hiv-treatment-as-prevention.pdf
- 7. Loutfy MR, Wu W, Letchumanan M et al Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV‐positive partner is fully suppressed on antiretroviral therapy. PLoS ONE 2013;8(2):e55747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carter M. High probability that HIV viral load remains suppressed between tests: implications for infectiousness debate [Internet]. aidsmap. 2009. Available from: http://www.aidsmap.com/High-probability-that-HIV-viral-load-remains-suppressed-between-tests-implications-for-infectiousness-debate/page/1435712/
- 9. Cohen MS, Chen YQ, McCauley M et al Prevention of HIV‐1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Beckhoven D, Lacor P, Moutschen M et al Factors associated with the continuum of care of HIV‐infected patients in Belgium. J Int AIDS Soc. 2014;4(Suppl 3):19534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helleberg M, Häggblom A, Sönnerborg A et al HIV Care in the Swedish‐Danish HIV Cohort 1995‐2010, Closing the Gaps. Vermund SH, editor.PLoS ONE [Internet]. Public Library of Science;2013;8(8):e72257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Supervie V, Marty L, Lacombe J‐M, Dray‐Spira R, Costagliola D, FHDH‐ANRS CO4 study group . Looking beyond the cascade of HIV care to end the AIDS epidemic: estimation of the time interval from HIV infection to viral suppression. J Acquir Immune Defic Syndr 2016;73(3):348–355. [DOI] [PubMed] [Google Scholar]
- 13. Tuite H, Horgan M, Mallon S et al Antiretroviral Treatment and Viral Load Responses in HIV‐infected Patients Accessing Specialist Care in Ireland. In: 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) [Internet]. London; 2012. Available at: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=145623&XNSPRACHE_ID=2&X
- 14. Van Sighem A, Gras L, Smit C et al Monitoring Report 2014 Human Immunodeficiency Virus (HIV) Infection in the Netherlands [Internet]. 2014 [cited 2016 Aug 29]. Available from: http://www.hiv-monitoring.nl/files/8914/1527/1076/SHM_Monitoring_report_2014.pdf
- 15. Pokrovskaya A, Popova A, Ladnaya N, Yurin O. The cascade of HIV care in Russia, 2011–2013. In: Journal of the International AIDS Society [Internet]. 2014 [cited 2016 Aug 29]. Available at http://www.jiasociety.org/index.php/jias/article/view/19506 [DOI] [PMC free article] [PubMed]
- 16. Diaz A, Sobrino P, Del Amo J, Moreno S, Diez M. La Cascada del Tratamiento en España: Primeras Estimaciones. In: VI Congreso Nacional de GESIDA [Internet]. 2014. p. P – 081. Available from: http://www.gesida-seimc.org/contenidos/congresos/anteriores/2014/gesida2014-VIcongresocomunicaciones.pdf
- 17. Delpech V. Metrics of Success: Avoiding the “Cascadista”/Micro Indicator Approach. In: Plenary presentation at TasP PrEP Evidence Summit [Internet]. London; 2013. Available from: http://www.iapac.org/tasp_prep/presentations/TPSlon13_Plenary11_Delpech.pdf
- 18. Raymond A, Hill A, Pozniak A. Large disparities in HIV treatment cascades between eight European and high‐income countries – analysis of break points. In: Journal of the International AIDS Society [Internet]. 2014 [cited 2016 Aug 29]. Available from: http://www.jiasociety.org/index.php/jias/article/view/19507 [DOI] [PMC free article] [PubMed]
- 19. UNAIDS . 90‐90‐90 An ambitious treatment target to help end the AIDS epidemic [Internet]. Geneva; 2014 [cited 2016 Aug 29]. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf
- 20. Kelly SL, Wilson DP. HIV cascade monitoring and simple modeling reveal potential for reductions in HIV incidence. J Acquir Immune Defic Syndr 2015;69(3):257–63. [DOI] [PubMed] [Google Scholar]
- 21. Del Rio C. Cascade of Care and its relevance to Seek, Test, Treat and Retain Strategy. In: CFAR [Internet]. Emory Center for AIDS Research; [cited 2016 Aug 29]. Available from: http://www.apa.org/about/gr/issues/substance-abuse/del-rio-nida.pdf
- 22. Wilton J, Broeckaert L. The HIV treatment cascade – patching the leaks to improve HIV prevention [Internet]. CATIE. 2013. Available at: http://www.catie.ca/en/pif/spring-2013/hiv-treatment-cascade-patching-leaks-improve-hiv-prevention
- 23. Dublin Declaration on Partnership to fight HIV/AIDS in Europe and Central Asia [Internet]. Breaking the Barriers – Partnership to fight HIV/AIDS in Europe and Central Asia; 2004. Available at: http://www.unicef.org/ceecis/The_Dublin_Declaration.pdf
- 24. Control EC for DP and. From Dublin to Rome: ten years of responding to HIV in Europe and Central Asia. Stockholm: ECDC; 2014.
- 25. ECDC . HIV modelling tool [Internet]. 2016. Available at: http://ecdc.europa.eu/en/healthtopics/aids/Pages/hiv-modelling-tool.aspx
- 26. WHO . WHO Guideline on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV [Internet]. WHO. Geneva: World Health Organization; 2015 [cited 2016 Aug 29]. Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Of the 55 countries contacted, 40 (73%) provided quantitative data for at least one element of the HIV continuum. Of the 32 EU/EEA (plus Switzerland) countries contacted, 28 (88%) provided quantitative data for at least one element, and 12 (52%) of the 23 non‐EU/EEA countries contacted provided data. Thirty‐two (58%) of all countries contacted provided data for at least four elements and 13 (24%) provided data for all six elements. The 13 countries were Armenia, Austria, Azerbaijan, Bulgaria, Denmark, Georgia, Luxembourg, the Netherlands, Romania, Serbia, Spain, Switzerland and the United Kingdom.
Among the 40 countries providing quantitative data, the elements most frequently reported were the number of people diagnosed (37 countries; 93%) and the number of people on ART (35 countries; 88%) (see Fig. 1). Of these 40 countries, 31 (77%) based their response on cumulative population‐based data, six (15%) on annual population‐based data, and three (8%) on cohort data.
Figure 1.

Percentage of European and Central Asian countries reporting quantitative data for different elements of the HIV continuum of care (n = 40). ART, antiretroviral therapy; EEA, European Economic Area; EU, European Union.


