Abstract
Aims
To determine the impact of race and ethnicity on the efficacy, body weight and hypoglycaemia incidence with vildagliptin treatment in patients with type 2 diabetes mellitus using patient‐level data from the vildagliptin clinical trial programme.
Methods
Data from 22 randomized, placebo‐controlled global and local (Japan, China) registration studies of vildagliptin (50 mg once‐daily or twice‐daily) of ≥12‐week duration were analysed by race (Caucasian [n = 2764] and Asian [n = 2232]) and by ethnicity (Japanese, Chinese, and Indian). The placebo‐subtracted differences in the change in glycated haemoglobin (HbA1c) and body weight from baseline to week 12 or week 24 were evaluated by race or ethnicity using repeated measure analysis of unstructured covariance. Hypoglycaemia incidences were summarized using descriptive statistics.
Results
The HbA1c reduction from baseline with vildagliptin was similar across the racial/ethnic subgroups (−0.83% ± 0.02% to −1.01% ± 0.05%). Placebo‐corrected HbA1c reduction was similar between Caucasian (−0.68% ± 0.03%) and Asian (−0.80% ± 0.03%) patients ( P value for interaction = .56); analysis by race and ethnicity showed better efficacy ( P < .02) in Japanese patients. Japanese patients were drug‐naïve and treated with a single oral anti‐diabetes drug only; they showed no response to placebo. Weight neutrality of vildagliptin was demonstrated in all groups (0.47 ± 0.11 kg to −0.29 ± 0.08 kg). Hypoglycaemic events (≥1) were infrequent in all ethnic subgroups.
Conclusions
The glycaemic efficacy of vildagliptin was similar in Caucasian and Asian patients. The slightly better efficacy observed in Japanese patients was driven by the absence of placebo effect and might be explained by their earlier stage of diabetes compared to other subgroups.
Keywords: Asia, Caucasian, China, DPP‐4 inhibitor, ethnicity, India, Japan, race
1. INTRODUCTION
The global epidemic of type 2 diabetes mellitus (T2DM) is growing at an alarming rate. The prevalence is estimated to further increase in the coming decades, with the biggest rise expected in developing countries.1 International guidelines advocate effective and safe treatment of diabetes, tailored to the individual patient profile, to achieve and maintain adequate glycaemic control without risk of hypoglycaemia or weight gain.2
It has been suggested that the pathophysiology of T2DM may vary between races in terms of differences in defects in insulin secretion and insulin resistance,3, 4, 5, 6, 7 and thus may have a differential impact on the response to treatment. Furthermore, the glycaemic response to anti‐diabetes drugs may vary among ethnic cohorts because of lifestyle as well as circumstantial factors,8 as does the risk of hypoglycaemia.9 Therefore, understanding the impact of race and ethnicity on the efficacy and safety of anti‐diabetes drugs in patients with T2DM is of both scientific and practical interest.10, 11, 12, 13
Recent meta‐analyses of studies with dipeptidyl peptidase‐4 inhibitors (DPP‐4 inhibitors) and glucagon‐like peptide‐1 (GLP‐1) receptor agonists14, 15, 16, 17 that evaluated the difference in glycaemic control between Caucasians and Asians suggested a slightly better efficacy in Asian patients with T2DM than in Caucasian patients. However, these meta‐analyses did not differentiate among patients from the Asian regions (except for Japan) and included a mixed population. Moreover, these publications did not report the potential differences in hypoglycaemia incidence, a common adverse effect of treatment intensification.
Vildagliptin, a DPP‐4 inhibitor, has been extensively studied globally as well as in trials conducted in patients with T2DM from India, China and Japan, and has demonstrated improved glycaemic control with a low incidence of hypoglycaemia and weight neutrality.18 The primary objective of the current analysis was to assess the impact of race and ethnicity on the glucose‐lowering efficacy of vildagliptin and, additionally, to assess the effects on body weight and incidence of hypoglycaemia in different racial/ethnic subgroups.
2. MATERIALS AND METHODS
2.1. Study design
This was a post‐hoc exploratory analysis including patient‐level data from 22 randomized, placebo‐controlled monotherapy or combination therapy (add‐on to oral anti‐diabetes drugs [OADs] or insulin) trials with vildagliptin, carried out between 2005 and 2013. Clinical trials with a duration of at least 12 weeks comparing vildagliptin (50 mg once daily [qd] or 50 mg twice daily [bid]) with placebo were pooled (Tables S1 and S2).19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Studies using active comparators were not included, because such studies were not performed in Japan, and only one active‐controlled study was performed in China (acarbose) while different comparators were used in global studies (sulphonylurea [SU], thiazolidinediones or metformin).
2.2. Statistical analysis
The patient‐level data were presented by race (Caucasian and Asian groups) and by ethnicity (Chinese, Japanese, and Indian subgroups) within the Asian population. Patient demographics and baseline characteristics were summarized using descriptive statistics. A repeated measure analysis of unstructured covariance model was used to evaluate the primary endpoint, the effect of race or ethnicity on the placebo‐subtracted differences in the change in glycated haemoglobin (HbA1c; Japanese Diabetes Society [JDS] HbA1c values were corrected to those of the National Glycohemoglobin Standardization Program [NGSP]) from baseline to week 12. A linear model was used, with age (years), gender, racial/ethnic subgroup, diabetes duration (years), baseline HbA1c, baseline fasting plasma glucose (FPG), background therapy at baseline (drug naïve, OADs alone or in combination, insulin alone or with OADs), study treatment, visit, study, treatment by visit interaction, treatment by racial/ethnic subgroup interaction and treatment by racial/ethnic group by visit interaction as fixed effects and patient as a random effect nested within treatment. A 12‐week cut‐off was used for the primary analysis (efficacy), irrespective of the individual study duration, to allow a uniform comparison across racial and ethnic subgroups. This timepoint was selected because of the duration of the Japanese studies (12 weeks, in line with local regulatory requirements). The same model used for the primary endpoint (without adjustment for background therapy at baseline) was used for analysis of the secondary endpoint, change in body weight at week 12 and 24. The incidence of hypoglycaemia (number of patients with hypoglycaemia, severe hypoglycaemia and discontinuations because of hypoglycaemia) during 12 and 24 weeks (except for Japanese studies) of treatment from the safety population was summarized using descriptive statistics. The power for race/ethnicity by treatment (vildagliptin 50 mg bid/qd and placebo) interaction at week 12 was calculated using two‐way analysis of variance.
2.3. Ethics and good clinical practice
All studies contributing to this pooled analysis were conducted in accordance with the International Conference on Harmonisation‐Good Clinical Practice (ICH‐GCP) guidelines and the principles of the Declaration of Helsinki. All study protocols were approved by the relevant independent ethics committee/institutional review board. All patients from each study provided written informed consent.
3. RESULTS
3.1. Patient population
The study population included 2764 Caucasian and 2232 Asian patients with T2DM. The Japanese and Chinese studies included 645 and 1005 patients, respectively; 436 patients from India participated in the global studies. Asians were younger (56.7 ± 9.9 years), had a shorter duration of T2DM (7.7 ± 6.4 years) and a lower body mass index (BMI, 25.6 ± 3.4 kg/m2) than Caucasians (Table 1). Patients from the Indian subgroup were youngest (53.1 ± 10.2 years) but had the highest HbA1c (8.7% ± 1.0%) and lowest FPG (8.9 ± 2.5 mmol/L) (Table 1). The Japanese subgroup had few female patients (32.4%) and the lowest baseline HbA1c (8.1% ± 0.8%). In this subgroup, patients were drug‐naïve or used 1 OAD prior to initiating vildagliptin, while more than 80% of patients in the Indian subgroup were on insulin or SU background therapy (Figure 1).
Table 1.
Baseline patient demographics and background characteristics by race and ethnicity (randomized set)
| Caucasians | Asians | Japanese 1 | Chinese | Indian | |
|---|---|---|---|---|---|
| (n = 2764) | (n = 2232) | (n = 645) | (n = 1005) | (n = 436) | |
| Age, years | 61.0 ± 11.2 | 56.7 ± 9.9 | 59.3 ± 9.1 | 56.7 ± 9.7 | 53.1 ± 10.2 |
| Women | 1253 (45.3) | 987 (44.2) | 209 (32.4) | 515 (51.2) | 185 (42.4) |
| BMI, kg/m2 | 31.9 ± 5.2 | 25.6 ± 3.4 | 24.7 ± 3.1 | 25.5 ± 3.1 | 26.5 ± 3.6 |
| HbA1c, % | 8.3 ± 0.9 | 8.4 ± 0.9 | 8.1 ± 0.8 | 8.5 ± 0.9 | 8.7 ± 1.0 |
| FPG, mmol/L | 9.9 ± 2.7 | 9.2 ± 2.2 | 9.0 ± 1.9 | 9.5 ± 2.3 | 8.9 ± 2.5 |
| Duration of T2DM, years | 8.3 ± 7.8 | 7.7 ± 6.4 | 7.2 ± 6.0 | 7.7 ± 6.1 | 7.5 ± 7.2 |
Values are expressed as mean ± SD or n (%).
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SD, standard deviation; T2DM, type 2 diabetes mellitus.
All Japanese studies were of 12‐weeks duration.
Figure 1.
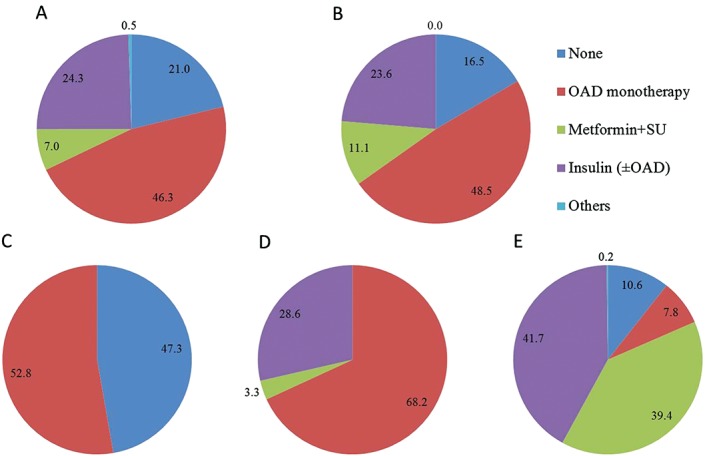
Proportion of patients (randomized set) receiving various background anti‐diabetes therapies by racial/ethnic subgroups: A, Caucasians (n=2764); B, Asians (n=2232); C, Japanese (n=645); D, Chinese (n=1005); and E, Indian (n=436). Abbreviations: OAD, oral anti‐diabetes drug; SU, sulphonylurea.
3.2. Glycaemic control
The change in HbA1c from baseline to week 12 with vildagliptin treatment was similar across the groups, ranging between −0.83% ± 0.02% and −1.01% ± 0.05%. However, the placebo responses differed; the Japanese subgroup had the smallest response to placebo (+0.05%) while the other groups showed a small (−0.14% in Caucasians) to moderate (−0.27% in Chinese) improvement with placebo. Consequently, the greatest placebo‐subtracted improvement in HbA1c was observed in the Japanese subgroup (−1.04% ± 0.06%) while the smallest treatment effect was observed in the Chinese and Caucasian groups (−0.66% ± 0.05% and −0.68% ± 0.03%, respectively) (Table 2).
Table 2.
Adjusted mean change in HbA1c (%) from baseline to 12 weeks with vildagliptin 50 mg qd/bid and placebo by racial/ethnic subgroups
| Race/ethnicity | Mean baseline HbA1c | Adjusted mean change from baseline (SE) | Difference in adjusted mean change (Vildagliptin − Placebo) | |||
|---|---|---|---|---|---|---|
| Vildagliptin; placebo, n | Vildagliptin/placebo | Vildagliptin 50 mg qd/bid | Placebo | Mean (SE) | 95% CI | |
| Caucasians | 1340; 939 | 8.3/8.2 | −0.83 (0.02) | −0.14 (0.02) | −0.68 (0.03) 1 | −0.74, −0.63 |
| Asians | 1123; 894 | 8.4/8.5 | −0.96 (0.02) | −0.16 (0.02) | −0.80 (0.03) 1 | −0.86, −0.74 |
| Japanese | 339; 285 | 8.1/8.1 | −0.99 (0.04) | 0.05 (0.04) | −1.04 (0.06) 1 | −1.15, −0.93 |
| Chinese | 529; 387 | 8.4/8.5 | −0.94 (0.03) | −0.27 (0.03) | −0.66 (0.05) 1 | −0.75, −0.57 |
| Indian | 187; 164 | 8.6/8.8 | −1.01 (0.05) | −0.19 (0.05) | −0.82 (0.07) 1 | −0.97, −0.68 |
All values are expressed as % units unless specified.
Abbreviations: bid, twice daily; CI, confidence interval; HbA1c, glycated haemoglobin; qd, once daily; SE, standard error.
P < .0001 compared to placebo. P‐value for interaction term = .019.
When only race (Caucasian and Asian) was included in the analytical model for evaluating the efficacy (placebo‐subtracted differences in the “change in HbA1c from baseline”), no significant race by treatment interaction was observed (P = .56). However, when the Japanese subgroup was excluded from the Asian pool and included in the model as a separate group, a significant association with efficacy emerged (race/ethnicity—efficacy interaction P = .017 for Caucasian, Asian [without the Japanese subgroup] and Japanese). This finding was maintained when the Caucasian, Chinese, Indian, and Japanese subgroups were analysed individually, confirming a significant association of ethnicity with efficacy (interaction P = .019 for all) driven by the Japanese subgroup. The power for race/ethnicity by treatment interaction at week 12 was 0.987, confirming the above associations.
3.3. Body weight
The mean changes from baseline in body weight at weeks 12 and 24 in the vildagliptin‐treated patients were comparable, with no significant increase in body weight across different subgroups (week 12: Caucasians, 0.15 ± 0.06 kg; Asians, 0.03 ± 0.06 kg; Japanese, 0.47 ± 0.11 kg) (Table 3).
Table 3.
Adjusted mean (SE) change from baseline in body weight (kg) at weeks 12 and 24 with vildagliptin 50 mg qd/bid and placebo by racial/ethnic subgroups
| Race/ethnicity | Adjusted mean change from baseline, Week 12 | Adjusted mean change from baseline, Week 24 | ||||||
|---|---|---|---|---|---|---|---|---|
| Vildagliptin | Vildagliptin | Placebo | Placebo | Vildagliptin | Vildagliptin | Placebo | Placebo | |
| n | 50 mg qd/bid | n | n | 50 mg qd/bid | n | |||
| Caucasians | 1317 | 0.15 (0.06) | 890 | −0.32 (0.06) | 1280 | 0.28 (0.07) | 842 | −0.34 (0.08) |
| Asians | 1097 | 0.03 (0.06) | 879 | −0.36 (0.07) | 786 | 0.04 (0.09) | 603 | −0.44 (0.10) |
| Japanese | 274 | 0.47 (0.11) | 222 | −0.33 (0.12) | N/A 1 | |||
| Chinese | 543 | −0.29 (0.08) | 411 | −0.44 (0.10) | 519 | −0.13 (0.11) | 375 | −0.55 (0.12) |
| Indian | 210 | 0.01 (0.14) | 185 | −0.26 (0.15) | 202 | 0.21 (0.17) | 170 | −0.27 (0.19) |
All values are expressed as mean (SE) unless specified. P‐value for interaction term at week 12 = 0.6245, and at week 24 = 0.3550.
Abbreviations: bid, twice daily; qd, once daily; N/A, not available; SE, standard error.
All Japanese studies were of 12‐weeks duration.
3.4. Hypoglycaemic events
Overall, hypoglycaemic events were infrequent in all the ethnic subgroups, and did not accumulate over time. The lowest incidence of hypoglycaemia at week 12 was reported in the Japanese subgroup (0.9%). While the addition of vildagliptin significantly reduced overall glycaemia, the incidence of hypoglycaemia between vildagliptin‐ and placebo‐treated patients was overall similar in all subgroups (Table 4). The highest overall incidence of hypoglycaemia at week 24 was reported in the Indian subgroup (7.1% of patients with ≥ 1 event, with a similar incidence in both vildagliptin and placebo groups). During the 24‐week study period, severe hypoglycaemic events were rare; 6 Caucasian patients (3 on vildagliptin, 3 on placebo) and 2 Indian patients (placebo‐treated, on insulin background therapy) reported severe hypoglycaemic events.
Table 4.
Hypoglycaemia incidences from baseline to 12 weeks and 24 weeks with vildagliptin 50 mg qd/bid and placebo by racial/ethnic subgroups
| Hypoglycaemic events (%) (number of patients with ≥ 1 event) 1 | Caucasians | Asians | Japanese | Chinese | Indian |
|---|---|---|---|---|---|
| Overall up to 12 weeks | 133/2754 (4.8) | 56/2230 (2.5) | 6/645 (0.9) | 17/1004 (1.7) | 26/435 (6.0) |
| Vildagliptin | 72/1614 (4.5) | 33/1228 (2.7) | 4/349 (1.1) | 8/568 (1.4) | 16/230 (7.0) |
| Placebo | 61/1140 (5.4) | 23/1002 (2.3) | 2/296 (0.7) | 9/436 (2.1) | 10/205 (4.9) |
| Overall up to 24 weeks | 162/2754 (5.9) | 59/2230 (2.6) | 19/1004 (1.9) | 31/435 (7.1) | |
| Vildagliptin | 87/1614 (5.4) | 35/1228 (2.9) | (N/A) 2 | 10/568 (1.8) | 18/230 (7.8) |
| Placebo | 75/1140 (6.6) | 24/1002 (2.4) | 9/436 (2.1) | 13/205 (6.3) |
Data are expressed as n (%).
Abbreviations: bid, twice daily; N/A, not available; qd, once daily.
Safety population set.
All Japanese studies were of 12‐weeks duration.
4. DISCUSSION
To our knowledge, this is the first study with incretin‐based therapies using patient‐level data from a large pool of randomized controlled trials to explore the efficacy and safety of a DPP‐4 inhibitor by race and ethnicity. We demonstrated that the efficacy of vildagliptin was overall similar in Asian and Caucasian patients. Analysis by ethnicity showed a slightly better efficacy in Japanese patients compared with the other Asian subgroups or Caucasians, primarily because of the absence of response to placebo in the Japanese studies. The study found no relevant differences in HbA1c reductions from baseline across the racial and ethnic subgroups, a finding consistent with an analysis of efficacy in drug‐naïve Caucasian and Japanese patients.41 One could argue that the observed similar reduction in HbA1c from baseline with vildagliptin across the racial/ethnic subgroups suggests better efficacy in Japanese patients, given that this subgroup had the lowest baseline HbA1c and, therefore, a smaller HbA1c reduction would be expected. However, there are differences between the Japanese and the other populations which may, to some extent, contribute to this observed difference in efficacy. The usage pattern of background anti‐diabetes therapy in the different subgroups, spanning from only drug‐naïve/single OAD treatment in the Japanese patients to dual OAD or insulin treatment in the Indian patients, suggests that patients may have been at different stages of disease, with more preserved β‐cell function in the Japanese subgroup than in the others. This might be supported by the fact that patients from India are diagnosed with T2DM substantially late in the natural course of the disease,42, 43 when β‐cell dysfunction has already set in.
The better placebo‐corrected efficacy in Japanese patients was driven by an absence of response to placebo, while there was a variable degree of response to placebo in Caucasian, Chinese and Indian subgroups. This might be attributed to the better adherence to dietary and treatment recommendations on the part of Japanese patients. A study assessing food‐intake patterns in Japanese patients with T2DM suggested that the diet composition of these patients was closer to dietary recommendations for T2DM than the typical diet of Western patients with diabetes.44 Similarly, better adherence to pharmacotherapy before and during clinical studies45 and a more stringent general follow‐up of patients with diabetes in Japan was reported.46 Another possible explanation for the better efficacy of vildagliptin in Japanese patients, that is, because of increased exposure to vildagliptin or better pharmacodynamics, is considered unlikely as the pharmacokinetic/pharmacodynamic studies with vildagliptin in Caucasian, Chinese and Japanese subjects showed similar results across the groups.47, 48, 49
Differences in genetics and pathophysiology of T2DM in Japanese and Caucasian patients could also play a role. Genetic studies revealed genes for susceptibility to T2DM and specific to the Japanese population.50 Such genetic variations between Japanese and Caucasian populations could be the root cause of differences in insulin sensitivity and β‐cell secretion, with a potential impact on treatment response. However, a recent study in native Japanese and Caucasian patients on the pathophysiology of glucose intolerance revealed that disposition indices, characterizing the interplay between insulin resistance and insulin secretion, were not different in Japanese and Caucasian patients with T2DM, suggesting a similar pathophysiology of the disease.4 Therefore, genetics and potentially associated differences in the pathophysiology of T2DM between Caucasians and Asians are less likely to explain the observed difference in the efficacy of vildagliptin between these populations.
The race and ethnicity of the study population were not associated with the placebo‐subtracted differences in change in body weight from baseline. While treatment with vildagliptin showed weight neutrality in all populations, the Asian population in our analysis had a lower baseline BMI vs the Caucasian population, as expected. A meta‐analysis14 comparing the HbA1c‐lowering efficacy of a DPP‐4 inhibitor in Asian and Caucasian populations revealed no correlation between BMI and HbA1c‐lowering with the DPP‐4 inhibitor in studies investigating a population with an average BMI ≥ 30 kg/m2; however, it revealed a significant correlation between BMI and HbA1c‐lowering with a DPP‐4 inhibitor in studies investigating a population with an average BMI < 30 kg/m2. In our analysis, the baseline BMI in the Japanese group was close to the BMI of the Indian and Chinese groups; thus, it is unlikely that lower BMI is driving the slightly higher efficacy of vildagliptin in the Japanese patients.
The slight variations in the incidence of hypoglycaemia with vildagliptin across the ethnic subgroups could be explained by differences in background anti‐diabetes medication; more than 80% of patients in the Indian subgroup were at a more advanced stage of T2DM, using insulin or SU in combination with metformin before the start of the study, whereas none from the Japanese subgroup was on such treatments. Furthermore, despite similar background treatments, the incidence of hypoglycaemia in the Chinese subgroup was lower than that in the Caucasian group. Cultural patterns in reporting adverse events, such as hypoglycaemia, might be a plausible reason for this difference.51, 52 Importantly, the incidences of hypoglycaemia between vildagliptin‐ and placebo‐treated patients were similar within the different racial/ethnic subgroups, indicating a low risk of hypoglycaemia with vildagliptin treatment.
The present pooled analysis has some limitations. The differences in background anti‐hyperglycaemic therapies among the populations might contribute to a degree of heterogeneity in the assessment of comparable glucose‐lowering efficacy, whereas studies performed in different populations with the same treatment have avoided this potential bias.14, 53 However, inclusion of background therapy as a covariate in the statistical model should have addressed this issue. In spite of this limitation, our findings are in line with the meta‐analysis by Park et al., who found an even greater difference in the efficacy of DPP‐4 inhibitors between Japanese vs non‐Japanese trials.16 Furthermore, β‐cell function was not measured systematically during the vildagliptin clinical trial programme; adherence to diet and exercise was not assessed, nor were data on treatment‐adherence prior to the study collected. Therefore, our assumptions of differences in lifestyle factors and compliance with treatment are not supported by the data but, rather, are based on published literature.
In conclusion, in this patient‐level pooled analysis, vildagliptin demonstrated a robust glucose‐lowering efficacy, weight neutrality and no increased incidence of hypoglycaemia as compared to placebo in patients with T2DM, independent of race. The slightly better glucose‐lowering efficacy observed in Japanese patients may be explained by differences in the patient population, that is, Japanese patients being at an earlier stage of diabetes with more preserved β‐cell function and/or better adherence to dietary and therapeutic recommendations.
Supporting information
Table S1. Vildagliptin studies contributing to the pooled analysis.
Table S2. Number of patients by race/ethnicity subgroup and treatment in each country (randomized set).
ACKNOWLEDGMENTS
The authors acknowledge the patients, investigators and staff at participating sites of all the studies. The authors also thank Sashi Kiran Goteti and Vaishali Bhosekar, Novartis Healthcare Pvt. Ltd., Hyderabad, India, for editorial assistance and statistical analysis, respectively, for this manuscript.
Conflict of interest
P. K., P. M. P., W. K. and V. L. are employed by and own stocks in Novartis. V. M. has received research or educational grants from MSD, Novartis, Novo Nordisk, Eli Lilly, USV, Lifescan, Johnson & Johnson, Sanofi Aventis, Merck and from several Indian pharmaceutical companies. M. F. is currently employed at Novo Nordisk Brazil. M. O. has received grants from Eli Lilly, Novo Nordisk, Boehringer Ingelheim, MSD, Novartis, Takeda, Kowa and Sanofi; and has received speaker's bureau fees from Eli Lilly, Novo Nordisk, Boehringer Ingelheim, MSD, Novartis, Takeda, Kowa and Sanofi.
Author contributions
All authors participated in the study design, data review and data interpretation. All authors were involved in manuscript outline and revisions and are responsible for intellectual content. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Kozlovski P, Fonseca M, Viswanathan M, Lukashevich V, Odawara M, Paldánius PM and Kothny W. Effect of race and ethnicity on vildagliptin efficacy: A pooled analysis of phase II and III studies, Diabetes Obes Metab, 2017;19(3):429–435.
Funding information The study was funded by Novartis Pharma AG, Basel, Switzerland. The study sponsor participated in the study design, data collection, data review, data analysis, writing of the report and decision to submit the article for publication.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. http://www.diabetesatlas.org/resources/2015‐atlas.html. Accessed December 2, 2016. [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 3. Møller JB, Dalla Man C, Overgaard RV, et al. Ethnic differences in insulin sensitivity, beta‐cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014;99:4273‐4280. [DOI] [PubMed] [Google Scholar]
- 4. Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37:796‐804. [DOI] [PubMed] [Google Scholar]
- 5. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66(suppl):S37‐S44. [DOI] [PubMed] [Google Scholar]
- 6. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi R. Metabolic syndrome – emerging clusters of the Indian phenotype. J Assoc Physicians India. 2003;51:445‐446. [PubMed] [Google Scholar]
- 8. Nakanishi S, Okubo M, Yoneda M, et al. A comparison between Japanese‐Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed Pharmacother. 2004;58:571‐577. [DOI] [PubMed] [Google Scholar]
- 9. Davidson JA, Lacaya LB, Jiang H, et al. Impact of race/ethnicity on the efficacy and safety of commonly used insulin regimens: a post hoc analysis of clinical trials in type 2 diabetes mellitus. Endocr Pract. 2010;16:818‐828. [DOI] [PubMed] [Google Scholar]
- 10. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129‐2140. [DOI] [PubMed] [Google Scholar]
- 12. Singh AK. Incretin response in Asian type 2 diabetes: are Indians different? Indian J Endocrinol Metab. 2015;19:30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louisa M, Takeuchi M, Takeuchi M, Nafrialdi N, Setiabudy R. Incretin‐based therapies for type 2 diabetes mellitus in Asian patients: analysis of clinical trials. Med J Indones. 2010;19:205‐212. [Google Scholar]
- 14. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia. 2013;56:696‐708. [DOI] [PubMed] [Google Scholar]
- 15. Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab. 2014;16:900‐909. [DOI] [PubMed] [Google Scholar]
- 16. Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: meta‐analysis. Ann Pharmacother. 2012;46:1453‐1469. [DOI] [PubMed] [Google Scholar]
- 17. Wong MC, Wang HH, Kwan MW, et al. Comparative effectiveness of dipeptidyl peptidase‐4 (DPP‐4) inhibitors and human glucagon‐like peptide‐1 (GLP‐1) analogue as add‐on therapies to among diabetes patients in the Asia‐Pacific region: a systematic review. PLoS One. 2014;9:e90963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2014;74:587‐610. [DOI] [PubMed] [Google Scholar]
- 19. Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug‐naïve patients with type 2 diabetes: a 24‐week, double‐blind, randomized, placebo‐controlled, multiple‐dose study. Horm Metab Res. 2007;39:218‐223. [DOI] [PubMed] [Google Scholar]
- 20. Scherbaum WA, Schweizer A, Mari A, et al. Efficacy and tolerability of vildagliptin in drug‐naïve patients with type 2 diabetes and mild hyperglycaemia.*. Diabetes Obes Metab. 2008;10:675‐682. [DOI] [PubMed] [Google Scholar]
- 21. Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model‐assessed beta‐cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab. 2008;93:103‐109. [DOI] [PubMed] [Google Scholar]
- 22. Foley JE, Bunck MC, Möller‐Goede DL, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug‐naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia. 2011;54:1985‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pi‐Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin in drug‐naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132‐138. [DOI] [PubMed] [Google Scholar]
- 24. Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose‐dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233‐240. [DOI] [PubMed] [Google Scholar]
- 25. Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890‐895. [DOI] [PubMed] [Google Scholar]
- 26. Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res. 2009;41:368‐373. [DOI] [PubMed] [Google Scholar]
- 27. Pan C, Xing X, Han P, et al. Efficacy and tolerability of vildagliptin as add‐on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:737‐744. [DOI] [PubMed] [Google Scholar]
- 28. Odawara M, Hamada I, Suzuki M. Efficacy and safety of vildagliptin as add‐on to metformin in Japanese patients with type 2 diabetes mellitus. Diabetes Ther. 2014;5:169‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo‐controlled study. Diabetes Obes Metab. 2007;9:166‐174. [DOI] [PubMed] [Google Scholar]
- 30. Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047‐1056. [DOI] [PubMed] [Google Scholar]
- 31. Kikuchi M, Haneda M, Koya D, et al. Efficacy and tolerability of vildagliptin as an add‐on to glimepiride in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89:216‐223. [DOI] [PubMed] [Google Scholar]
- 32. Yang W, Xing X, Lv X, et al. Vildagliptin added to sulfonylurea improves glycemic control without hypoglycemia and weight gain in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7:174‐181. [DOI] [PubMed] [Google Scholar]
- 33. Lukashevich V, Del Prato S, Araga M, Kothny W. Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea. Diabetes Obes Metab. 2014;16:403‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148‐1155. [DOI] [PubMed] [Google Scholar]
- 35. Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252‐257. [DOI] [PubMed] [Google Scholar]
- 36. Ning G, Wang W, Li L, et al. Vildagliptin as add‐on therapy to insulin improves glycemic control without increasing risk of hypoglycemia in Asian, predominantly Chinese, patients with type 2 diabetes mellitus. J Diabetes. 2016;8:345‐353. [DOI] [PubMed] [Google Scholar]
- 37. Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24‐week randomized placebo‐controlled trial. Diabetes Obes Metab. 2011;13:947‐954. [DOI] [PubMed] [Google Scholar]
- 38. Kothny W, Shao Q, Groop PH, Lukashevich V. One‐year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab. 2012;14:1032‐1039. [DOI] [PubMed] [Google Scholar]
- 39. McMurray JJV, Ponikowski P, Bolli GB et al. The Vildagliptin in Ventricular Dysfunction Diabetes trial (VIVIDD) [late breaking abstract]. Heart Failure Congress May 2013, 25–28; Lisbon, Portugal. Sophia Antipolis: European Society of Cardiology; 2013, p. 99. http://spo.escardio.org/SessionDetails.aspx?eevtid=61&sessId=10923&subSessId=0#.WGNHPY00OM8. Accessed December 2, 2016.
- 40. Krum H, Lukashevich V, Bolli GB, Kozlovski P, Kothny W, Ponikowski P. No significant difference in risk of heart failure hospitalization with vildagliptin in diabetic patients with systolic chronic heart failure: VIVIDD study. Diabetes. 2014;63(suppl 1):A212‐A343. [Google Scholar]
- 41. Foley JE, Bhosekar V, Kawamori R. Does the treatment of type 2 diabetes mellitus with the DPP‐4 inhibitor vildagliptin reduce HbA1c to a greater extent in Japanese patients than in Caucasian patients? Vasc Health Risk Manag. 2016;12:9‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rayappa PH, Raju KN, Kapur A, Bjork S, Sylvest C, Kumar KM. The impact of socio‐economic factors on diabetes care. Int J Diab Dev Countries. 1999;19:8‐16. [Google Scholar]
- 43. Ramachandran A, Snehalatha C, Vijay V, Viswanathan M. Diabetic retinopathy at the time of diagnosis of NIDDM in south Indian subjects. Diabetes Res Clin Pract. 1996;32:111‐114. [DOI] [PubMed] [Google Scholar]
- 44. Horikawa C, Yoshimura Y, Kamada C, et al. Dietary intake in Japanese patients with type 2 diabetes: analysis from Japan diabetes complications study. J Diabetes Invest. 2014;5:176‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Obika M, Trence DL. Comparison of type 2 diabetes care in the United States and Japan. Endocr Pract. 2010;16:707‐711. [DOI] [PubMed] [Google Scholar]
- 46. Mashitani T, Hayashino Y, Okamura S, et al. Patient‐reported adherence to insulin regimen is associated with glycemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 3). Diabetes Res Clin Pract. 2013;100:189‐194. [DOI] [PubMed] [Google Scholar]
- 47. Hu P, Yin Q, Deckert F, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J Clin Pharmacol. 2009;49:39‐49. [DOI] [PubMed] [Google Scholar]
- 48. He YL, Yamaguchi M, Ito H, Terao S, Sekiguchi K. Pharmacokinetics and pharmacodynamics of vildagliptin in Japanese patients with type 2 diabetes. Int J Clin Pharmacol Ther. 2010;48:582‐595. [DOI] [PubMed] [Google Scholar]
- 49. He YL, Ito H, Yamaguchi M, et al. Effects of meal timing relative to dosing on the pharmacokinetics and pharmacodynamics of vildagliptin in Japanese patients with type 2 diabetes. Int J Clin Pharmacol Ther. 2012;50:237‐247. [DOI] [PubMed] [Google Scholar]
- 50. Imamura M, Takahashi A, Yamauchi T, et al. Genome‐wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nat Commun. 2016;7:10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barko R, Corbett CF, Allen CB, Shultz JA. Perceptions of diabetes symptoms and self‐management strategies: a cross‐cultural comparison. J Transcult Nurs. 2011;22:274‐281. [DOI] [PubMed] [Google Scholar]
- 52. Kalra S, Balhara YP, Mithal A. Cross‐cultural variation in symptom perception of hypoglycemia. J Midlife Health. 2013;4:176‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mohan V, Yang W, Son HY, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106‐116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Vildagliptin studies contributing to the pooled analysis.
Table S2. Number of patients by race/ethnicity subgroup and treatment in each country (randomized set).


