Abstract
Distraction osteogenesis (DO) is a surgical procedure used to correct various skeletal disorders. Improving the technique by reducing the healing time would be of clinical relevance. The aim of this study was to determine the angiogenic and regenerative potential of conditioned media (CMs) collected from human dental pulp cells (hDPCs) grown under different culture conditions. CM collected from cells under hypoxia was used to improve bone healing and the DO procedure in vivo. The angiogenic potentials of CMs collected from hDPCs grown under normoxic (−Nor) and hypoxic (−Hyp) conditions were evaluated by quantitative PCR (VEGF‐A, angiopoietin‐1, angiopoietin‐2, interleukin‐6 (IL‐6) and CXCL12), ELISA assays (VEGF‐A, Ang‐2), tube‐formation and wound‐healing assays, using human umbilical vein endothelial cells. The results demonstrated that hypoxic CM had significantly higher angiogenic potential than normoxic CM. Human fetal osteoblasts (hFOBs) were exposed to CM, followed by alizarin red staining, to assess the osteogenic potential. It was found that CM did not enhance the mineralization capacity of hFOBs. DO was performed in the tibiae of 30 mice, followed by a local injection of 20 µl CM (CM–Nor and CM–Hyp groups) or serum‐free DMEM (control group) into the distraction zone every second day. The mice were sacrificed at days 13 and 27. The CM–Hyp treatment revealed a higher X‐ray density than the control group (p < 0.05). Our study suggests that the angiogenic effect promoted by hypoxic culture conditions is dependent on VEGF‐A and Ang‐2 released from hDPCs. Furthermore, CM–Hyp treatment may thus improve the DO procedure, accelerating bone healing. © 2015 The Authors. Journal of Tissue Engineering and Regenerative Medicine published by John Wiley & Sons, Ltd.
Keywords: distraction osteogenesis, conditioned media, regeneration, bone, angiogenesis
1. Introduction
Distraction osteogenesis (DO) is a surgical procedure to correct a variety of skeletal disorders, such as limb length discrepancies, bone defects, limb deformities and for the reunion of fractures in various congenital and post‐traumatic conditions (Watson, 2006). The DO model consists of three phases: the latency phase, after osteotomy and placement of the external distractor; the distraction phase, in which the separated bone ends are gradually and continuously distracted; and the consolidation phase (Ilizarov, 1989). Serial DO treatment allows the formation of new hard and soft tissues. Although this technique is useful in the treatment of orthopaedic disorders, the main disadvantages are the considerable time required to lengthen the bone and the use of distraction devices for extended periods (Abbaspour et al., 2009). Therefore, improving the DO technique by reducing the healing period would be of clinical importance.
Stem cell therapy has been shown to have beneficial effects on bone tissue regeneration (Yamada et al., 2004). Promising results have been reported in both animals and humans, although the level of engraftment and differentiation of the cells could be improved (Ide et al., 2010; Toma et al., 2002). Recently, the role played by paracrine factors produced by stem cells in tissue regeneration and healing has been investigated and reports have demonstrated that angiogenesis and osteogenesis are promoted by conditioned medium (CM) collected from bone marrow mesenchymal stem cells (BMMSCs) (Chen et al., 2008; Osugi et al., 2012).
It is well known that primitive stimuli, such as hypoxic conditions and mechanical stress, can change the characteristics of cells and the factors they secrete (Wang and Thampatty, 2006; Wang et al., 2007). For example, the hypoxia‐inducible factor (HIF‐1) pathway senses changes in local oxygen availability and responds via transcriptional activation of two pro‐angiogenic factors, vascular endothelial growth factor (VEGF)‐A and angiopoietins (Ang). Mechanical stress is also known to be a stimulus that influences bone metabolism, with demonstration that bone tissue and osteoprecursor cells respond to mechanical stress both in vitro and in vivo (Rubin et al., 2006).
Dental pulp stem cells, which can be collected from dental pulp, have phenotypic characteristics similar to those of BMMSCs (Shi et al., 2001). These cells can differentiate into the dentinogenic lineage, the mesodermal lineage or the ectodermal lineage cell (Egusa et al., 2012) and secrete various cytokines and chemokines (Aranha et al., 2010; Gong et al., 2010). Moreover, these cells can be collected without adverse health problems. Thus, these previous reports support the use of dental pulp cells as a unique cellular resource for regeneration therapies. However, their possibilities for bone regeneration in DO is mostly unknown. In vitro experiments were performed, aiming to determine the angiogenic and regenerative potential of conditioned media (CMs) collected from human dental pulp cells grown under normoxic and hypoxic culture conditions. Furthermore, CMs collected from cells cultured under hypoxia were used to accelerate bone healing and improve the DO surgical procedure in vivo.
2. Materials and methods
The overall experimental design is summarized in Figure 1A–C.
Figure 1.
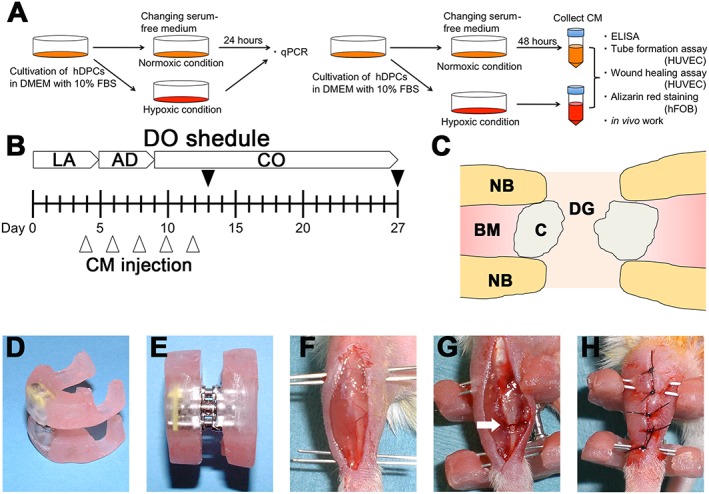
Outline of the study: (A) in vitro experiments and analyses; (B) in vivo distraction schedule and experimental design; after a 5 day latency period, distraction was started at a rate of 0.4 mm/12 h and continued for 4 days, increasing the length of the bone defect by 3.2 mm: black arrowheads, time points of sacrifice; white arrowheads, time points for injecting 20 µl of either control medium (control), CM–Nor or CM–Hyp; LA, latency period; AD, active distraction period; CO, consolidation period. (C) Schematic drawing of distraction site: NB, native bone; BM, bone marrow; C, callus; DG, distraction gap. (D–H) A custom‐made distractor was composed of two incomplete acrylic resin rings and an expansion screw: an anterior longitudinal incision was made on the right lower limb of each mouse; needles were inserted through the skin into the proximal and distal metaphysis of the tibia; subsequently, sets of needles were fixed to the custom‐made fixator with acrylic resin; after polymerization of the resin, osteotomy was carried out at the middle of the diaphysis (white arrow); the wound was closed with a 4–0 nylon suture. [Colour figure can be viewed at wileyonlinelibrary.com]
2.1. Isolation of hDPC and cell culture
hDPCs were isolated as previously described (Gronthos et al., 2000). Briefly, adult third molars or premolars (from patients aged 18–25 years) scheduled for extraction were collected (six teeth in total). Informed consent was obtained from each patient, and the Ethical Committee at the University of Bergen, Norway, approved the research protocol. After separation of the crowns from the roots, dental pulps were isolated and then digested in a solution of 3 mg/ml collagenase type I and 4 mg/ml dispase for 1 h at 37 °C. Single‐cell suspensions (1 × 104–2 × 104 cells/ml) were plated on culture dishes in Dulbecco's modified Eagle's medium (DMEM; PAA, GE Healthcare, Little Chalfont, UK) supplemented with 10% fetal calf serum (FCS) and 1% antibiotic mixture comprising penicillin–streptomycin, before incubation at 37 °C in 5% CO2. Cells at passages 4–8 were used for all experiments.
2.2. CM preparation
When the hDPCs reached 80% confluence, the media were replaced with serum‐free DMEM containing penicillin–streptomycin and cultured for 48 h at 37 °C in an atmosphere of 21% O2 and 5% CO2 (normoxic cells) or in a hypoxic chamber (Stem Cell Technology, Vancouver, BC, Canada) containing 1% O2 and 5% CO2, (hypoxic cells). Then the CMs were collected and centrifuged for 5 min at room temperature. The collected CMs were defined as CM–Nor and CM–Hyp, respectively. The collected CMs were stored at 4 °C before being used for the following experiments.
2.3. RNA expression assay in hDPC
Cells at passages 4–6 were plated at a density of 5.0 × 105 in 10 cm culture dishes (Nunc, Roskilde, Denmark). After the cells had reached 80% confluence, they were exposed to normoxic or hypoxic conditions for 24 h. The total RNA collected from hDPCs was extracted using a Maxwell® 16 LEV Simply RNA Cells Kit and a Maxwell® 16 Instrument (Promega, Fitchburg, WI, USA), according to the manufacturer's instructions. RNA purity and quantification were determined by spectrophotometry (Nanodrop Spectrophotometer, ThermoScientific NanoDrop Technologies, Wilmington, DE, USA). First‐strand cDNA synthesis was carried out using a High‐Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA, USA) from 1 µg total RNA.
Quantitative real‐time transcription–polymerase chain reaction (qPCR) was performed in StepOne Plus (Applied Biosystems), with fast enzyme and cycling conditions, on a StepOne real‐time PCR system, using TaqMan® gene expression assays (Applied Biosystems). Data of these targeted genes were normalized to the internal control glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) to obtain ΔC t. The final quantities of the genes of interest, relative to those seen in samples from normoxic cells, were reported by 2–ΔΔCt method. Primer sets are listed in Table 1.
Table 1.
Primer Accession Nos used in qPCR
| Species | Gene | Taqman assay ID |
|---|---|---|
| Human | GAPDH | Hs02758991_g1 |
| Human | VEGFA | Hs00900055_m1 |
| Human | ANG‐1 | Hs00375822_m1 |
| Human | ANG‐2 | Hs01048042_m1 |
| Human | IL‐6 | Hs00985639_m1 |
| Human | CXCL12 | Hs03676656_mH |
| Mouse | β‐Actin | Mm00607939_s1 |
| Mouse | Runx2 | Mm00501584_m1 |
| Mouse | Sp7 | Mm04209856_m1 |
| Mouse | Sox5 | Mm01264584_m1 |
| Mouse | Sox9 | Mm00448840_m1 |
| Mouse | F8 | Mm01215675_m1 |
GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; VEGFA, vascular endothelial growth factor‐A; ANG‐1, angiopoietin‐1; ANG‐2, angiopoietin‐2; IL‐6, interleukin‐6; CXCL12, C–X–C motif chemokine 12; Runx2, runt‐related transcription factor 2; Sp7, Osterix; F8, factor 8
2.4. CM and angiogenic growth factor expression
The levels of VEGF‐A and Ang‐2 in CMs collected from hDPCs grown under normoxic or hypoxic conditions for 48 h were investigated using enzyme‐linked immunosorbent assay (ELISA). The concentration of these growth factors was measured using a Human Quantikine ELISA Kit (DVE00 and DANG20, R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
2.5. Tube formation assay
Passage 2–3 human umbilical vein endothelial cells (HUVECs; C2517A; Lonza, Walkersville, MD, USA) at 80% confluence were suspended in CM–Nor or CM–Hyp and cultured at a density of 1.0 × 105, in duplicate, in 96‐well plates coated with Growth Factor Reduced Matrigel (356230, BD Biosciences, San Jose, CA, USA). EGM‐2 BulletKit (CC‐3162, Lonza) and serum‐free DMEM were used as positive and negative controls, respectively. After 8 h, the formation of tube‐like structures was examined using a microscope. Photographs were taken and the number of branch points, total tube lengths and tube formation areas (see supporting information, Figure S1A) were measured and assayed, using the NIS‐Elements software program BR 3.07 (Nikon, Tokyo, Japan).
2.6. Endothelial cell wound‐healing assay
Passage 2–3 HUVECs were cultured in Culture‐Insert 24 (80241, Ibidi, Martinsried, Planegg, Germany) at a concentration of 3.0 × 104 cells/well, in duplicate. After the cells had reached 80% confluence, the culture inserts were gently removed using sterile tweezers. The wounded monolayers were then washed three times with phosphate‐buffered saline (PBS) to remove cell debris and incubated with normoxic and hypoxic CMs and positive/negative control media. After 24 h of incubation, the cells were fixed and stained with crystal violet. The area of cell‐free wound was recorded using a microscope. Photographs were taken of each well, measured and assayed using Photoshop CS5 (Adobe Systems, San Jose, CA, USA) and the ImageJ software program v. 1.43 (NIH, Bethesda, MD, USA). The wound‐healing effect was calculated as the percentage of the remaining cell‐free area compared with the area of the initial wound.
2.7. Alizarin red staining
Human fetal osteoblasts (hFOBs; CRL‐11372, ATCC, Manassas, VA, USA) were cultured in 24‐well plates at a concentration of 5.0 × 104 cells/well, with DMEM supplemented with 10% FBS at 34 °C in an atmosphere containing 21% O2 and 5% CO2. When the cells had reached 80% confluence, the media were replaced with DMEM, normoxic or hypoxic CM, all media supplemented with 1% FBS. After 14 days of culture, the cells were stained with a saturated solution of alizarin red S adjusted to pH 4.2 (A5533, Sigma‐Aldrich, St. Louis, MI, USA).
2.8. Mouse DO model
All animal experiments were performed in accordance with the Guidelines for Animal Experiments at the University of Bergen. Thirty 8–10 week‐old female NMRI mice were used. The surgical DO method was performed as previously described (Fujio et al., 2011; Isefuku et al., 2000). The fixator was composed of two incomplete acrylic resin rings (outer diameter 20 mm, inner diameter 10 mm, thickness 5 mm) and an expansion screw (600‐301‐30, Ortho Dentaurum, Berlin, Germany) (Figure 1D, E). The total weight of the device, including the needles inserted into the tibia, was 2.7 g. The animals were anaesthetized by isoflurane (Isoba Vet, Schering‐Plough, Lysaker, Norway). The right limb was carefully shaved and disinfected with 10% iodine solution. A longitudinal skin incision was made on the right leg and the underlying muscles were bluntly separated, taking care not to remove all of the periosteum. The fibula was fractured by direct lateral pressure. The tibia was fixed to the device with one pair of 25 gauge needles proximally and one pair of 27 gauge needles distally, glued with acrylic resin (Figure 1 F). After complete polymerization (ca. 5 min), an osteotomy was performed at the middle of the diaphysis (Figure 1G). This was done using very small cutting forceps. The wound was closed with a 4–0 nylon suture (Figure 1H).
After a latency period of 5 days, distraction was started at a rate of 0.4 mm/12 h. The lengthening was continued for 4 days, resulting in a gap of 3.2 mm in total; 20 µl CM or serum‐free DMEM as control were injected transcutaneously into the centre of the distraction gap every second day during days 4–12 after operation (five injections in total), using a syringe (Hamilton, Bonaduz, Switzerland) (Figure 1C). We defined the following groups, based on the injection protocol: control group (n = 5); CM–Nor (n = 5); CM–Hyp (n = 5). The mice were sacrificed at days 13 and 27 after surgery (n = 5/group) (Figure 1B).
2.9. Assessment of transcription factors during DO
Tissue collected from the distal distraction gap was excised and stored in liquid nitrogen until RNA extraction at day 13 (n = 5). Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). RNA purity and quantification were determined by Nanodrop spectrophotometry. First‐strand cDNA synthesis was carried out using the High‐Capacity cDNA Archive Kit (Applied Biosystems) from 1 µg total RNA. qPCR was performed in StepOne Plus (Applied Biosystems), with fast enzyme and cycling conditions, on a StepOne real‐time PCR system, using TaqMan® gene expression assays (Applied Biosystems). Data of these targeted genes were normalized to the internal control β‐actin to obtain ΔC t. The final quantities of the genes of interest, relative to control samples, were reported by the 2–ΔΔCt method. Primer sets are listed in Table 1.
2.10. Radiographic and histological analysis
Digital X‐ray images were taken at days 13 and 27 after surgery, using a Kodak Multispectral FX instrument (Carestream Health, Toronto, Canada). The distraction gap was outlined from the outside corners of the two proximal and the two distal cortices, forming a quadrilateral region of interest (ROI). The X‐ray density of newly formed bone was analysed using Bruker MI SE software, v. 7.1.3 (Scrum, Osaka, Japan). After the animals had been sacrificed, the distracted tibias were fixed in 10% neutral buffered formalin for 24 h at room temperature. Samples were decalcified with Morse's solution (10% sodium citrate, 20% formic acid) overnight at 4 °C. The samples were then washed in PBS and embedded with OCTTM (Sakura Finetek, Tokyo, Japan), after which 8 µm sections were made on a cryostat (Leica CM 3050S, Leica Microsystems, Wetzlar, Germany). The cryosections were stained with haematoxylin and eosin (H&E) and safranin O–fast green. In the sections of each sample, the percentage area of newly formed cartilage and bone was measured. The pixel number of each area in the distraction zone was counted using Image J, v. 1.43 (NIH, Bethesda, MD, USA) (see supporting information, Figure S1B).
2.11. Immunohistochemistry
The sections were washed in PBS, blocked with 1% bovine serum albumin (BSA)/PBS for 30 min and then incubated with the primary antibody [rabbit polyclonal antibody (Ab) α‐SMA; ab5694, Abcam, Cambridge, UK) in blocking buffer for 1 h. The sections were then incubated with the secondary antibody, conjugated with AlexaFluor 555 (Life Technologies, Waltham, MA, USA) for 30 min. The cell nuclei were labelled with diamidinophenylidole (D1306; Life Technologies). The sections were finally mounted with Prolong Gold Antifade Reagent (P36934, Life Technologies). Vascular density was assessed morphometrically by examining five fields/section of the distraction gap, in three successive sections, after immunofluorescence staining using the anti‐α‐SMA Ab. Images were captured using an Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan).
2.12. Statistical analysis
Statistical analysis was performed using the SPSS statistical package v. 19.0 (IBM, Armonk, New York, USA). Paired Student's t‐test was used for comparisons. One‐way analysis of variance (ANOVA) was applied to compare two or more means, followed by the Tukey HSD test. The results were expressed as mean ± standard deviation (SD); p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of hypoxia on the angiogenic properties of CMs from hDPCs in vitro
Results obtained from qPCR (Figure 2A) and ELISA (Figure 2B, C) showed that VEGF‐A and Ang‐2 were upregulated in hDPCs grown under hypoxic conditions. The mRNA expressions of VEGF‐A and Ang‐2 were approximately two‐ and 1.5‐fold higher, respectively, when compared with their expression under normoxic conditions (p < 0.05). On the other hand, Ang‐1 and CXCL12 expressions were three‐ to four‐fold lower under hypoxic than under normoxic conditions (p < 0.01). IL‐6 expression was not significantly different under hypoxic and normoxic conditions (Figure 2A). ELISA was performed to confirm the qPCR results at the protein level. Both VEGF‐A165 and Ang‐2 protein expression increased significantly under hypoxic conditions (p < 0.05) compared with the normoxic group (Figure 2B, C).
Figure 2.
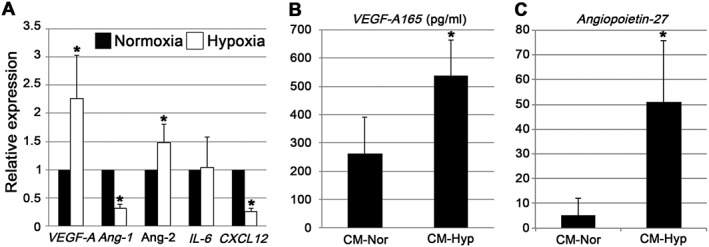
Expression of angiogenic factors from hDPCs under normoxic and hypoxic culture conditions. (A) qPCR assay of mRNA extracted from hDPCs 24 h after normoxic and hypoxic incubation conditions (n = 6); VEGF‐A and Ang‐2 were both upregulated under hypoxic culture conditions. (B, C) CMs were collected after incubation under nomoxic and hypoxic conditions for 48 h (n = 6); VEGF‐A 165 (B) and Ang‐2 (C) were both upregulated under hypoxic culture conditions. Data represent mean ± SD; *p < 0.05
3.2. Effect of CM from hDPC on angiogenesis and mineralization in vitro
To evaluate the angiogenic activity of CM, we performed both the Matrigel tube formation assay and the endothelial cell wound‐healing assay, using HUVECs. In the control group, HUVECs demonstrated little tube formation (Figure 3A). In contrast, the CM group demonstrated increased endothelial tube formation (Figure 3B, C). The numbers of branch points, total tube lengths and tube formation areas were significantly greater in the hypoxic than in the normoxic and control groups (Figure 3D–F). To further evaluate the angiogenic properties of CM, we performed an endothelial cell wound‐healing assay using HUVECs. Hypoxic conditions significantly accelerated wound closure compared to the control and normoxic group (Figure 4A–D). To assess the mineralization capacity of CM, we performed alizarin red staining with hFOBs, which have a high mineralization capacity. hFOBs produced mineral deposits when cultured in DMEM supplemented with 10% FBS (Figure 5A). Mineral deposition was decreased by low serum concentrations (Figure 5B). CM–Nor and CM–Hyp did not enhance the mineralization capacity of hFOBs (Figure 5C, D).
Figure 3.
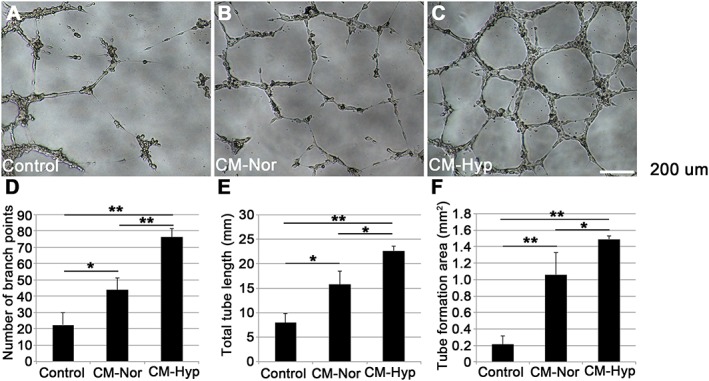
CMs from hDPCs enhance endothelial cell tube formation in vitro. The angiogenic potentials of CMs were tested by Matrigel tube formation assay (n = 6); HUVECs were suspended in CM–Nor or CM–Hyp in duplicate; tube formation was also observed after culture in serum‐free DMEM and EGM‐2 Bullet Kit as negative and positive controls (data not shown), respectively; photographs are from (A) control, (B) CM–Nor and (C) CM–Hyp cultures. Number of branch points (D), total tube length (E) and tube formation area (F) were calculated using NIS elements software: the CM–Nor cultures showed higher tube formation than the control cultures; the CM–Hyp cultures showed greater angiogenic potential than CM–Nor cultures; data represent mean ± SD; **p < 0.01, *p < 0.05; bar = 200 µm. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
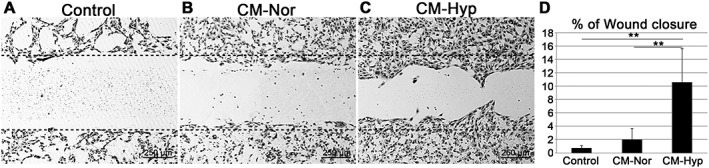
CM from hypoxic conditions promotes endothelial cell migration in vitro. Wound‐healing assays were performed to assess the angiogenic potentials of CMs from hDPCs. HUVECs were cultured in Culture‐Insert 24‐well plates, in duplicate; after the cells had reached 80% confluence, the Culture‐Insert was gently removed; the wounded monolayers were incubated with CM–Nor (B) and CM–Hyp (C) and both positive (data not shown) and negative (A) control media. After 24 h of incubation, the cells were fixed and stained with crystal violet; the percentage of wound closure was calculated using ImageJ software. (D) CM–Hyp cultures showed greater angiogenic potential compared with the others; data represent mean ± SD; **p < 0.01; bar = 250 µm; line of black dots represents base line
Figure 5.
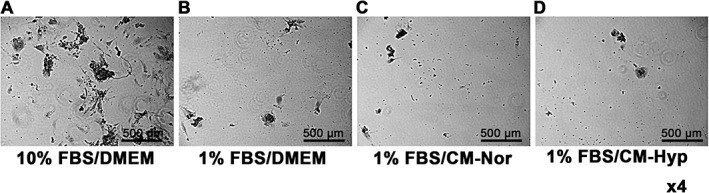
Effect of CM on the mineralization capacity of hFOBs: these were cultured with (A) DMEM supplemented with 10% FBS; (B) DMEM supplemented with 1% FBS; (C) CM–Nor supplemented with 1% FBS; and (D) CM–Hyp supplemented with 1% FBS; bar = 500 µm
3.3. Effect of CM on DO healing in mouse tibiae
Histological evaluation of the distraction sites showed less newly formed callus and cartilage in the control group than in the CM groups at day 13 (Figure 6A–F). The centre of the distraction site of the control group was filled with fibrous tissue. In contrast, the hypoxic CM group showed massive callus formation at the centre of the distraction site and some cartilage formation in the periosteal region. Interestingly, the normoxic CM group showed greater cartilage formation at the periosteal and distraction sites. To further investigate the role of CM, we performed qPCR assays on day 13 samples (Figure 6G). Osteoblastic and chondrogenic markers were consistent with the histology seen, with the animals exposed to hypoxic CM showing twice as much Osterix expression as the control group (p < 0.05). However, there was no significant difference seen for osteoblastic and chondrogenic markers between the normoxic and hypoxic CM groups, which showed approximately 2.5‐ and two‐fold higher Sox 5 expression, respectively, than the control group. Only the normoxic group was significantly different from the control group (p < 0.05). Significantly upregulated Factor 8 gene was observed in the hypoxic group compared to the control (p < 0.01) and normoxic (p < 0.05) groups. Histological and qPCR findings were confirmed by immunohistochemistry. Injection of CM increased α‐SMA‐positive mature blood vessels at the site of distraction. Furthermore, the hypoxic CMs showed increased blood vessel formation compared with the normoxic group (p < 0.01) (Figure 7A–D).
Figure 6.
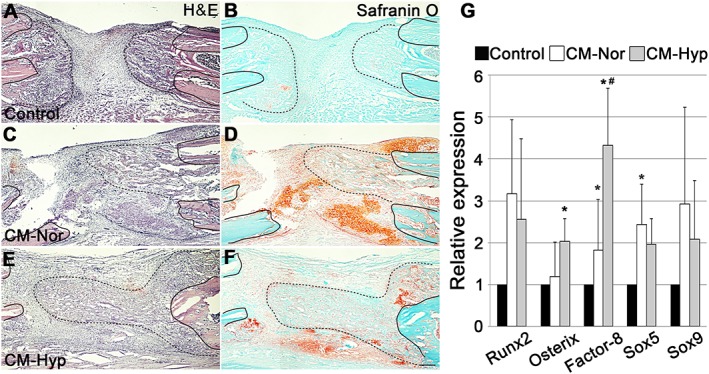
Local administration of CM–Hyp promotes new callus formation in the DO gap: representative micrographs of sections displaying the DO gap stained with H&E (A, C, E) and safranin O–fast green (B, D, F); n = 5. Black line on each figure indicates the end of the distal (left side) and proximal (right side) bone fragment; line of black dots on each figure shows newly formed callus: neocallus formation and much fibrous tissue were evident within the DO gap in the control group at day 13 (A, B); little cartilage was observed in the control group (B); the DO gap in the CM–Hyp group showed well generated newly formed callus and periosteum‐derived cartilage (E, F); the gap in the CM–Nor group showed massive cartilage and a little callus formation (C, D). To evaluate the effect of the CM on mRNA, DO gap tissue was taken from each group at day 13 (n = 5); qPCR results showed significant differences between exposure to CM–Nor and CM–Hyp in Factor 8 gene mRNA (G). Data represent mean ± SD; *p < 0.05 vs control, # p < 0.05 vs CM–Nor; bar = 250 µm. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 7.
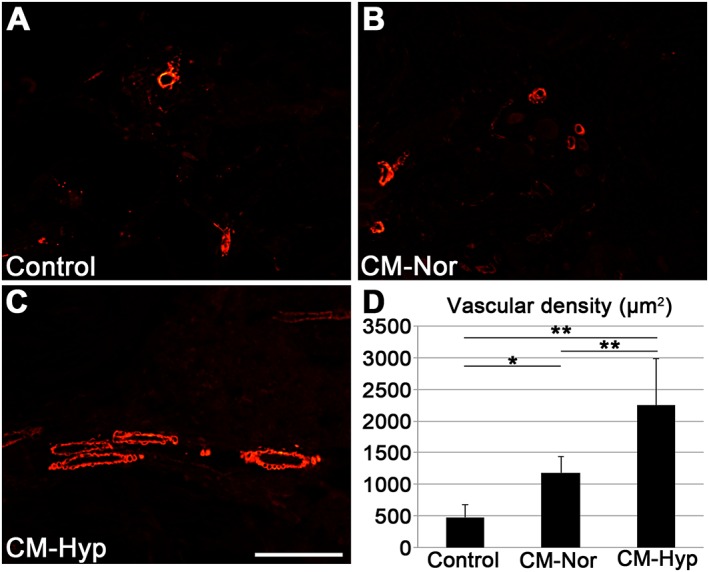
Injection of CM promotes mature blood vessel regeneration. Blood vessel regeneration was assessed by immunofluorescence staining for α‐SMA (n = 5). Both CM–Nor (B) and CM–Hyp (C) showed significantly higher vascular density (D) compared with control (A); moreover, CM–Hyp showed greater blood vessel regeneration than CM–Nor. Data represent mean ± SD; **p < 0.01, *p < 0.05; bar = 100 µm. [Colour figure can be viewed at wileyonlinelibrary.com]
To evaluate DO healing, newly formed bone was assessed radiologically on days 13 and 27. On day 13 there was little bone formation in any of the groups (Figure 8A, C, E). On day 27 the control group showed weakly calcified and narrow newly formed bone at the distraction site (Figure 8B, D, F). In contrast, the hypoxic CM group revealed strongly calcified bone at the distraction site (Figure 8F). In addition, the X‐ray density of the DO gap was higher in this group than in the control group (Figure 8G, p < 0.05). Histological findings also revealed that the CM–Hyp group showed a significantly higher bone formation area compared with the control group at day 27 (Figure 9A–C, G). There was no significant difference between the three groups in the cartilage formation areas at day 27 (Figure 9D–F, G).
Figure 8.
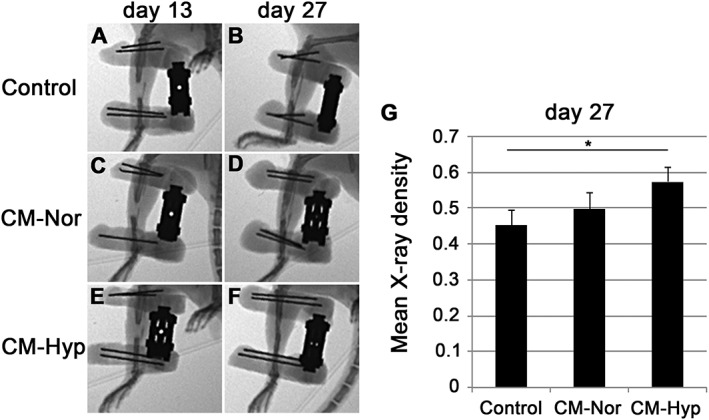
CM from hypoxia promotes healing during DO: newly formed bone was assessed radiologically on days 13 (A, C, E) and 27 (B, D, F) (n = 5). There was no significant difference between CMs on day 13; on day 27 the controls (B) showed some calcified and narrow bone formation in the distraction site; the CM–Nor group showed calcified bone formation in the DO gap (D); the CM–Hyp group (F) had strongly calcified bone formation with high X‐ray density in the distraction site compared with controls (G). Data represent mean ± SD; *p < 0.05
Figure 9.
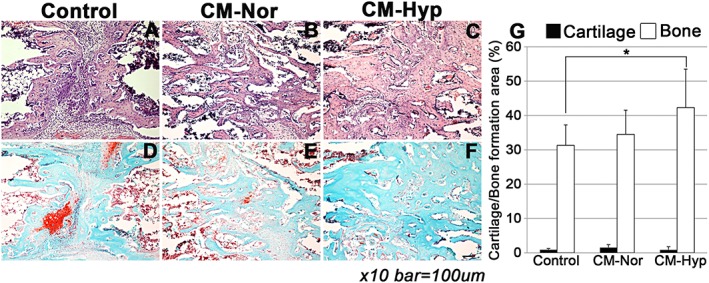
Local administration of CM enhances bone formation: newly formed bone and cartilage were assessed by H&E (A–C) and safranin O–fast green (D, E, F) staining (n = 5). The CM–Hyp group showed a significantly higher bone‐formation area compared with the control group at day 27 (G); there were no significant differences in the three groups in cartilage formation areas on day 27. Bar = 100 µm; data represent mean ± SD; *p < 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
4. Discussion
In previous studies, our group has shown that CM from BMMSCs has regenerative potential in several animal bone defect models (Osugi et al., 2012; Inukai et al., 2013), including DO healing through recruitment of BMMSCs, endothelial cells (ECs) and endothelial progenitor cells (EPCs) (Ando et al., 2014). The latter authors focused on monocyte chemoattractant protein (MCP)‐1 and MCP‐3 in CM from BMMSCs and stated that these chemokines are essential factors in recruiting BMMSCs and ECs/EPCs. In the present study, human dental pulp cells (hDPCs) derived from healthy third molars or premolars extracted during routine surgery were prepared and used according to the protocol described previously (Gronthos et al., 2000). Dental pulp is a heterogeneous mixture of various cells, such as ECs, odontoblasts, fibroblasts and mesenchymal stem cells. The 'stemness' of dental pulp cells has been evaluated in several studies (Al‐Sharabi et al., 2014; Sakai et al., 2012). Although these cells have stem cell properties, such as multi‐differential and self‐renewal capacity comparable to other adult stem cells (Egusa et al., 2012), hDPCs have higher proliferative capacity than BMMSCs (Alge et al., 2010). Furthermore, hDPCs can be obtained easily from teeth that are scheduled for ordinary extraction, and therefore with fewer ethical considerations. The results obtained from this study suggest that hDPCs have advantages as a cell source for regenerative therapy.
Hung et al. (2007) investigated in vitro the release of cytokines from BMMSCs grown under normoxic and hypoxic conditions, using cytokine arrays. In their study, higher expression of angiogenic factors such as VEGF, MCP‐1 and angiogenin was found in the CMs collected from cells cultured under hypoxic conditions. Chen et al. (2008) also revealed an advantage of hypoxic culture conditions on the secretion of angiogenic factors from BMMSCs.
Accordingly, the in vitro and in vivo experiments performed in the present study clearly demonstrated profound stimulatory effects of hypoxia on angiogenesis. This indicates that hypoxia may induce the angiogenic properties of stem cell in general.
We evaluated the expression of angiogenic factors derived from hDPCs cultured under hypoxic conditions. It has previously been suggested that the angiogenic potential of hDPCs is enhanced by hypoxic culture conditions (Ceradini et al., 2004; Aranha et al., 2010). It was demonstrated that hypoxic‐cultured hDPCs increased the expression of HIF‐1, recognized as a master gene of angiogenesis, and VEGF, reported to be a major regulator of angiogenesis at the protein level. In this study, we showed that hypoxia induced not only VEGF but also Ang‐2 upregulation at both the gene and protein levels. Moreover, we demonstrated the angiogenic potential of hypoxic CMs by using both in vitro and in vivo models. However, we did not observe CXCL12 gene upregulation in hDPCs, a result that is consistent with previous findings (Gong et al., 2010). The latter authors examined the regulation of the CXCL12–CXCR4 axis in hDPC cultured under hypoxic conditions and found an increased expression of HIF‐1 and CXCR4 mRNA, but a decreased expression of CXCL12 mRNA over a 24 h culture period. We evaluated the expression of Ang‐1, which has been previously reported as an angiogenic growth factor generating a stable and functional vasculature (Suri et al., 1996). In this in vitro study, the Ang‐1 level of hDPCs was reduced by hypoxia. Generally, it is known that hypoxia induces Ang‐2 mRNA expression in endothelial cells (Pichiule et al., 2004). In contrast, Ang‐1 is reported to be downregulated by hypoxia (Enholm et al., 1997). However, Park et al. (2003) reported that hypoxia upregulated Ang‐1 mRNA in retinal pericytes. Further studies are needed to elucidate the relationship between hypoxia and angiopoietic agents.
The in vitro angiogenic potential of CM was tested by Matrigel tube formation and wound‐healing assays, using HUVEC. These assays have been widely used to evaluate angiogenic potential in vitro (Ashton et al., 1999). In both assays, CM–Hyp had a greater angiogenic effect than control medium and CM–Nor. We examined the mRNA expression of basic fibroblast growth factor, hepatocyte growth factor, epidermal growth factor and chemokine (C–C motif) ligand 2 (data not shown), as these factors have previously been reported to be angiogenic (Bikfalvi et al., 1997; Morishita et al., 1999; Shen et al., 2010; Stamatovic et al., 2006). However, the mRNA expression for these growth factors was reduced under hypoxic culture conditions. This suggests that VEGF‐A and Ang‐2 were the main growth factors responsible for the enhanced in vitro angiogenesis found in this study. Previous in vitro reports have also suggested that both VEGF‐A and Ang‐2 are important for angiogenesis (Halin et al., 2008; Kim et al., 2009). We therefore suggest that these factors enhanced bone formation through blood vessel formation in our DO model. Angiogenesis involves the sprouting and remodelling of the primitive vessels and the subsequent stabilization of the sprouts by α‐SMA‐positive mural cells (pericytes in medium‐sized vessels and smooth muscle cells in larger ones). It is well known that expression of VEGF‐A and Ang‐2 is observed at sites of vascular remodelling. Moreover, Ang‐2 has been shown to have a VEGF‐dependent modulation of capillary structure and endothelial cell survival in vivo (Lobov et al., 2002; Maisonpierre et al., 1997). Our study suggests that the angiogenic effect seen in vivo is promoted by an increased amount of VEGF‐A and Ang‐2 in the hypoxic CM. In this study, CM–Hyp did not change the mineralization capacity of hFOBs in vitro. Therefore, we suggest that CM–Hyp promotes DO healing through enhanced angiogenesis. Further studies are needed to elucidate the possible osteogenic effects of CM–Hyp in vivo.
As bone formation interacts strongly with blood vessel formation (Carano and Filvaroff, 2003; Carmeliet, 2003; Kanczler and Oreffo, 2008), we propose that the effect of conditioned media on DO healing is effected primarily through blood vessel formation. The newly formed blood vessels can transport oxygen, nutrients, soluble growth factors and various types of cells, which collectively play a key role in bone formation. It is also well known that, during DO healing, bone regeneration is induced by blood vessel regeneration (Li, 1999). The qPCR results at day 13 showed no significant difference in the expression of osteogenic and chondrogenic markers between the normoxic and hypoxic groups in vivo. However, we observed significantly higher expression of Factor‐8, a marker for blood vessel formation. Further, the immunohistochemistry results also showed that the average α‐SMA‐positive mature vascular density in the hypoxic group was about twice that in the normoxic group. Additional evidence supporting the concept that the bone healing observed in the present study is dependent on angiogenesis comes from the finding that CM did not enhance mineralization in vitro. These results suggest that injection of CM enhanced DO healing through the formation of α‐SMA‐positive blood vessels. A recent report, using a cell‐based transplantation approach, showed that the transplantation of stem cells from human dental pulp into the distraction site promoted DO healing (Alkaisi et al., 2013). Taken together, our data suggest that the paracrine angiogenic signalling/growth factors derived from dental pulp cells may have a significant role in bone healing.
Although stem cell implantation has beneficial effects on specific diseases, the implanted stem cells cannot survive for a long time (Ide et al., 2010; Toma et al., 2002). As a consequence, it has been proposed that paracrine factors secreted by the implanted stem cells are of crucial importance for the beneficial effects that are observed after stem cell implantation. Therefore, we tested whether CM from hDPCs contains factors that promote DO healing. Local administration of CM–Hyp may improve the DO procedure by a reduction in healing time.
In summary, the biological effect of CM from hDPCs can be modified by culture conditions. Hypoxic culture conditions stimulated an upregulation of angiogenic factors secreted from hDPCs. Local administration of CM from hDPCs cultured under hypoxic conditions promoted blood vessels and bone formation in the DO gap. Our results suggest that paracrine factors secreted from hDPCs can be optimized for use in DO treatment by altering the culture conditions.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Supporting information
Figure S1. (A) Schematic drawing of the branch points, tube length and tube formation area defined in this study: white, branch point; blue, tube length; yellow, tube formation area. (B) The distraction zone was defined as the area enclosed in a quadrangle, with its four end points being the osteotomized lines. The cartilage‐/bone‐formation areas were calculated using the equation:
Newly formed cartilage area (%) = (new cartilage area/distraction zone) × 100
Supporting info item
Acknowledgements
The authors thank Dr Yuji Ando and Ms Siren Østvold for their encouragement to complete this study, which was supported by the University of Bergen and the Meltzer Foundation.
Fujio, M. , Xing, Z. , Sharabi, N. , Xue, Y. , Yamamoto, A. , Hibi, H. , Ueda, M. , Fristad, I. , and Mustafa, K. (2017) Conditioned media from hypoxic‐cultured human dental pulp cells promotes bone healing during distraction osteogenesis. J Tissue Eng Regen Med, 11: 2116–2126. doi: 10.1002/term.2109.
Contributor Information
Masahito Fujio, Email: m-fujio@med.nagoya-u.ac.jp.
Kamal Mustafa, Email: kamal.mustafa@iko.uib.no.
References
- Abbaspour A, Takahashi M, Sairyo K et al 2009; Optimal increase in bone mass by continuous local infusion of alendronate during distraction osteogenesis in rabbits. Bone 44: 917–923. [DOI] [PubMed] [Google Scholar]
- Alge DL, Zhou D, Adams LL et al 2010; Donor‐matched comparison of dental pulp stem cells and bone marrow‐derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 4: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkaisi A, Ismail AR, Mutum SS et al 2013; Transplantation of human dental pulp stem cells: enhance bone consolidation in mandibular distraction osteogenesis. J Oral Maxillofac Surg 711758: e1–13. [DOI] [PubMed] [Google Scholar]
- Al‐Sharabi N, Xue Y, Fujio M et al 2014; Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng A 20: 3063–3072. [DOI] [PubMed] [Google Scholar]
- Ando Y, Matsubara K, Ishikawa J et al 2014; Stem cell‐conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 61: 82–90. [DOI] [PubMed] [Google Scholar]
- Aranha AM, Zhang Z, Neiva KG et al 2010; Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod 36: 1633–1637. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Yokota R, John G et al 1999; Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). J Biol Chem 274: 35562–35570. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G et al 1997; Biological roles of fibroblast growth factor‐2. Endocr Rev 18: 26–45. [DOI] [PubMed] [Google Scholar]
- Carano RA, Filvaroff EH. 2003; Angiogenesis and bone repair. Drug Discov Today 8: 980–989. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. 2003; Angiogenesis in health and disease. Nat Med 9: 653–660. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ et al 2004; Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med 10: 858–864. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY et al 2008; Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3: e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egusa H, Sonoyama W, Nishimura M et al 2012; Stem cells in dentistry‐part I: stem cell sources. J Prosthodont Res 56: 151–165. [DOI] [PubMed] [Google Scholar]
- Enholm B, Paavonen K, Ristimäki A et al 1997; Comparison of VEGF, VEGF‐B, VEGF‐C and Ang‐1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 14: 2475–2483. [DOI] [PubMed] [Google Scholar]
- Fujio M, Yamamoto A, Ando Y et al 2011; Stromal cell‐derived factor‐1 enhances distraction osteogenesis‐mediated skeletal tissue regeneration through the recruitment of endothelial precursors. Bone 49: 693–700. [DOI] [PubMed] [Google Scholar]
- Gong QM, Quan JJ, Jiang HW et al 2010; Regulation of the stromal cell‐derived factor‐1α–CXCR4 axis in human dental pulp cells. J Endod 36: 1499–1503. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J et al 2000; Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc Natl Acad Sci USA 97: 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin C, Fahrngruber H, Meingassner JG et al 2008; Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am J Pathol 173: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Pochampally RR, Chen SC, et al 2007; Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K–Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 25: 2363–2370. [DOI] [PubMed] [Google Scholar]
- Ide C, Nakai Y, Nakano N et al 2010; Bone marrow stromal cell transplantation for treatment of sub‐acute spinal cord injury in the rat. Brain Res 1332: 32–47. [DOI] [PubMed] [Google Scholar]
- Ilizarov GA. 1989; The tension‐stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res 239: 263–285. [PubMed] [Google Scholar]
- Inukai T, Katagiri W, Yoshimi R et al 2013; Novel application of stem cell‐derived factors for periodontal regeneration. Biochem Biophys Res Commun 430: 763–768. [DOI] [PubMed] [Google Scholar]
- Isefuku S, Joyner CJ, Simpson AH. 2000; A murine model of distraction osteogenesis. Bone 27: 661–665. [DOI] [PubMed] [Google Scholar]
- Kanczler JM, Oreffo RO. 2008; Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15: 100–114. [DOI] [PubMed] [Google Scholar]
- Kim HZ, Jung K, Kim HM et al 2009; A designed angiopoietin‐2 variant, pentameric COMP‐Ang2, strongly activates Tie2 receptor and stimulates angiogenesis. Biochim Biophys Acta 1793: 772–780. [DOI] [PubMed] [Google Scholar]
- Li G, Simpson AH, Kenwright J et al 1999; Effect of lengthening rate on angiogenesis during distraction osteogenesis. J Orthop Res 17: 362–367. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. 2002; Angiopoietin‐2 displays VEGF‐dependent modulation of capillary structure and endothelial cell survival in vivo . Proc Natl Acad Sci USA 99: 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF et al 1997; Angiopoietin‐2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60. [DOI] [PubMed] [Google Scholar]
- Morishita R, Nakamura S, Hayashi S et al 1999; Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 33: 1379–1384. [DOI] [PubMed] [Google Scholar]
- Osugi M, Katagiri W, Yoshimi R et al 2012; Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng A 18: 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Kim NH, Jo I. 2003; Hypoxia and vascular endothelial growth factor acutely upregulate angiopoietin‐1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res 65: 125–131. [DOI] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, LaManna JC. 2004; Hypoxic regulation of angiopoietin‐2 expression in endothelial cells. J Biol Chem 279: 12171–12180. [DOI] [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR. 2006; Molecular pathways mediating mechanical signaling in bone. Gene 367: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yamamoto A, Matsubara K et al 2012; Human dental pulp‐derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro‐regenerative mechanisms. J Clin Invest 122: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Sheng Y, Ji L et al 2010; Involvement of c‐Jun N‐terminal kinase and extracellular signal‐regulated kinase 1/2 in EGF‐induced angiogenesis. Cell Biol Int 34: 1213–1218. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronths S. 2001; Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29: 532–539. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Mostarica‐Stojkovic M et al 2006; CCL2 regulates angiogenesis via activation of Ets‐1 transcription factor. J Immunol 177: 2651–2661. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S et al 1996; Requisite role of angiopoietin‐1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–80. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS et al 2002; Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105: 93–98. [DOI] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. 2006; An introductory review of cell mechanobiology. Biomech Model Mechanobiol 5: 1–16. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L et al 2007; The hypoxia‐inducible factor‐α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117: 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT. 2006; Distraction osteogenesis. J Am Acad Orthop Surg 14: S168–174. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ueda M, Naiki T et al 2004; Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet‐rich plasma: tissue‐engineered bone regeneration. Tissue Eng 10: 955–964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Schematic drawing of the branch points, tube length and tube formation area defined in this study: white, branch point; blue, tube length; yellow, tube formation area. (B) The distraction zone was defined as the area enclosed in a quadrangle, with its four end points being the osteotomized lines. The cartilage‐/bone‐formation areas were calculated using the equation:
Newly formed cartilage area (%) = (new cartilage area/distraction zone) × 100
Supporting info item


