Abstract
Background
Oral submucous fibrosis (OSF) is a chronic progressive disease of the oral cavity that is considered a common potentially malignant disorder in South Asia. Areca nut chewing is the main etiological factor, but its carcinogenic mechanism has yet to be proven. The purpose of this study was to identify the useful biomarkers in predicting high‐risk patients with OSF.
Methods
Thirty‐six cases of OSF and six cases of normal oral mucosa (NOM) were used for this study. Immunohistochemical staining was performed for Ki67, cyclin D1, p16, p53, β‐catenin, c‐Jun, c‐Met, and insulin‐like growth factor II mRNA‐binding protein 3 (IMP3). The expression patterns of NOM served as guidelines for the scoring system.
Results
The expression of Ki67, cyclin D1, c‐Met, IMP3, and β‐catenin showed a significant difference between OSF and NOM samples. The combined biomarkers of Ki67 and p16 showed significantly different expression between the transformation and non‐transformation groups. With discriminant analysis, we proposed a noble formula and cutoff value for predicting high‐risk patients with OSF.
Conclusion
The notable biomarkers in our present study were Ki67 and p16 showing significantly different expression levels between the transformation and non‐transformation groups. With the identification of high‐risk patients with OSF, we can expect to develop more intensive treatment modalities, leading to the reduction in cancer transformation rate from OSF.
Keywords: combined biomarkers, oral submucous fibrosis, risk assessment, squamous cell carcinoma
Introduction
Oral submucous fibrosis (OSF) is a chronic progressive oral mucosal disease with a potential for malignant transformation 1. Clinical presentation depends on the stage of the disease. Initially, most patients present with a burning sensation or intolerance to spicy food, and they may have vesicles, particularly on the palate. Ulceration and dryness of the mouth are later followed by fibrosis of the oral mucosa, leading to rigidity of the lips, tongue, and palate and causing trismus 2. The important histopathologic features consist of the deposition of dense collagen in the lamina propria associated with epithelial atrophy. Initially, there is juxta‐epithelial inflammation followed by hyalinization 3. OSF is frequently noted in South‐East Asian countries where areca nut chewing is popular, suggesting that this habit is the most important etiological factor in the pathogenesis of OSF 4.
Importantly, the malignant transformation rate was shown to be 7.6% over a period of 17 years 5, 6. Furthermore, more than 2400 new cases of oral squamous cell carcinoma (OSCC) arising from OSF have been diagnosed each year in Taiwan due to the prevalent use of betel quid 5. Therefore, the early detection of potentially malignant OSF has been crucial in the inhibition of oral cancer.
Many efforts have been made to explore carcinogenesis and identify predictable diagnostic biomarkers in OSF. However, no useful marker has been identified to date. To identify the useful biomarkers in predicting oral cancer development in patients with OSF, we performed immunohistochemical staining for eight candidate biomarkers. Every biomarker has its own special attribution to be selected.
Ki67 and cyclin D1 can be used to evaluate the cell proliferation 7. p16 and p53 were investigated as tumor‐suppressor genes known to be affected in head‐and‐neck squamous cell carcinomas (HNSCCs). In HNSCCs, the incidence of p16 inactivation due to mutation was reported to be 10% 8 and that by homozygous deletion was 33% 9. Thus, point mutations in p53 occur in 10–17% of precancerous disease and in 35–67% of OSCC 10. To further investigate the transcriptional activity in OSF tissue, the expression levels of β‐catenin and c‐Jun were evaluated 11, 12. Growth factor receptors c‐Met and insulin‐like growth factor II mRNA‐binding protein 3 (IMP3) were reported to play pivotal roles in tumor invasion 13, 14.
The aim of our study was to generate a prediction model for risk assessment using combined biomarkers. Although no single biomarker has been satisfactory in predicting carcinomatous transformation of potentially malignant OSF, the combined biomarkers identified in our study may be clinically useful to detect high‐risk OSF and reduce the incidence of OSCC, thus guiding the clinician's decision on the diagnosis, treatment, and prevention strategies.
Materials and methods
Human tissue samples
Thirty‐six formalin‐fixed, paraffin‐embedded biopsy specimens with clinically and pathologically confirmed OSF were obtained from the Department of Oral Pathology, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka (Table 1). Six samples of normal oral mucosa (NOM) were used as controls. The study has been approved by an ethical committee from the Institutional Research Board of the Faculty of Dental Sciences, University of Peradeniya, Sri Lanka (No: FDS‐FRC/2014/18).
Table 1.
Clinical information of patients
| Parameters | No. of patients (N = 36) | Without transformation to cancer | With transformation to cancer | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | |||||
| Female | 12 | 9 | 29 | 3 | 60 |
| Male | 24 | 22 | 71 | 2 | 40 |
| Age (years) | |||||
| <50 | 22 | 19 | 61.2 | 3 | 60 |
| ≥50 | 14 | 12 | 38.8 | 2 | 40 |
| Tumor site | |||||
| Buccal mucosa | 32 | 28 | 90.4 | 4 | 80 |
| Lip | 2 | 1 | 3.2 | 1 | 20 |
| Tongue | 1 | 1 | 3.2 | ||
| Undefined | 1 | 1 | 3.2 | ||
| Histology | |||||
| With dysplasia | 12 | 9 | 29 | 3 | 60 |
| Without dysplasia | 24 | 22 | 71 | 2 | 40 |
Hematoxylin and eosin staining
Formalin‐fixed, paraffin‐embedded specimens were cut into 4‐μm‐thick sections and were stained with hematoxylin and eosin for histologic confirmation of clinical diagnosis and to grade epithelial dysplasia. Additional sequential sections were prepared for immunohistochemical studies.
Immunohistochemistry protocol
All 36 cases of OSF were available for high‐quality immunohistochemical staining except one tissue sample in which epithelial tissue was lost for p53 staining. Immunohistochemical staining was performed on 4‐μm‐thick sections using an EnVision‐HRP detection system (Dako, Carpinteria, CA, USA). All of the procedures were performed at room temperature. The sections were deparaffinized through a series of xylene baths and rehydrated in graded concentrations of alcohol. To retrieve antigenicity, the slides were steamed with 10 mM citrate buffer (pH 6.0; Dako REAL™). Tissue sections were treated with 3% hydrogen peroxide to block endogenous peroxidase activity, followed by incubation in 5% bovine serum albumin. The sections were then incubated with primary antibodies diluted with phosphate‐buffered saline for 90 min in a humid chamber. The dilution ratio of each antibody is as follows: Ki67, p53, and c‐Jun were used in 1:100, cyclin D1 in 1:400, IMP3 in 1:50, and β‐catenin in 1:80, respectively, which were purchased from Abcam (Cambridge, UK); p16 and c‐Met were used in 1:50 and 1:200, respectively, which were purchased from (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
The slides were then incubated with secondary EnVision reagent (Dako, Carpinteria, CA, USA) for 30 min, followed by incubation with 3,3‐diaminobenzidine tetrachloride (Dako). Next, the sections were counterstained with Mayer's hematoxylin for visualization. The dilution solution without antibody treatment was applied as the negative control. The expression pattern of OSF was compared with that of NOM.
Scoring system
Immunostained sections for all antibodies were independently studied in detail by three oral pathologists, and the tissue sections were graded as follows:
The expression patterns of NOM served as guidelines for the scoring system. The scoring methods were defined based on different criteria for each antibody depending on the expression pattern and cellular localization of NOM. The expression was scored from 0 to 3. The expression pattern of NOM was given a score 1. Score 0 represented a lower expression than that of NOM; Score 1 represented the same expression pattern as NOM; Score 2 or 3 represented higher expression than that of NOM. The scoring criteria are shown in Table 2.
Table 2.
Evaluation criteria of expression patterns of immunohistochemical staining
| Biomarkers | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Ki67 | Negative or less than NOM | 2–8% immunoreactive cells in NOM | More than 8% | – |
| Cyclin D1 | Negative or less than NOM | 4–9% immunoreactive cells in NOM | – | – |
| p16 | – | 0–5% cytoplasmic and nucleus immunoreactive cells in NOM | More than 5% | – |
| IMP3 | – | Less than 30% of epithelial cells with cytoplasmic and perinuclear immunoreactivity in NOM | 30–60% of epithelial cells with cytoplasmic and perinuclear immunoreactivity in NOM | More than 60% epithelial with cytoplasmic and perinuclear immunoreactivity in NOM |
| β‐Catenin | Positive cells found less than 50% of epithelium membranous immunoreactivity | 50–100% membranous immunoreactivity in NOM | – | – |
| p53 | – | No expression in NOM | Nucleus expression in basal cell layer of epithelium | – |
| c‐Met | – | No expression in NOM | Cytoplasmic expression in basal and suprabasal layer of epithelium | – |
| c‐Jun | Negative | Suprabasal area nuclear expression in NOM | Nucleus expression cells in basal cell layer | – |
NOM, normal oral mucosa.
Statistical analysis
Correlation between the clinical findings of patients and cancer risk was analyzed by Fisher's exact test. We applied Fisher's exact test to compare the biomarkers determining between NOM and OSF, between the absence and presence of epithelial dysplasia and between cancerous transformation and non‐transformation OSFs. Furthermore, discriminant analysis was used to identify a combined biomarker model. The expression of combined biomarker was compared between cancerous transformation and non‐transformation samples by Mann–Whitney U‐test. All of the statistical analyses were performed using statistical software (SPSS ver. 21.0; SPSS Inc., Chicago, IL, USA), and the level of statistical significance was set at P < 0.05.
Results
Characteristics of the patients and oral cancer risk
The medium follow‐up period for the patient population was 6.5 years, with five (13.8%) of 36 patients transformed to invasive carcinoma. The clinicopathological characteristics of the patients are listed in Table 1.
Of 12 cases showing epithelial dysplasia, three were transformed to invasive carcinoma. Using statistical analysis, neither any clinical factor nor the presence of epithelial dysplasia was related to cancerous transformation (data not shown).
Biomarkers for determining NOM and OSF
To identify the biomarkers to differentiate between OSF and NOM, we used Fisher's exact test. The expression of Ki67, cyclin D1, c‐Met, IMP3, and β‐catenin showed a significant difference in the OSF, as compared with the NOM (Table 3 and Fig. 1). To examine the proliferating activity, positive cells for Ki67 and cyclin D1 were counted, and the proliferation index was measured by calculating the percentage of positive cells from the total number of cells. The proliferating indices of cyclin D1 and Ki67 were 4–9% and 2–8% in the NOM, respectively. In contrast, most cases of OSF showed no positive reaction for both biomarkers with the exception that Ki67 expression was found to be increased in OSF cases with malignant transformation (Fig. 1C). The expression of c‐Met and IMP3 showed higher expression in the OSF than in the NOM showing undetectable and weak expression (Fig. 1G–L), whereas β‐catenin showed reduced expression in the OSF, as compared with the NOM (Fig. 1M–O).
Table 3.
Statistical analysis according to differently expressed gene in tissue groups
| Biomarkers expression pattern | Comparison between NOM and OSF | Comparison between with and without dysplasia | Comparison between with and without transformation to cancer |
|---|---|---|---|
| Ki67 | 0.00 | 0.30 | 0.02 |
| Cyclin D1 | 0.00 | 0.60 | 0.40 |
| c‐Met | 0.01 | 0.24 | 0.03 |
| IMP3 | 0.00 | 0.27 | 0.34 |
| β‐Catenin | 0.02 | 1.00 | 0.63 |
| c‐Jun | 0.90 | 0.79 | 0.56 |
| p16 | 0.55 | 0.04 | 0.00 |
| p53 | 0.19 | 0.00 | 0.87 |
NOM, normal oral mucosa; OSF, oral submucous fibrosis; IMP3, insulin‐like growth factor II mRNA‐binding protein 3.
P values from Fisher's exact test are provided.
Figure 1.
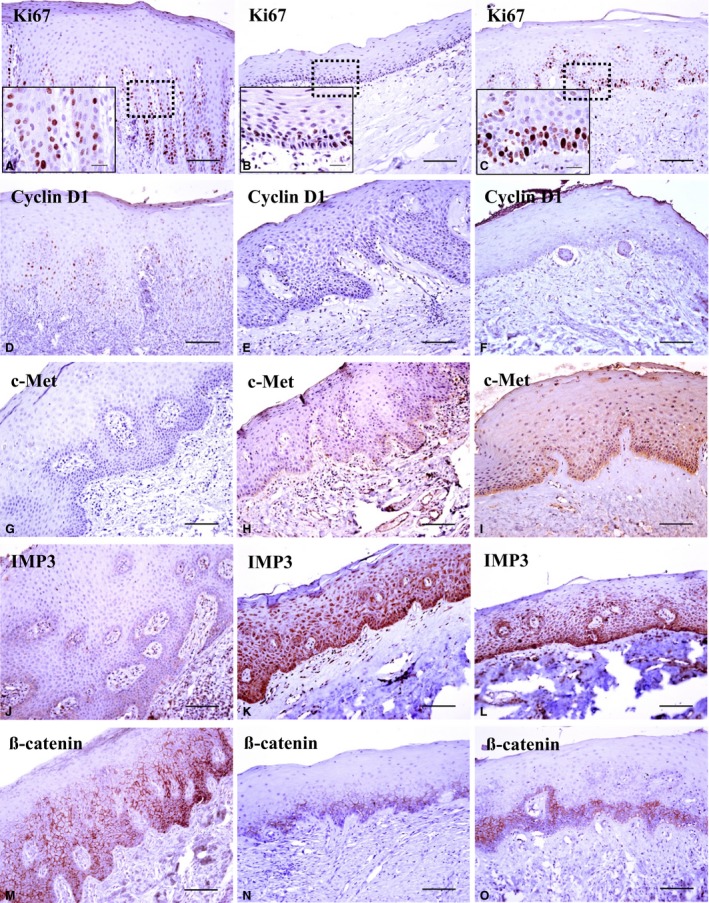
Immunohistochemical expression pattern for each antibody in the normal oral mucosa group (A, D, G, J, M); Oral submucous fibrosis (OSF) samples without cancer transformation (B, E, H, K, N); OSF samples with cancer transformation (C, F, I, L, O). Ki67 (A–C); the dark brown nuclear staining was considered to be positive for Ki67. Ki67 expression reduced in OSF samples without cancer transformation (B), whereas its positivity increased in OSF samples with cancer transformation (C). Cyclin D1 expression (D–F) was reduced in both OSF samples with and without cancer transformation. c‐Met expression (G–I) increased in OSF samples with cancer transformation. IMP3 expression (J–L) increased in both OSF samples with and without cancer transformation. β‐Catenin expression (M–O) reduced in OSF samples with and without cancer transformation. The original magnification of all figures was taken at ×200. Higher magnification (×1000) views of Ki67 expression are presented in the inset micrograph (scale bar of all figures, 50 μm).
Correlation between biomarker expression and epithelial dysplasia
The correlation between the expression of biomarkers and histologic status is shown in Table 3. The biomarkers of p53 and p16 were found to be positively related to epithelial dysplasia in the OSF (P = 0.00 and P = 0.04, respectively). p53 expression showed no positive response in the NOM (Fig. 2D). By contrast, p53 overexpression was found in six of 12 dysplastic samples, regardless of malignant transformation (Fig. 2E,F). p16 expression showed the positivity of less than 5% in the epithelium of NOM samples (Fig. 2A). p16 showed both nuclear and cytoplasmic expression in OSF samples. Particularly, two of three cases of malignant transformed OSF with epithelial dysplasia revealed high p16 expression scored as 2 (Fig. 2C).
Figure 2.
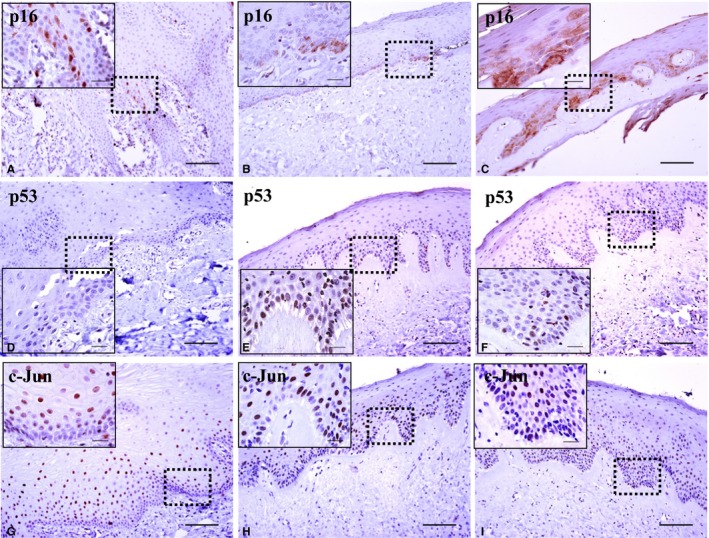
Immunohistochemical expression pattern for each antibody in the normal oral mucosa (NOM) group (A, D, G); Oral submucous fibrosis (OSF) samples without cancer transformation (B, E, H); OSF samples with cancer transformation (C, F, I). p16 expression (A–C) increased in dysplastic OSF samples with cancer transformation (C). p53 expression (D–F) showed no response in NOM samples (D). OSF samples with dysplasia showed a positive reaction regardless of cancer transformation (E, F). c‐Jun expression (G–I) showed no difference between OSF samples with and without cancer transformation (original magnification, ×200; magnification of inset micrographs, ×1000; scale bar of all figures, 50 μm).
Biomarkers for determining cancerous transformed and non‐transformed OSFs
According to the Fisher's exact test, Ki67, c‐Met, and p16 were the most useful biomarkers to determine high‐risk OSF (P = 0.02, P = 0.03, and P = 0.00, respectively) (Table 3).
As described, NOM showed 2–8% of positive activity by Ki67 expression (Fig. 1A). In contrast, 26 (83.9%) of 31 OSF samples without malignant transformation showed negative to markedly reduced expression (Fig. 1B). Of five OSF samples with transformation, two (40%) were scored as 0 and two (40%) were scored as 1. A single case was scored as 2 showing an increased proliferating activity compared with NOM cases (Fig. 1C).
c‐Met expression was undetectable in the NOM (Fig. 1G). Sixteen (51.6%) of 31 OSF samples without transformation exhibited the same expression pattern as that of the NOM and were scored as 1 (Fig. 1H). In contrast, all cases of OSF with transformation showed a definite cytoplasmic staining in the basal and suprabasal layers and were scored as 2 (Fig. 1I).
p16‐positive cells were observed in less than 5% of all epithelium with nuclear and cytoplasmic immunoreactivity in the NOM (Fig. 2A). Surprisingly, all 31 (100%) OSF samples without transformation were observed to have the same expression as NOM and were scored as 1 (Fig. 2B). In contrast, the same expression as NOM was found in three samples (60%) of OSF with transformation, and they were scored as 1. The higher expression was detected in two (40%) OSF samples with transformation than in the NOM and was scored as 2 (Fig. 2C).
Combined biomarker model to predict high‐risk OSF
To improve the prognostic predictability of the biomarkers, we used the combined biomarker model. By discriminant analysis, the combination of the Ki67 and p16 biomarkers was identified to show the highest predictability. The combined expression levels were calculated as 0.688 × Ki67 + 0.888 × p16, and they were significantly different between the groups (P = 0.00108). The combined expression levels were distributed as shown in Fig. 3, and they could be classified into high‐ and low‐risk groups based on the cutoff value of 0.9. Furthermore, the predictive accuracy was 94.4%. The sensitivity and specificity were 0.6 and 1, respectively.
Figure 3.
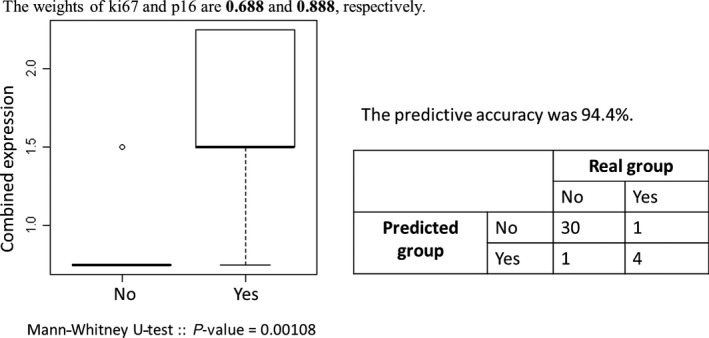
Results of discriminant analysis, Ki67 and p16 were identified as combined biomarkers for the high‐risk oral submucous fibrosis groups.
Discussion
Recent epidemiological data indicate that the OSF prevalence has increased since 2000 (2.42 in 2000 to 6.42/1000/year in 2004) 15. One study recognized that OSCC originating from OSF is clinically more invasive and also exhibits a higher metastasis and recurrence rate than OSCC not originating from OSF 16. Therefore, there has been much focus on investigating biomarkers for the prevention and early detection of carcinomatous transformation. In this study, we examined several biomarkers to investigate the useful biomarkers in predicting oral cancer development in patients with OSF. The selection of biomarkers was based on the understanding of the physiological/pathological features of OSF tissue. For detecting clinically feasible biomarkers in the routine diagnostic level, this study did not include DNA‐targeting methods such as DNA aneuploidy, but it was confined to examine the protein expression by immunohistochemical staining.
We have observed that Ki67, cyclin D1, c‐Met, IMP3, and β‐catenin were found to show significantly different expression between the NOM and the OSF. All of these biomarkers have gained attention as prognostic biomarkers to predict the proliferation activity and transcriptional activity, as indicators for metastasis and poor prognosis and as good targets for therapeutic inhibition. Ki67 and cyclin D1 were used to evaluate the cell proliferation. Ki67 is a nuclear marker expressed in all phases of the cell cycle other than the G0 phase and is widely used as a surrogate marker for proliferation in tumor samples 17, 18. Cyclin D1 modulates cell cycle transition from G1 to S phase and also plays a role in apoptosis 19. In our study, proliferating activity was found to be reduced in OSF samples compared with the NOM through the expression of cyclin D1 and Ki67. Contradictory results to our study were found by the research group of Ranganathan and Kavitha 20 observing that Ki67 expression in the OSF was significantly higher than that of NOM, but less than that of OSCC. This different result may be explained as the proliferating activity of the OSF being largely dependent on the developmental stage. The OSF at the early stage may show a lower proliferating activity, as evidenced by atrophic epithelium. In our study, the proliferation activity of OSF samples with cancerous transformation showed higher proliferating activity than that of the NOM and OSF samples without transformation, suggesting that the switch toward the upregulation of the cell cycle from atrophic epithelium could represent transformation.
Recent studies have shown that IMP3 is an important protein for tumor cell proliferation and invasion, indicating that IMP3 is an oncofetal protein that may play a critical role in malignant transformation and tumor progression 21. IMP3 was found to be expressed in carcinoma lesions and oral leukoplakia with dysplasia 22. Previous studies have suggested that the IMP3 staining pattern should be a useful adjunct in distinguishing benign from malignant squamous epithelia. In our study, IMP3 expression increased in the OSF compared with the NOM, whereas we could not find a clear relationship with malignant transformation or epithelial dysplasia. c‐Met plays a crucial role in morphogenic organization during embryonic development and in the control of the structure and function of adult tissues, including cell migration and proliferation necessary for injury repair 23. Specifically, a significant increase in the expression of c‐Met was noted in the transformation from NOM to epithelial dysplasia and to OSCC 24. In our study, both IMP3 and c‐Met were found to show higher expression in OSF samples than in NOM samples, suggesting that these two proteins may play roles in developing OSF. In addition, β‐catenin signaling has been implicated in promoting many human squamous cell carcinomas 25. β‐Catenin expression was reported to show reduced expression in OSCC 26. In our study, we found the reduced expression of β‐catenin in OSF samples, reflecting the disruption of the E‐cadherin–catenin complex. Taken together, the insulted epithelium by carcinogens, such as areca nut, undergoes genetic alterations, as evidenced by the increased expression of IMP3 and c‐Met and reduced expression of β‐catenin.
Notable biomarkers in our present study were Ki67 and p16, which showed significantly different expression between the transformation and non‐transformation OSFs. According to the discriminant analysis, the combination of Ki67 and p16 was identified to show the highest predictability for high‐risk OSF. Co‐expression of both proteins was determined as the combined biomarker model (P = 0.00108). Accordingly, we propose that these two markers may play a role in predicting malignant transformation and could be used as biomarkers for high‐risk OSF. p16 represents a negative regulator of the cell cycle that ensures the control of the cellular passage from G1 phase to S phase. Mitogenic stimuli, as well as growth factors, determine the activation of cyclin D, resulting in retinoblastoma protein phosphorylation, followed by the release of a transcription factor that ensures cell proliferation 27. The relationship between p16 expression and its biologic behavior has been debatable, although the relationship between human papillomavirus infection and p16 expression has been highly correlated 28. In our study, we demonstrated that p16 positivity could provide evidence for assessing high‐risk OSF. Taken together, the proliferating activity of more than 8% and p16 expression of more than 5% can serve as promising biomarkers to assess the high risk of OSF. Furthermore, the proposed formula and cutoff value is the first trial to predict risk assessment using combined biomarkers in potentially malignant OSF. With the identification of high‐risk patients with OSF, more intensive treatment modalities can be developed, resulting in a reduced incidence of OSCC.
The positive relationship between epithelial dysplasia and malignant transformation in OSF has been investigated 29. In our study, we could not find the positive correlation between epithelial dysplasia and malignant transformation in OSF. This discrepancy can be explained by insufficient number of the study samples and the subjectivity of determining epithelial dysplasia.
Limitations of our current study might be considered as follows. First, only five cases in the OSF with malignant transformation were enrolled in this study. To confirm our data, a prospective clinical study should be conducted. Second, with regard to the multistep carcinogenic process, protein expression may peak at different stages proven by sequential expression of p53 during transformation from precancer to cancer 30. Considering the dynamic process of human disease state, our prediction model with proposed formula should be validated by large‐scale studies. Third, this study did not detect human papillomavirus infection in OSF cases. Further study of the observing expression pattern of markers according to human papillomavirus infection in OSF may contribute to the consolidation of our results for clinical application.
Conflict of interest
All of the authors have declared no conflicts of interest.
Acknowledgements
We thank Mr. Priyanka Bandara Tennakoon and Ms. Inoka Krishanthi Rambukewela, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka, for preparing the slide sections from human samples. This work was supported by the S & T Support Program for Developing Countries funded by the Ministry of Sciences, ICT, and Future plan (2013K1A3A9A01044071) in Korea.
J Oral Pathol Med (2017) 46: 431–438
Contributor Information
Wanninayake M Tilakaratne, Email: wmtilak@pdn.ac.lk.
Jin Kim, Email: jink@yuhs.ac.
References
- 1. Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol 2006; 42: 561–8. [DOI] [PubMed] [Google Scholar]
- 2. Arakeri G, Brennan PA. Oral submucous fibrosis: an overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg 2013; 51: 587–93. [DOI] [PubMed] [Google Scholar]
- 3. Angadi PV, Rekha KP. Oral submucous fibrosis: a clinicopathologic review of 205 cases in Indians. Oral Maxillofac Surg 2011; 15: 15–9. [DOI] [PubMed] [Google Scholar]
- 4. Sumeth Perera MW, Gunasinghe D, Perera PA, et al. Development of an in vivo mouse model to study oral submucous fibrosis. J Oral Pathol Med 2007; 36: 273–80. [DOI] [PubMed] [Google Scholar]
- 5. Li N, Jian X, Hu Y, Xu C, Yao Z, Zhong X. Discovery of novel biomarkers in oral submucous fibrosis by microarray analysis. Cancer Epidemiol Biomarkers Prev 2008; 17: 2249–59. [DOI] [PubMed] [Google Scholar]
- 6. Chang MC, Lin LD, Wu HL, et al. Areca nut‐induced buccal mucosa fibroblast contraction and its signaling: a potential role in oral submucous fibrosis – a precancer condition. Carcinogenesis 2013; 34: 1096–104. [DOI] [PubMed] [Google Scholar]
- 7. Carlos de Vicente J, Herrero‐Zapatero A, Fresno MF, Lopez‐Arranz JS. Expression of cyclin D1 and Ki‐67 in squamous cell carcinoma of the oral cavity: clinicopathological and prognostic significance. Oral Oncol 2002; 38: 301–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhang SY, Klein‐Szanto AJ, Sauter ER, et al. Higher frequency of alterations in the p16/CDKN2 gene in squamous cell carcinoma cell lines than in primary tumors of the head and neck. Cancer Res 1994; 54: 5050–3. [PubMed] [Google Scholar]
- 9. Cairns P, Polascik TJ, Eby Y, et al. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet 1995; 11: 210–2. [DOI] [PubMed] [Google Scholar]
- 10. Cruz I, Napier SS, van der Waal I, et al. Suprabasal p53 immunoexpression is strongly associated with high grade dysplasia and risk for malignant transformation in potentially malignant oral lesions from Northern Ireland. J Clin Pathol 2002; 55: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez‐Moles MA, Ruiz‐Avila I, Gil‐Montoya JA, Plaza‐Campillo J, Scully C. β‐Catenin in oral cancer: an update on current knowledge. Oral Oncol 2014; 50: 818–24. [DOI] [PubMed] [Google Scholar]
- 12. Lee SS, Tsai CH, Tsai LL, Chou MC, Chou MY, Chang YC. β‐Catenin expression in areca quid chewing‐associated oral squamous cell carcinomas and upregulated by arecoline in human oral epithelial cells. J Formos Med Assoc 2012; 111: 194–200. [DOI] [PubMed] [Google Scholar]
- 13. Laser‐Azogui A, Diamant‐Levi T, Israeli S, Roytman Y, Tsarfaty I. Met‐induced membrane blebbing leads to amoeboid cell motility and invasion. Oncogene 2014; 33: 1788–98. [DOI] [PubMed] [Google Scholar]
- 14. Lu D, Yang X, Jiang NY, et al. IMP3, a new biomarker to predict progression of cervical intraepithelial neoplasia into invasive cancer. Am J Surg Pathol 2011; 35: 1638–45. [DOI] [PubMed] [Google Scholar]
- 15. Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN. Oral submucous fibrosis: study of 1000 cases from central India. J Oral Pathol Med 2007; 36: 12–7. [DOI] [PubMed] [Google Scholar]
- 16. Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis 2004; 19: 251–62. [DOI] [PubMed] [Google Scholar]
- 17. Pich A, Chiusa L, Navone R. Prognostic relevance of cell proliferation in head and neck tumors. Ann Oncol 2004; 15: 1319–29. [DOI] [PubMed] [Google Scholar]
- 18. Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011; 103: 1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dionne KR, Warnakulasuriya S, Zain RB, Cheong SC. Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer 2015; 136: 503–15. [DOI] [PubMed] [Google Scholar]
- 20. Ranganathan K, Kavitha R. Proliferation and apoptosis markers in oral submucous fibrosis. J Oral Maxillofac Pathol 2011; 15: 148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan RH, Wang CC, Chou CC, Chang KJ, Lee PH, Jeng YM. Diffuse expression of RNA‐binding protein IMP3 predicts high‐stage lymph node metastasis and poor prognosis in colorectal adenocarcinoma. Ann Surg Oncol 2009; 16: 1711–9. [DOI] [PubMed] [Google Scholar]
- 22. Li HG, Han JJ, Huang ZQ, Wang L, Chen WL, Shen XM. IMP3 is a novel biomarker to predict metastasis and prognosis of tongue squamous cell carcinoma. J Craniofac Surg 2011; 22: 2022–5. [DOI] [PubMed] [Google Scholar]
- 23. Brusevold IJ, Soland TM, Khuu C, Christoffersen T, Bryne M. Nuclear and cytoplasmic expression of Met in oral squamous cell carcinoma and in an organotypic oral cancer model. Eur J Oral Sci 2010; 118: 342–9. [DOI] [PubMed] [Google Scholar]
- 24. Chen YS, Wang JT, Chang YF, et al. Expression of hepatocyte growth factor and c‐met protein is significantly associated with the progression of oral squamous cell carcinoma in Taiwan. J Oral Pathol Med 2004; 33: 209–17. [DOI] [PubMed] [Google Scholar]
- 25. Bian YS, Osterheld MC, Bosman FT, Fontolliet C, Benhattar J. Nuclear accumulation of beta‐catenin is a common and early event during neoplastic progression of Barrett esophagus. Am J Clin Pathol 2000; 114: 583–90. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka N, Odajima T, Ogi K, Ikeda T, Satoh M. Expression of E‐cadherin, alpha‐catenin, and beta‐catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br J Cancer 2003; 89: 557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pande P, Mathur M, Shukla NK, Ralhan R. pRb and p16 protein alterations in human oral tumorigenesis. Oral Oncol 1998; 34: 396–403. [DOI] [PubMed] [Google Scholar]
- 28. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol 2014; 32: 3930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res 1984; 92: 224–9. [DOI] [PubMed] [Google Scholar]
- 30. Murti PR, Warnakulasuriya KA, Johnson NW, et al. p53 expression in oral precancer as a marker for malignant potential. J Oral Pathol Med 1998; 27: 191–6. [DOI] [PubMed] [Google Scholar]


