Abstract
Background
Anemia has been associated with adverse cerebrovascular outcomes, particularly after cardiac operations. This study was conducted to determine if hemoglobin levels during and after cardiopulmonary bypass (CPB) predict postoperative stroke in cardiac surgical patients, independent of transfusion requirements.
Methods
Individuals who had sustained a clinical postoperative stroke (cases) were matched 1:2 with controls by age, sex, surgical procedure, and year of operation. In 617 patients, conditional logistic regression was performed to analyze associations between hemoglobin levels before and after CPB, and stroke.
Results
After adjustment for potentially confounding vascular risk factors, lower hemoglobin after CPB was associated with a higher risk of stroke, even after adjustment for the amount of packed red blood cells transfused (adjusted odds ratio, 1.28; 95% confidence interval, 1.00 to 1.64, per point of lower hemoglobin level; adjusted odds ratio for stroke per higher quartile of packed red blood cells transfused in this model, 1.37; 95% confidence interval, 1.02 to 1.83). Similar associations were not found for hemoglobin concentrations preoperatively nor change in hemoglobin from before to after CPB. A post-CPB hemoglobin level below the median was associated with 37% increased odds of a postoperative stroke occurring (p = 0.02).
Conclusions
Lower postoperative hemoglobin levels and higher intraoperative transfusion requirements are each independently associated with a higher risk of stroke. Reduced cerebral oxygen delivery due to anemia may contribute to cerebral injury after coronary artery bypass grafting.
Individuals who undergo cardiac operations are often exposed to large fluctuations in blood volume, both as a result of blood loss and infusion of large volumes of fluid. Recent literature has focused on anemia as a potential marker of adverse neurologic outcomes, but the nature of this relationship, and whether it is purely a marker of poor outcomes or is a cause of these outcomes is still not well understood [1].
Preoperative anemia has been associated with adverse outcomes after cardiac operations. Individuals who are anemic preoperatively have an increased risk of postoperative kidney failure [2] and death, among other postoperative complications [3, 4], although other studies have failed to find similar results [5]. Some studies have emphasized the importance of the preoperative hemoglobin level [6] in predicting postoperative complications, with others emphasizing the nadir hematocrit level [7] as being the most important. In other studies, a low hematocrit during cardiopulmonary bypass (CPB) has been associated with higher odds of postoperative stroke [1].
In this case-control study of cardiac surgical patients, we compared hemoglobin levels before and after CPB in individuals with and without stroke. We hypothesized that hemoglobin level would be a predictor of postoperative stroke and specifically hypothesized that change in hemoglobin would predict postoperative stroke more strongly than either value at the start or the end of CPB.
Material and Methods
Since 1992, all individuals undergoing cardiac operations at Johns Hopkins Hospital have been monitored for the development of any new neurologic deficits, including stroke. In patients in whom a new postoperative neurologic deficit was identified, a neurologic consultation was requested. If this consultant diagnosed a stroke, this patient and the diagnosis were entered into the Cardiac Surgery Stroke database, from which “cases” were selected. Controls were patients not in the stroke database, but in the Cardiac Surgery database, which includes all individuals who have undergone cardiac operations at our institution. These controls were matched 2:1 to the cases for a 20-year age band, sex, nature of operation, and year of the procedure. An individual could be the control for more than one case. Controls were available for all cases from the past 5 years (from a total of 5,279 individuals undergoing cardiac operations at our institution during that time period). The study was approved by the Institutional Review Board.
Records were reviewed for 689 patients (cases and controls) who had undergone any form of cardiac operation with CPB at Johns Hopkins Hospital from 2001 to 2006. Demographics, vascular history (peripheral vascular disease, myocardial infarction, prior stroke or transient ischemic attack, diabetes mellitus, hypercholesterolemia, morbid obesity, smoking history, and hypertension), and intraoperative factors (CPB time) were recorded from hospital records, perfusion records, and electronic databases.
Statistical Analyses
The primary independent variable was hemoglobin level before the initiation of CPB (pre-CPB), after stopping CPB (post-CPB), and the difference between the pre-CPB and post-CPB values. Hemoglobin level was also dichotomized at its median (8.8 g/dL), for separate analyses. Descriptive statistics were completed using χ2 statistics for categoric variables and t tests for continuous variables. Potential confounders (see covariates listed above) were included in the multivariate model; a backwards stepwise regression was used with a significance level of p < 0.10 for inclusion. Age was included both as a linear and quadratic variable. Conditional logistic regression was used to determine the independent effects of these variables on case status (stroke vs no stroke). Model goodness-of-fit of the standard logistic regression models of the variables was checked using the Hosmer-Lemeshow statistic.
Separate models were created analyzing the association between stroke status and (1) pre-CPB hemoglobin, (2) post-CPB hemoglobin, (3) hemoglobin level change, and (4) below vs above the median hemoglobin values, each. All models were repeated with and without adjustment for number of packed red blood cells (pRBC) transfused during the operation. Number of pRBC transfused was categorized into quartiles as 0 to 1 units, 2 to 3 units, 4 units, or 5 or more units. Stata 10.0 software (StataCorp, College Station, TX) was used for the analyses.
Secondary Analyses
Anemia was defined based on pre-CPB values (<12 g/dL for women, <13 g/DL for men), and analyzed as a predictor of stroke. In addition, hemoglobin values were analyzed both as linear and nonlinear predictors. Finally, the lower of the two hemoglobin values (before and after CPB) was used as a predictor in a secondary analysis.
Results
The study consisted originally of 689 patients; after exclusions, 617 were available for our final analyses. Individuals were excluded if they were missing hemoglobin level data or had no available control with complete hemoglobin data (29 were missing pre-CPB hemoglobin values, with 14 individuals missing post-CPB hemoglobin values). For the conditional logistic regression, only cases with at least one corresponding control, and only controls with a corresponding case were included. An additional 29 individuals were excluded because either the case or both controls were missing hemoglobin data, leaving 617 individuals in whom a primary univariate case-control analysis of hemoglobin level could be analyzed: 213 cases and 404 controls. Some covariate information was missing on more individuals, so numbers available for the full multivariate regression were smaller (see numbers listed in data tables). Demographic information is reported in Table 1.
Table 1.
Demographics and Medical History of 617 Patients, Comprising 213 Cases and 404 Controlsa
| Cases (stroke) (n = 213) | Controls (no stroke) (n = 404) | p Valueb | |
|---|---|---|---|
| Sex, % male | 60.1 | 59.9 | 0.96 |
| Mean ± SD age, years | 64.9 ± 13.3 | 64.8 ± 13.3 | 0.93 |
| Hypertension, % | 75 | 68.2 | 0.12 |
| Diabetes mellitus, % | 28.7 | 26.9 | 0.63 |
| Hypercholesterolemia, % | 53.8 | 57.6 | 0.42 |
| Peripheral vascular disease, % | 18.4 | 9.3 | 0.001 |
| Myocardial infarction, % | 35.9 | 31.3 | 0.25 |
| COPD, % | 10.3 | 7.4 | 0.22 |
| Obesity, % | 11.9 | 14.4 | 0.41 |
| Smoking, % | 40.6 | 47.5 | 0.16 |
| Prior stroke, % | 27.4 | 18.8 | 0.016 |
| Family history of CAD, % | 33 | 30.9 | 0.63 |
| Type of operation, % | |||
| Redo CABG | 3.3 | 3 | 0.83 |
| CABG (including redo) | 34.7 | 34.7 | 0.98 |
| Aortic | 21.6 | / | … |
| Valve | 14.1 | 14.9 | … |
| CABG + valve | 16.4 | 16.8 | … |
| CABG + other operation | 6.1 | 6.2 | … |
| Other operation | 7 | 5.5 | … |
| Pre-op MAP, mean ± SD, mm Hg | 92.6 ± 16.3 | 90.4 ± 15.6 | 0.1 |
| CPB, mean ± SD, min | 136.7 ± 61.5 | 125.1 ± 58.1 | 0.02 |
Controls were matched based on sex, 20-year age band, type of surgical procedure, and year of procedure.
The p values are calculated by Pearson χ2 for categoric variables and by t test for continuous variables. All of these variables, with the exception of sex and operation type (because controls were matched on these factors) were entered into the multivariate models.
CABG = coronary artery bypass grafting; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disorder; CPB = cardiopulmonary bypass; MAP = mean arterial pressure; SD = standard deviation.
Univariate Analyses
The average pre-CPB hemoglobin values were higher than post-CPB values (11.2 vs 8.8 g/dL, p < 0.0001). Mean hemoglobin level pre-CPB was 11.1 g/dL in cases and 11.3 g/dL in controls. In univariate analyses, pre-CPB hemoglobin was not associated with presence or absence of stroke (odds ratio [OR], 1.08 per 1-point [g/dL] lower hemoglobin value; 95% confidence interval [CI], 0.99 to 1.18). Hemoglobin values post-CPB averaged 8.7 g/dL in cases and 9.0 g/dL in controls (Table 2). The univariate OR for stroke associated with each 1-point decrease (g/dL) in post-CPB hemoglobin level was 1.34 (95% CI, 1.14 to 1.57). Additionally, change in hemoglobin level was not associated with case status (OR, 1.02; 95% CI, 0.94 to 1.12, per 1-point larger change in hemoglobin level). All hemoglobin values (pre-CPB, post-CPB, and hemoglobin difference) were in the direction of lower values or a larger drop in hemoglobin in the cases.
Table 2.
Hemoglobin Variables for Cardiopulmonary Bypass
| Variable | Cases (stroke)
|
Controls (no stroke)
|
p Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | ||
| Hemoglobin level | |||||||
| Pre-CPB | 11.1 | 4 to 16.2 | 2.08 | 11.4 | 5.7 to 17.3 | 1.93 | 0.08 |
| Post-CPB | 8.7 | 4 to 11 | 1.09 | 9.0 | 5 to 12 | 1.09 | 0.0002 |
| Difference | 2.4 | −3 to 7 | 1.97 | 2.4 | −4.1 to 10.2 | 1.94 | 0.75 |
CPB = cardiopulmonary bypass; SD = standard deviation.
In the univariate analysis of the effect of pRBC transfusion on case status, each higher quartile of pRBC transfused was associated with a 1.36-times higher odds (95% CI, 1.14 to 1.63) of postoperative stroke. Each unit of pRBC was associated with an unadjusted 1.16-times higher odds of stroke (95% CI, 1.07 to 1.25).
Multivariate Analyses
A stepwise multivariate conditional logistic regression analysis was performed using the variables listed in Table 1. The final multivariate analysis included variables that, in a stepwise regression, were significant at p < 0.10 (Table 3): first (model 1), without inclusion of transfusion, and second (model 2), with inclusion of pRBC transfusion quartile. The association between post-CPB hemoglobin level and stroke is partially attenuated, but the association remains significant (p < 0.05). Quartile of pRBC transfused remains significant in all models. Results were similar when number of units transfused was entered instead of pRBC quartile.
Table 3.
Results of the Multivariate Conditional Logistic Regression Analysis Using Different Hemoglobin Values as Predictors
| Variable | Model 1b (n = 379) | Model 2c (n = 292) |
|---|---|---|
| Hemoglobin levela | OR (95% CI) | OR (95% CI) |
| A. Pre-CPB (per g/dL decrease) | 1.12 (0.99–1.27) | 1.08 (0.92–1.25) |
| pRBC quartile | 1.37 (1.01–1.84) | |
| B. Post-CPB (per g/dL decrease) | 1.41 (1.13–1.75) | 1.28 (1.00–1.64) |
| pRBC quartile | 1.37 (1.02–1.83) | |
| C. Difference (per 1 g/dL larger diff) | 0.98 (0.87–1.11) | 0.96 (0.84–1.10) |
| pRBC quartile | 1.66 (1.26–2.18) | |
| D. Post-CPB below median vs above median | 1.86 (1.17–2.96) | 1.85 (1.07–3.18) |
| pRBC quartile | 1.39 (1.05–1.86) |
(A) hemoglobin 1; (B) hemoglobin 2; (C) [hemoglobin 1 – hemoglobin 2]; and (D) post-CPB hemoglobin dichotomized at its median value (8.8 g/dL), comparing those below the median with those above the median.
Variables with p < 0.1 in stepwise logistic regression without effect of transfusion: A, B, and D: peripheral vascular disease, CPB, preoperative mean arterial pressure in mm Hg; C: same but also history of obesity.
Same variables entered into model, plus quartile of number of units of pRBC transfused as a covariate. Variables included in final models (p < 0.1 in each stepwise regression): age, squared age, CPB, history of peripheral vascular disease, preoperative mean arterial pressure.
CI = confidence interval; CPB = cardiopulmonary bypass; OR = odds ratio; pRBC = packed red blood cells.
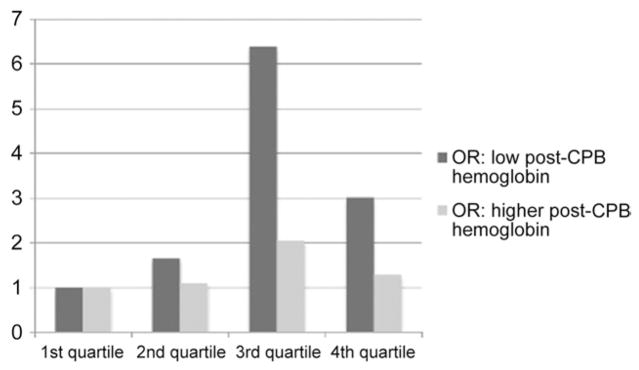
When effect of transfusion quartile on postoperative stroke was stratified depending on post-CPB hemoglobin level, increasing transfusion requirement was a stronger predictor of stroke when post-CPB hemoglobin level was below the median (unadjusted OR, 1.59; 95% CI, 1.09 to 2.32, per increasing quartile of RBC transfusion) than when it was above the median (unadjusted OR, 1.15; 95% CI, 0.80 to 1.65; Fig 1). When the inclusion cutoff for remaining in the conditional logistic regression model was liberalized to p < 0.2, the adjusted OR per increasing quartile of RBC transfusion was 2.79 (95% CI, 1.27 to 6.17) when the post-CPB hemoglobin level was below the median but was not associated (OR, 0.93; 95% CI, 0.41 to 2.12) when the post-CPB hemoglobin level was above the median. The interaction term for transfusion quartile X post-CPB above or below its median value neared but did not reach statistical significance (adjusted p = 0.057). Transfusion requirements were, however, higher in individuals with lower post-CPB hemoglobin levels (3.3 pRBC in those with post-CPB below the median vs 2.9 in those with post-CPB levels above the median).
Fig. 1.
Odds ratios (OR) for postoperative stroke based on quartile of transfusion, comparing individuals with postcardiopulmonary bypass (CPB) hemoglobin levels below (dark bars) and above (light bars) the median.
Secondary Analyses
Although all individuals would have been considered anemic after CPB, 72.1% (72.8% of men and 71.8% of women) were anemic at the start of the operation by standard definitions. Presence of pre-CPB anemia was not associated with postoperative stroke in the absence of transfusion as a covariate (adjusted OR, 1.21; 95% CI, 0.73 to 2.01) or when pRBC transfusion quartile was included as a covariate (adjusted OR, 1.32; 95% CI, 0.70 to 2.50).
Analyzing hemoglobin with nonlinear techniques (eg, cubic splines) did not improve the models.
Lowest Hemoglobin Level as Predictor
When the lower of the two hemoglobin values (before or after CPB) was used as the predictor, quartile of pRBC transfusion remained significant (adjusted OR, 1.36; 95% CI, 1.01 to 1.81), but this new variable, “lower” hemoglobin, did not (adjusted OR, 1.26; 95% CI, 0.99 to 1.60).
Comment
This single-institution retrospective study found that lower post-CPB hemoglobin levels and amount of transfusion during the operation were each independently associated with a higher risk of stroke after cardiac operations. Pre-CPB hemoglobin levels were not similarly associated, nor was pre-CPB to post-CPB change in hemoglobin levels. Our analytic technique, which used a case-control study design, thus decreasing potential confounding from time, age, and surgical procedure effects, strengthens this relationship. We adjusted for other variables with a role in the relationship between hemoglobin level and stroke to minimize the likelihood of this association being due to a confounder. Previous studies have emphasized the importance of hemoglobin values from before or during CPB in predicting postoperative outcome (including stroke), but our results emphasize that the degree of anemia experienced at the end of CPB may be at least as important, if not more, in predicting stroke, and that this appears to be independent of transfusion.
Our study found that transfusion was an important predictor of stroke and attenuated the relationship between post-CPB hemoglobin and stroke but did not eliminate the association. There was a suggestion that transfusion was a stronger predictor of stroke when the post-CPB hemoglobin level was below the median. Although having lower post-CPB hemoglobin levels is certainly associated with higher transfusion requirements, the combination of the two may be more important. This may be because individuals with low post-CPB hemoglobin values who have also received a higher amount of transfused units of pRBC actually had an even lower hemoglobin nadir during the operation.
A low hemoglobin level may be important in risk for stroke because of resultant cerebral hypoperfusion resulting directly from the low hemoglobin and reduced cerebral oxygenation, or because it may be a reflection of a higher acuity patient, with the need for more transfusions, [8] or a more complicated operative course. Similarly, it may reflect hemodilution from bypass. The typical response to anemia is an increase in cardiac output, with additional cerebral vasodilation in an attempt to maintain adequate cerebral blood flow and oxygen transport [9]. Ultimately, these compensations may fail and ischemia can result.
We hypothesize that inadequate oxygen transport might be the mechanism of cerebral injury resulting from intraoperative anemia. Our results showed a relatively modest association, however, and thus do not strongly support a particular mechanism of injury. We hypothesize, however, that a relatively small injury, whether from embolization or hypoperfusion, might be associated with a surrounding ischemic penumbra of at-risk brain tissue, and the presence of lower hemoglobin levels would be associated with expansion of the infarct. Many very small embolic lesions to the brain might not be clinically detected, but if these infarcts expand, they might be associated with clinical symptoms and thus be clinically identified as strokes in our sample.
Our findings were in contrast to prior findings [5] emphasizing the importance of change from baseline of hemoglobin values in predicting postoperative complications. Similarly, a number of studies have identified the importance of intraoperative hemoglobin values, or extent of hemodilution, in predicting risk of major postoperative complications, including renal failure [10, 11]. One study demonstrated that patients maintained during CPB at a hematocrit of less than 24% had a greater likelihood of renal injury and failure, and the effects were even more severe with intraoperative transfusion and prolonged CPB [12]. Similarly, a study by Swaminathan and colleagues [13] concluded that lower intraoperative hematocrit levels might contribute to postoperative renal injury after cardiac operations.
Independent of the hemoglobin level, the number of pRBC transfused was associated with a higher risk of postoperative stroke. Determining the exact relationship between hemoglobin level and transfusion in the association with adverse outcomes is difficult. Most other studies assessing the role of transfusion during cardiac operations use transfusion as a surrogate for blood loss and thus identify adverse outcomes associated with transfusion [14, 15]. This may partially explain our results as well, but our finding that hemoglobin level is associated with stroke independent of transfusion amount suggests this is not the full nature of the relationship. Transfusions are associated with increased death after cardiac operations [16] and in nonsurgical populations [17]. Data are still lacking on the utility of pRBC transfusion in individuals with otherwise equivalent operative courses or hematocrit values.
The formal recommendations of the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists include routine transfusion at a hemoglobin level below 7 g/dL [18]. When we analyzed this cutoff as a predictor for stroke, although the odds for stroke was increased among individuals with a hemoglobin level (either pre-CPB or post-CPB) below 7, it was nonsignificant based on small numbers of individuals with values in this range, probably because transfusing to a higher level is already part of standard practice.
In a small randomized trial of 54 coronary artery bypass grafting patients, in which individuals received transfusions to maintain a hematocrit above 25% vs 20%, no differences in clinical outcomes nor oxygen delivery were found [19]. In another study of 428 coronary artery bypass graft patients randomized to transfusion for goal hemoglobin exceeding 8 g/dL vs exceeding 9 g/dL, clinical outcomes and mortality did not differ between the groups [8].
Only one study to our knowledge, The Erythropoietin NeuroProtective Effect: Assessment in CABG Surgery trial, in which patients were given erythropoietin preoperatively rather than transfusions, suggested improved postoperative neurologic outcomes, with slightly lower rates of neurocognitive dysfunction at 2 months postoperatively, among those individuals given the study drug compared with placebo [20]. This is one of very few published studies showing that correction of a low hemoglobin level might actually reduce adverse postoperative outcomes. Johnson and colleagues randomized patients into two different blood transfusion groups, one “liberal” (goal hematocrit > 32%) and the other “conservative” (goal hematocrit > 25%), and found no difference in adverse outcomes between these two groups [21].
Our study has several limitations. Potential unmeasured confounders could have influenced our results. Low postoperative hemoglobin and higher transfusion requirements may both be markers of sicker patients or of more complex surgical courses. In addition, the low hemoglobin values could reflect a greater need for fluid resuscitation, as might occur for an individual undergoing hemodynamic instability, such as a drop in blood pressure, which could be associated with certain types of strokes [22]. The stroke patients also had longer CPB times, which are associated with more hemodilution and therefore lower hemoglobin values (although duration of CPB time was adjusted for in the multivariate analysis).
Another limitation is the definition of strokes. These were all defined clinically, and patients were only referred for a postoperative neurologic assessment if clinical symptoms suggested a neurologic complication. It is likely that our stroke ascertainment was not complete, and it is also possible that some of the controls actually had some neurologic injury or even undiagnosed stroke.
Because transfusion is such an important factor in any analysis of the association between hemoglobin level and stroke, any limitations in data pertaining to transfusion will limit our primary results. We only included analysis of transfusion during the procedure itself. This does not fully account for the whole time range of potential neurologic injury: the early postoperative period, when individuals are often unstable and experiencing bleeding complications in the recovery room or intensive care unit, is a likely time when transfusion might be related to postoperative stroke. In addition, more hemoglobin level fluctuations might take place beyond what is reflected in two values, one before and one after CPB. Further studies will need to examine these two aspects (transfusion and the range of hemoglobin levels) in more detail to elucidate this association.
Finally, our sample size was relatively modest, so we may not have had the power to detect associations between the preoperative hemoglobin or change in hemoglobin levels and stroke. Larger prospective studies including standardized neurologic assessment, as well as neuroimaging, would help address some of these concerns.
Despite these limitations, our study is strengthened by the matching of our controls. Owing to the large volume of cardiac operations done at our institution, we were able to match by specific procedure and year of procedure. In addition, the cardiac anesthesiologists record hemoglobin values before and after CPB. These values were directly recorded from the anesthesiologists’ and perfusionists’ paperwork to assure comparability. Our use of multivariate adjustment also accounts for many major confounders, minimizing the likelihood of bias accounting for the results.
In conclusion, our findings suggest that cardiac surgical patients with lower post-CPB hemoglobin values have a greater likelihood of postoperative stroke. Each percentage drop in post-CPB hemoglobin level was independently associated with higher odds of postoperative stroke, independent of transfusion requirements. Further studies are needed to determine whether interventions to raise postoperative hemoglobin levels could reduce stroke rates and whether similar relationships are present with other forms of neurologic injury. In addition, studies with an emphasis on neuroimaging to further elucidate the mechanism of the injury associated with lower hemoglobin levels are needed.
Acknowledgments
This study was supported by National Institutes of Health grant RO1-NS035610 (GM McKhann) and the Dana Foundation (GM McKhann). We would like to acknowledge the assistance of the Cardiac Surgical Intensive Care Unit nursing staff for their assistance with data collection and their unwavering support of our patient population.
References
- 1.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–7. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 2.De Santo L, Romano G, Della Corte A, et al. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg. 2009;138:965–70. doi: 10.1016/j.jtcvs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–9. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 4.Surgenor SD, Kramer RS, Olmstead EM, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108:1741–6. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- 5.Bell ML, Grunwald GK, Baltz JH, et al. Does preoperative hemoglobin independently predict short-term outcomes after coronary artery bypass graft surgery? Ann Thorac Surg. 2008;86:1415–23. doi: 10.1016/j.athoracsur.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 6.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, van Rensburg A, Beattie WS. The influence of baseline hemoglobin concentration on tolerance of anemia in cardiac surgery. Transfusion. 2008;48:666–72. doi: 10.1111/j.1537-2995.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 7.Ranucci M, Conti D, Castelvecchio S, et al. Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. Ann Thoracic Surgery. 2010;89:11–7. doi: 10.1016/j.athoracsur.2009.07.078. [DOI] [PubMed] [Google Scholar]
- 8.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–7. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 9.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89. doi: 10.1186/cc7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 11.Karkouti K, Beattie WS, Wijeysundera DN, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005;129:391–400. doi: 10.1016/j.jtcvs.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Habib RH, Zacharias A, Schwann TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33:1749–56. doi: 10.1097/01.ccm.0000171531.06133.b0. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. 2003;76:784–91. doi: 10.1016/s0003-4975(03)00558-7. [DOI] [PubMed] [Google Scholar]
- 14.Whitson BA, Huddleston SJ, Savik K, Shumway SJ. Bloodless cardiac surgery is associated with decreased morbidity and mortality. J Card Surg. 2007;22:373–8. doi: 10.1111/j.1540-8191.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 15.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culiford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 16.Kuduvalli M, Oo AY, Newall N, et al. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27:592–8. doi: 10.1016/j.ejcts.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: The Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists Clinical Practice Guideline. Ann Thorac Surg. 2007;83(5 suppl 1):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 19.von Heymann C, Sander M, Foer A, et al. The impact of an hematocrit of 20% during normothermic cardiopulmonary bypass for elective low risk coronary artery bypass graft surgery on oxygen delivery and clinical outcome—a randomized controlled study [ISRCTN35655335] Crit Care. 2006;10:R58. doi: 10.1186/cc4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haljan G, Maitland A, Buchan A, et al. The Erythropoietin NeuroProtective Effect: Assessment in CABG Surgery (TENPEAKS): a randomized, double-blind, placebo controlled, proof-of-concept clinical trial. Stroke. 2009;40:2769–75. doi: 10.1161/STROKEAHA.109.549436. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RG, Thurer RL, Kruskall MS, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg. 1992;104:307–14. [PubMed] [Google Scholar]
- 22.Gottesman RF, Sherman PM, Grega MA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306–11. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]