Abstract
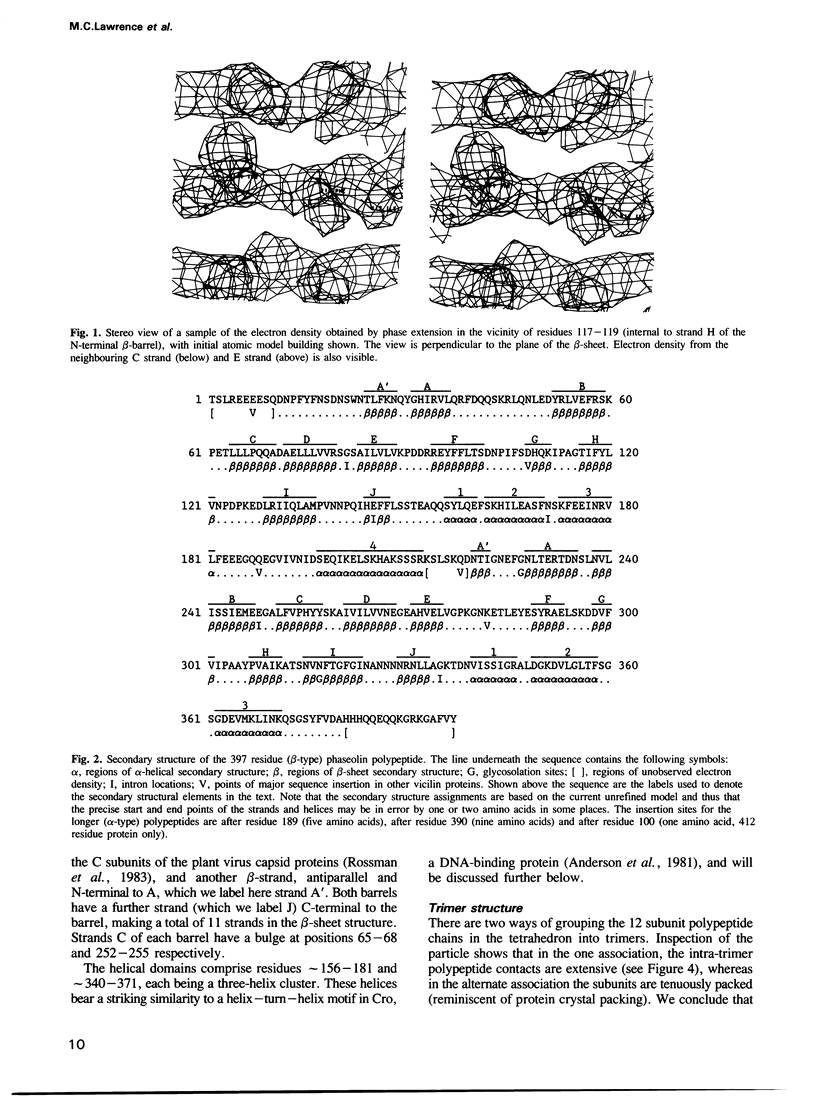
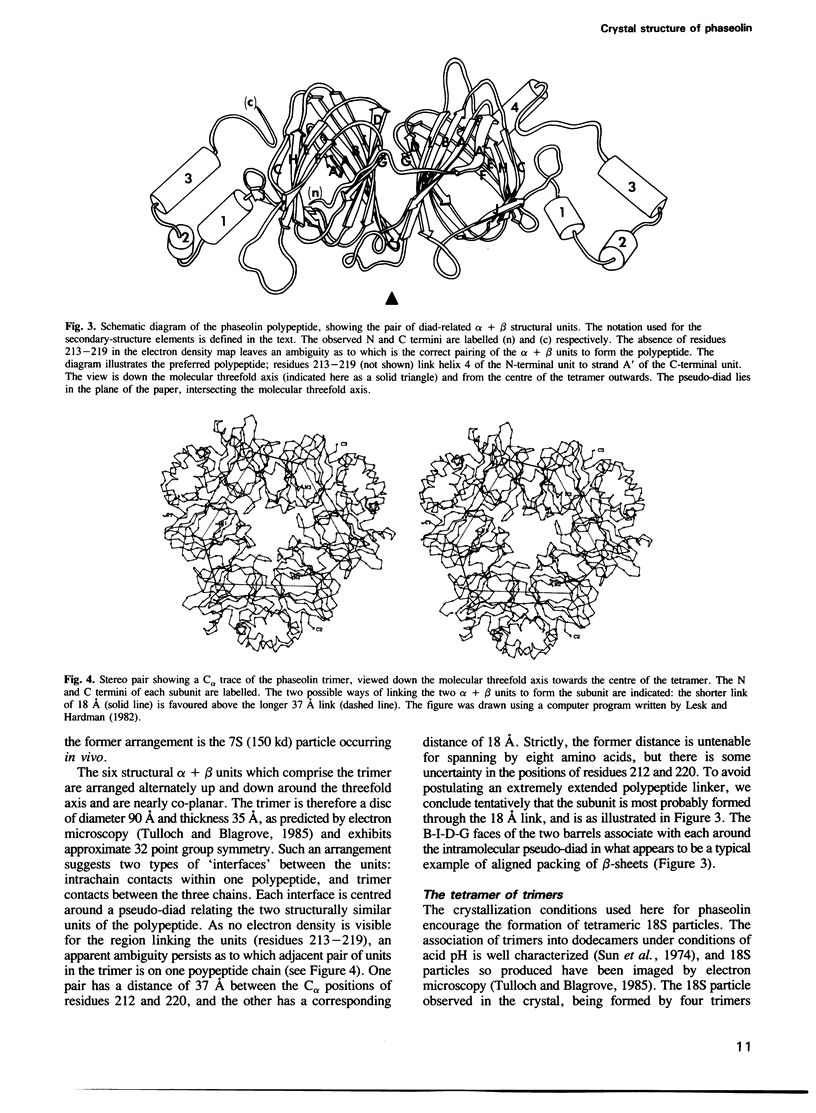
The polypeptides of the trimeric seed storage protein phaseolin comprise two structurally similar units each made up of a beta-barrel and an alpha-helical domain. The beta-barrel has the 'jelly-roll' folding topology of the viral coat proteins and the alpha-helical domain shows structural similarity to the helix-turn-helix motif found in certain DNA-binding proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Ohlendorf D. H., Takeda Y., Matthews B. W. Structure of the cro repressor from bacteriophage lambda and its interaction with DNA. Nature. 1981 Apr 30;290(5809):754–758. doi: 10.1038/290754a0. [DOI] [PubMed] [Google Scholar]
- Argos P., Narayana S. V., Nielsen N. C. Structural similarity between legumin and vicilin storage proteins from legumes. EMBO J. 1985 May;4(5):1111–1117. doi: 10.1002/j.1460-2075.1985.tb03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Tsukihara T., Rossmann M. G. A structural comparison of concanavalin A and tomato bushy stunt virus protein. J Mol Evol. 1980 Jul;15(3):169–179. doi: 10.1007/BF01732946. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bollini R., Vitale A., Chrispeels M. J. In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: evidence for two glycosylation steps. J Cell Biol. 1983 Apr;96(4):999–1007. doi: 10.1083/jcb.96.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Colman P. M., Suzuki E., Van Donkelaar A. The structure of cucurbitin: subunit symmetry and organization in situ. Eur J Biochem. 1980 Feb;103(3):585–588. doi: 10.1111/j.1432-1033.1980.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Dickinson C. D., Floener L. A., Lilley G. G., Nielsen N. C. Self-assembly of proglycinin and hybrid proglycinin synthesized in vitro from cDNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5525–5529. doi: 10.1073/pnas.84.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Schuler M. A., Godette W. D., Zenger V., Beachy R. N., Slightom J. L. The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. Structural homologies of genes and proteins. J Biol Chem. 1986 Jul 15;261(20):9228–9238. [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- Hall T. C., McLeester R. C., Bliss F. A. Equal Expression of the Maternal and Paternal Alleles for the Polypeptide Subunits of the Major Storage Protein of the Bean Phaseolus vulgaris L. Plant Physiol. 1977 Jun;59(6):1122–1124. doi: 10.1104/pp.59.6.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Tatchell K., Hall B. D., Nasmyth K. A. Isolation of a gene from Drosophila by complementation in yeast. Nature. 1981 Jan 1;289(5793):33–37. doi: 10.1038/289033a0. [DOI] [PubMed] [Google Scholar]
- Hirano H., Fukazawa C., Harada K. The complete amino acid sequence of the A3 subunit of the glycinin seed storage protein of the soybean (Glycine max (L.) Merrill). J Biol Chem. 1984 Dec 10;259(23):14371–14377. [PubMed] [Google Scholar]
- Johnson S., Grayson G., Robinson L., Chahade R., McPherson A. Biochemical and crystallographic data for phaseolin, the storage protein from Phaseolus vulgaris. Biochemistry. 1982 Sep 28;21(20):4839–4843. doi: 10.1021/bi00263a002. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Stuart D. I., Walker N. P. Structure of tumour necrosis factor. Nature. 1989 Mar 16;338(6212):225–228. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Eriksson S., Liljas A. Crystal structure of a ribosomal component at 2.6 A resolution. Nature. 1980 Aug 21;286(5775):824–826. doi: 10.1038/286824a0. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Hardman K. D. Computer-generated schematic diagrams of protein structures. Science. 1982 Apr 30;216(4545):539–540. doi: 10.1126/science.7071602. [DOI] [PubMed] [Google Scholar]
- Marco Y. A., Thanh V. H., Tumer N. E., Scallon B. J., Nielsen N. C. Cloning and structural analysis of DNA encoding an A2B1a subunit of glycinin. J Biol Chem. 1984 Nov 10;259(21):13436–13441. [PubMed] [Google Scholar]
- McKay D. B., Weber I. T., Steitz T. A. Structure of catabolite gene activator protein at 2.9-A resolution. Incorporation of amino acid sequence and interactions with cyclic AMP. J Biol Chem. 1982 Aug 25;257(16):9518–9524. [PubMed] [Google Scholar]
- Poulos T. L., Freer S. T., Alden R. A., Edwards S. L., Skogland U., Takio K., Eriksson B., Xuong N., Yonetani T., Kraut J. The crystal structure of cytochrome c peroxidase. J Biol Chem. 1980 Jan 25;255(2):575–580. [PubMed] [Google Scholar]
- Roberts M. M., White J. L., Grütter M. G., Burnett R. M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986 May 30;232(4754):1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Abad-Zapatero C., Murthy M. R., Liljas L., Jones T. A., Strandberg B. Structural comparisons of some small spherical plant viruses. J Mol Biol. 1983 Apr 25;165(4):711–736. doi: 10.1016/s0022-2836(83)80276-9. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The evolution of RNA viruses. Bioessays. 1987 Sep;7(3):99–103. doi: 10.1002/bies.950070302. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Drong R. F., Klassy R. C., Hoffman L. M. Nucleotide sequences from phaseolin cDNA clones: the major storage proteins from Phaseolus vulgaris are encoded by two unique gene families. Nucleic Acids Res. 1985 Sep 25;13(18):6483–6498. doi: 10.1093/nar/13.18.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A., Van Kuik J. A., Vliegenthart J. F., Chrispeels M. J. Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem. 1987 Oct 5;262(28):13392–13403. [PubMed] [Google Scholar]
- Sun S. M., McLeester R. C., Bliss F. A., Hall T. C. Reversible and irreversible dissociation of globulins from Phaseolus vulgaris seed. J Biol Chem. 1974 Apr 10;249(7):2118–2121. [PubMed] [Google Scholar]
- Suzuki E., Van Donkelaar A., Varghese J. N., Lilley G. G., Blagrove R. J., Colman P. M. Crystallization of phaseolin from Phaseolus vulgaris. J Biol Chem. 1983 Feb 25;258(4):2634–2636. [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Tulloch P. A., Blagrove R. J. Electron microscopy of seed-storage globulins. Arch Biochem Biophys. 1985 Sep;241(2):521–532. doi: 10.1016/0003-9861(85)90577-6. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]


