Abstract
Bone metastases in patients with solid tumours (ST) and bone lesions in patients with haematological malignancies (HM) are common. Associated skeletal‐related events (SREs) cause severe pain, reduced quality of life and place a burden on health care resources. Bone‐targeted agents can reduce the risk of SREs. We evaluated the management of bone metastasis/lesions in five European countries (France, Germany, Italy, Spain and the UK) by an observational chart audit. In total, 881 physicians completed brief questionnaires on 17 193 patients during the observation period, and detailed questionnaires for a further 9303 individuals. Patient cases were weighted according to the probability of inclusion. Although a large proportion of patients with bone metastases/lesions were receiving bisphosphonates, many had their treatment stopped (ST, 19%; HM, 36%) or will never be treated (ST, 18%; HM, 13%). The results were generally similar across the countries, although German patients were more likely to have asymptomatic bone lesions detected during routine imaging. In conclusion, many patients who could benefit from bone‐targeted agents do not receive bisphosphonates and many have their treatment stopped when they could benefit from continued treatment. Developing treatment guidelines, educating physicians and increasing the availability of new agents could benefit patients and reduce costs.
Keywords: bisphosphonate, bone metastases, bone‐targeted agent, haematological malignancy, skeletal‐related event, solid tumour
Introduction
Bone complications are common in patients with advanced solid tumours (ST) and haematological malignancies (HM; Coleman 2001). Skeletal metastases are most frequently found in individuals with advanced breast cancer or prostate cancer, affecting 65–90% of patients, and bone lesions are present in up to 96% of patients with newly diagnosed multiple myeloma (Coleman 2001; Kyle et al. 2003; Parker et al. 2013). Bone metastases also occur frequently in patients with other ST such as advanced lung and kidney cancer, but are rare in patients with gastrointestinal tumours. This pattern of occurrence may result from the route of blood drainage from the breast and pelvis to the skeleton. In addition, interactions between cancer cells and the bone marrow stroma may result in the promotion of secondary tumour growth (Coleman 2001).
As the treatment of cancer improves and patients live longer, the prevalence of bone involvement is predicted to rise. Bone metastases and lesions are associated with severe pain and debilitating skeletal‐related events (SREs), which include pathologic fractures, spinal cord compression and the need for therapeutic radiation or surgery to bone (Coleman 2001; Ibrahim et al. 2013). SREs are associated with increased mortality (Saad et al. 2007b; Norgaard et al. 2010) and reduced quality of life (Weinfurt et al. 2004, 2005; Depuy et al. 2007; Costa et al. 2008; von Moos et al. 2013), and place a considerable burden on hospital resources (Delea et al. 2006; Lage et al. 2008; Pockett et al. 2010; Hechmati et al. 2011; von Moos et al. 2013; Hoefeler et al. 2014; Body et al. 2015). Therefore, the effective management of metastatic bone disease and bone lesions is of increasing importance in order to improve clinical outcomes and the quality of life of patients. This management requires a multidisciplinary approach involving specialists in areas such as oncology, radiotherapy, orthopaedics, nursing and palliative care (Ibrahim et al. 2009, 2013).
Bisphosphonates are bone‐targeted drugs that have been shown to reduce the risk of SREs in patients with bone metastases secondary to ST (Body 2003; Lipton 2007) and in patients with multiple myeloma and bone involvement (Berenson et al. 1996; Rosen et al. 2003). The use of bisphosphonates, particularly nitrogen‐containing agents such as pamidronate and zoledronate, is associated with side effects which, although rare, should be considered when initiating treatment. Osteonecrosis of the jaw (Woo et al. 2006; EMA 2015a) and a modest risk of renal toxicity (<10%) (Conte & Guarneri 2004) have been associated with bisphosphonate use. The benefit–risk ratio should be taken into consideration before initiating bisphosphonate treatment and careful monitoring should be carried out once treatment has commenced. The risks are typically outweighed by the fact that bone‐targeted agents can delay the onset of the first and subsequent SREs (Lipton 2007; Saad et al. 2007a), therefore the clinical benefit for patients with bone metastases or lesions is considerable.
The aim of this patient chart survey was to assess the management of bone metastases and lesions in patients with ST and HM across five European countries, in order to evaluate the use of bisphosphonates and to identify any barriers to their use.
Patients and methods
This was an observational chart audit performed over 2–3 weeks in March 2010 in France, Germany, Italy, Spain and the UK. In total, 881 physicians (307 oncologists, 164 haematologists, 196 urologists, 120 oncological haematologists, 41 pulmonologists, 31 gynaecologists, 12 radiation oncologists and 10 internists) who treated patients with advanced cancer were selected to form a representative sample. Eligible physicians had to manage at least five patients per week with advanced cancer involving bone. The number of patients intended to be included in this survey was 16 500.
The audit was divided into two parts that were completed concurrently by participating physicians. In the first part, physicians completed a brief questionnaire (Appendix 1) for every patient they saw with bone metastases associated with ST (prostate cancer, breast cancer, lung cancer, renal cell carcinoma, bladder cancer, colorectal cancer or other ST), or with bone lesions associated with HM [multiple myeloma, or non‐Hodgkin lymphoma (NHL)], and for all additional patients who were being treated with a bisphosphonate for cancer‐related reasons. Disease was staged using a scale of I–IV (Ann Arbor staging system) in patients with ST or NHL, and a scale of I to III in patients with multiple myeloma (Durie and Salmon staging system). The observation period was 2–3 weeks, as dictated by the caseload of the physician. In the second part of the audit, physicians completed a detailed questionnaire (Appendix 2) on the next 11 consecutive patients that they saw who met the pre‐defined criteria. These criteria were: the first six patients treated with bisphosphonates for cancer‐related reasons (to treat or prevent bone lesions); the first two patients who had discontinued cancer‐related treatment with bisphosphonates; and the first three patients with bone involvement who had never received bisphosphonates. Data on these patients were collected retrospectively via chart survey.
The frequency with which a patient visits their physician is usually determined by the type of cancer they are being treated for. Therefore, in order to avoid an under‐estimation or over‐estimation of the prevalence of different cancers, cases from the first part of the audit were weighted according to the probability of inclusion in the study. The weighting coefficient was estimated based on the consultation frequency and the length of the observation period (i.e. the probability of the patient seeing the physician within the observation period; patients seeing the physician more frequently, e.g. those receiving chemotherapy every 3 weeks, were more likely to be included than those seeing the physician less frequently, e.g. those taking oral targeted therapy). To correct for any recruitment bias, data were weighted by the date of next consultation, with patients returning sooner allocated a lower coefficient than those returning later.
In each country, the evaluation an individual physician's caseload obtained in the first part of the audit was also used to weight each detailed patient case appropriately in the second part. This ensured that the representative proportions of the patient populations according to treatment status (currently treated with bisphosphonates, previously treated, treatment planned, or will never be treated) measured in the first part of the audit were maintained in the second part, and in any analysis based on data from the detailed questionnaires.
Results
Physicians and patients
In the first part of the audit (brief questionnaires), the 881 participating physicians provided data on 17 193 patients. These data showed that patient characteristics were largely consistent across the five countries (Table 1). The median ages of patients in each malignancy group were similar (ST, 67.7 years; HM, 67.5 years). For patients with ST, the youngest were in Germany (median age, 62.6 years) and the oldest in Italy (70.3 years). The median age of patients with HM was consistent across countries (64.3–68.3 years) (Table 1).
Table 1.
Patient characteristics by malignancy type and by country: data from the brief questionnaire
| Total (N = 17 193) | France (n = 3559) | Germany (n = 4450) | Italy (n = 3272) | Spain (n = 2538) | UK (n = 3374) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| Male (%) | 61 | 53 | 67 | 63 | 59 | 51 | 65 | 55 | 77 | 64 | 62 | 62 |
| Median age (years) | 67.7 | 67.5 | 69.6 | 67.5 | 62.6 | 67.3 | 70.3 | 68.3 | 66.8 | 64.3 | 69.6 | 68.2 |
| Median age range (years) | 15.1–100.3 | 10.5–99.6 | 26.7–100.3 | 20.4–94.2 | 24.8–100.3 | 29.8–89.2 | 19.6–100.3 | 10.5–89.1 | 16.8–99.8 | 20.4–99.6 | 15.1–99.9 | 20.0–97.6 |
HM, haematological malignancy; ST, solid tumour.
Detailed data were provided for 9303 patients who were included in the second part of the audit. From these questionnaires, the most common cancer types associated with bone metastases or lesions (n = 8768) were prostate cancer, breast cancer, lung cancer and multiple myeloma (Table 2). In Germany, there was a slightly higher proportion of patients with bone metastases who had lung cancer (20%) than in the other countries (Spain, 13%; France, 12%; Italy, 9%, UK, 8%; Table 2). Eastern Cooperative Oncology Group (ECOG) status and life expectancy (according to the treating physician's opinion) in patients with bone metastases or lesions are listed in Table 3. Most patients had an ECOG status of 1 (ST, 47%; HM, 38%) or 2 (ST, 27%; HM, 22%). Only 1% of all patients had an ECOG status of 4. Most patients with ST (82%) and HM (96%) had a life expectancy of 1 year or more (Table 3).
Table 2.
Type of cancer in patients with bone metastases/lesions: data from the detailed questionnaire
| Cancer type (%) | Total (N = 8768) | France (n = 1665) | Germany (n = 2326) | Italy (n = 1680) | Spain (n = 1534) | UK (n = 1563) |
|---|---|---|---|---|---|---|
| Prostate cancer | 31 | 33 | 20 | 35 | 37 | 34 |
| Breast cancer | 22 | 22 | 21 | 21 | 19 | 27 |
| Lung cancer | 13 | 12 | 20 | 9 | 13 | 8 |
| Renal cell carcinoma | 7 | 6 | 10 | 5 | 5 | 7 |
| Bladder cancer | 4 | 2 | 9 | 3 | 4 | 2 |
| Colorectal cancer | 3 | 1 | 7 | 2 | 2 | 2 |
| Other solid tumour | 2 | 3 | 3 | 2 | 2 | 1 |
| Multiple myeloma | 15 | 18 | 9 | 20 | 16 | 16 |
| Non‐Hodgkin lymphoma | 2 | 3 | 3 | 3 | 2 | 1 |
HM, haematological malignancy; ST, solid tumour.
Table 3.
ECOG performance status and life expectancy (according to treating physician's opinion) in patients with bone metastases/lesions: data from the detailed questionnaire
| Total (N = 8768) | France (n = 1665) | Germany (n = 2326) | Italy (n = 1680) | Spain (n = 1534) | UK (n = 1563) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| Performance status (%) | ||||||||||||
| ECOG 0 | 16 | 31 | 16 | 38 | 9 | 9 | 25 | 42 | 18 | 20 | 15 | 40 |
| ECOG 1 | 47 | 38 | 48 | 36 | 52 | 54 | 40 | 33 | 47 | 31 | 45 | 40 |
| ECOG 2 | 27 | 22 | 28 | 18 | 29 | 34 | 25 | 19 | 26 | 30 | 26 | 14 |
| ECOG 3 | 9 | 6 | 7 | 6 | 9 | 2 | 8 | 5 | 7 | 15 | 12 | 4 |
| ECOG 4 | 1 | 1 | 1 | 3 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 2 |
| Life expectancy (%) | ||||||||||||
| <1 year | 17 | 4 | 15 | 3 | 10 | 6 | 20 | 5 | 24 | 3 | 22 | 4 |
| 1–3 years | 46 | 34 | 42 | 25 | 43 | 41 | 47 | 33 | 48 | 50 | 53 | 26 |
| >3 years | 36 | 60 | 42 | 69 | 47 | 52 | 33 | 62 | 27 | 47 | 26 | 69 |
ECOG, Eastern Cooperative Oncology Group; HM, haematological malignancy; ST, solid tumour.
Data from the detailed questionnaire showed that disease staging at initial diagnosis was consistent across countries (data not shown). Almost half of the 7558 patients with ST were diagnosed with stage IV disease (stage I, 5%; stage II, 23%; stage III, 25%; stage IV, 46%). Similarly, for patients with NHL (n = 227), most patients were diagnosed with the latest stage disease (stage I, 3%; stage II, 10%; stage 3, 13%; stage IV, 74%). Of the 1528 patients with multiple myeloma, most were diagnosed with stage III disease (stage I, 10%; stage 2, 18%, stage III, 71%).
Detection of bone metastases/lesions
From the detailed questionnaire, almost all patients had bone metastases or bone lesions (94%; n = 8768). Of these patients, 82% had ST and 17% had HM (Table 2). The largest proportion of patients with bone involvement had their metastases or lesions identified during staging at diagnosis of the primary cancer (ST, 38%; HM, 65%) or as a result of bone pain (ST, 35%; HM, 36%) (Table 4). Germany was an exception because routine screening during follow‐up was the main method of detection of bone metastases and lesions in patients with ST (41%). Furthermore, metastases or lesions were less frequently identified in patients with ST as a result of bone pain in Germany (20%) than in other countries (range, 34–48%) (Table 4). Across most countries, patients were most likely to have bone metastases or lesions identified at multiple disseminated sites (ST, 36%; HM, 48%; Fig. 1). Again, Germany was the exception because patients with ST were more likely to have solitary bone metastasis at diagnosis (44%) than to have metastases at multiple disseminated sites (27%). The circumstances of diagnosis of bone lesions in patients with HM in Germany were similar to those in other countries.
Table 4.
Circumstances of discovery of bone metastases and lesions, by malignancy type and by country: data from the detailed questionnaire
| Response given (%) | Total (N = 8768) | France (n = 1665) | Germany (n = 2326) | Italy (n = 1680) | Spain (n = 1534) | UK (n = 1563) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| Staging at diagnosis | 38 | 65 | 41 | 74 | 31 | 75 | 39 | 57 | 37 | 52 | 45 | 68 |
| Bone pain | 35 | 36 | 48 | 52 | 20 | 7 | 34 | 37 | 37 | 46 | 42 | 34 |
| Routine metastases screening | 25 | 5 | 14 | 2 | 41 | 12 | 30 | 6 | 21 | 7 | 6 | 1 |
| Investigation following an eventa | 7 | 18 | 9 | 20 | 5 | 10 | 6 | 23 | 5 | 20 | 9 | 13 |
| Hypercalcaemia | 5 | 9 | 6 | 15 | 3 | 1 | 4 | 7 | 5 | 9 | 6 | 13 |
| Accidental discovery | 5 | 3 | 5 | 1 | 5 | 1 | 5 | 3 | 6 | 9 | 4 | 1 |
| Otherb | 3 | 0 | 6 | 0 | 0 | 0 | 3 | 0 | 4 | 0 | 3 | 0 |
HM, haematological malignancy; PSA, prostate‐specific antigen; ST, solid tumour.
Events included confirmed or suspected pathologic fracture or spinal compression.
Including increase in tumour markers (e.g. PSA), check on spread of metastases/restaging for any metastases, extensive examination after finding lumps, worsening of general condition and pain. Investigators could give more than one response.
Figure 1.
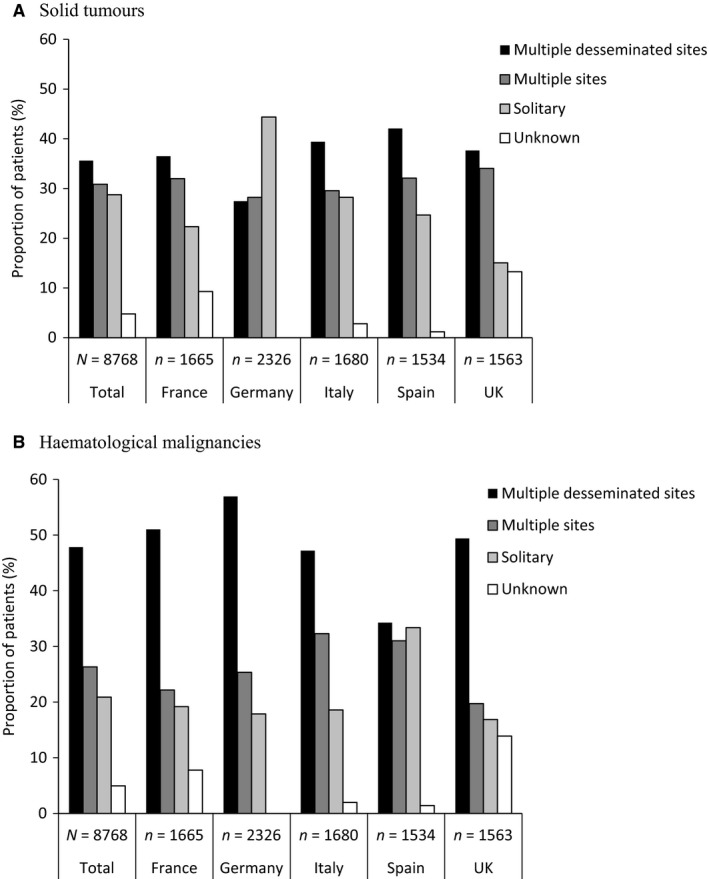
Description of bone metastases and lesions at discovery, by malignancy type and by country in patients with (A) solid tumours and (B) haematological malignancies: data from the detailed questionnaire. The difference between multiple disseminated sites and multiple sites was subjective and determined by the treating physician.
Treatment of bone metastases/lesions
In the first part of the audit (brief questionnaire), most patients had bone metastases or lesions at the time of consultation (86%). Although a large proportion of these patients were currently receiving bisphosphonate treatment (ST, 68%; HM, 60%), many had their treatment stopped (ST, 19%; HM, 36%) or would never receive bisphosphonates (ST, 18%; HM, 13%). Treatment with bisphosphonates was planned in a small proportion of patients (ST, 10%; HM, 7%) (Fig. 2).
Figure 2.
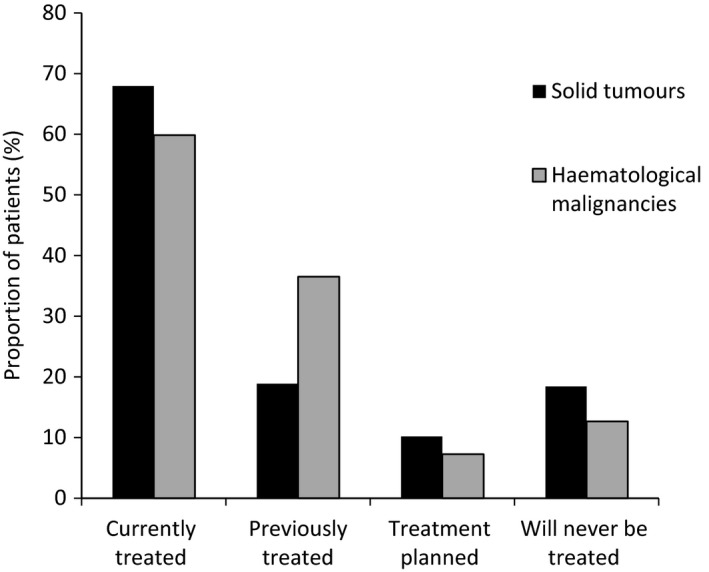
Bisphosphonate treatment rates in patients with bone metastases/lesions across all countries: data from the brief questionnaire (n = 14 871).
Almost one‐fifth of patients with ST and bone metastases or lesions were expected never to receive bisphosphonates (ST, 18%; HM, 13%) although most patients in this group had a moderate‐to‐high estimated risk of developing an SRE (evaluated by the physician) (data not shown). The proportions of patients with ST who were expected never to receive bisphosphonates were reasonably consistent across countries with the exception of the UK, where almost twice as many patients were expected never to receive bisphosphonates (26%) as in other countries (range, 13–14%). Zoledronic acid was the most commonly used bisphosphonate in both malignancy types (ST, 75%; HM, 66%). Pamidronate, clodronate and ibandronate were the next most common bisphosphonates and their use ranged from 5% to 9% in patients with ST, and from 4% to 14% in those with HM.
The main rationales for bisphosphonate treatment were: to prevent SREs (ST, 71%; HM, 66%), to treat or prevent pain (ST, 55%; HM, 42%), to prevent the development of new bone metastases or lesions (ST, 38%; HM, 47%) and to treat bone metastases or lesions at the original site (ST, 38%; HM, 36%) (Table 5). Compared with other countries, Germany reported more frequently that the rationale for bisphosphonate use was based on the assumption that they prevent new metastases or lesions (ST, 69%; HM, 67%), whereas in France, this reason was given infrequently (ST, 15%; HM, 33%) (Table 5).
Table 5.
Reason for initial treatment with bisphosphonates in patients with bone metastases/lesions, by malignancy type and by country: data from the detailed questionnaire
| Response given (%) | Total (N = 7368) | France (n = 1483) | Germany (n = 1973) | Italy (n = 1427) | Spain (n = 1325) | UK (n = 1160) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| Prevent SREs | 71 | 66 | 66 | 67 | 84 | 87 | 64 | 62 | 65 | 57 | 67 | 61 |
| Treat/prevent pain | 55 | 42 | 57 | 44 | 68 | 72 | 43 | 32 | 49 | 42 | 48 | 29 |
| Prevent new bone metastases/bone lesions | 38 | 47 | 15 | 33 | 69 | 67 | 32 | 47 | 26 | 42 | 26 | 49 |
| Treat bone metastases/lesion at original site(s) | 38 | 36 | 30 | 26 | 59 | 47 | 36 | 38 | 19 | 43 | 33 | 31 |
| Patient's disease has high risk factors | 5 | 7 | 9 | 20 | 1 | 0 | 5 | 6 | 4 | 3 | 10 | 4 |
| End of anti‐tumour treatment | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 3 | 3 | 1 | 2 | 0 |
| Other | 1 | 2 | 3 | 3 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 4 |
HM, haematological malignancy; ST, solid tumour.
Investigators could give more than one response.
The most common reason for discontinuing bisphosphonate treatment was the ‘end of treatment as planned’ (ST, 51%; HM, 72%, Table 6). Overall, patients were most often treated with bisphosphonates for a duration of 13–24 months (ST, 37%; HM, 38%) (Table 7). In Germany, a larger proportion of patients were treated with bisphosphonates for 13–24 months (ST, 55%; HM, 51%) than in the other countries [range, 9–37% (ST); 33–43% (HM)] (Table 7). Some patients who had their treatment stopped as planned were treated for only 6 months or less (ST, 15%; HM, 18%) (Table 8). Of those who had their treatment stopped owing to reaching the end of their planned treatment period, there was considerable variation between the countries: only 3% of patients with ST in Germany received bisphosphonates for 6 months or less, in contrast to 51% in the UK (Table 8).
Table 6.
Reasons for discontinuation of bisphosphonates in patients with bone metastases/lesions, by malignancy type and by country: data from the detailed questionnaire
| Response given (%) | Total (N = 1838) | France (n = 392) | Germany (n = 516) | Italy (n = 363) | Spain (n = 309) | UK (n = 258) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| End of treatment as planneda | 51 | 72 | 48 | 77 | 67 | 60 | 38 | 68 | 44 | 85 | 36 | 56 |
| Toxicity | 16 | 11 | 25 | 7 | 7 | 18 | 20 | 15 | 34 | 9 | 3 | 5 |
| Risk of toxicity | 7 | 6 | 10 | 3 | 9 | 17 | 5 | 9 | 5 | 1 | 6 | 9 |
| Lack of efficacyb | 12 | 4 | 7 | 7 | 13 | 9 | 20 | 1 | 7 | 1 | 10 | 3 |
| Contraindication due to concomitant treatment | 3 | 1 | 1 | 1 | 3 | 8 | 0 | 0 | 3 | 1 | 1 | 0 |
| Other | 9 | 7 | 18 | 11 | 4 | 10 | 16 | 6 | 5 | 0 | 7 | 8 |
Investigators could give more than one response.
HM, haematological malignancy; ST, solid tumour.
Planned duration of treatment was determined by treating physician.
As determined by treating physician.
Table 7.
Duration of treatment with bisphosphonates for those patients with bone metastases/lesions who had treatment stopped, by malignancy type and by country: data from the detailed questionnaire
| Treatment duration (%) | Total (N = 947) | France (n = 220) | Germany (n = 329) | Italy (n = 167) | Spain (n = 155) | UK (n = 74) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| ≤6 months | 15 | 18 | 27 | 19 | 3 | 0 | 11 | 25 | 26 | 15 | 51 | 21 |
| 7–12 months | 18 | 21 | 18 | 29 | 12 | 0 | 14 | 11 | 36 | 25 | 37 | 34 |
| 13–24 months | 52 | 41 | 33 | 42 | 70 | 65 | 58 | 31 | 28 | 48 | 6 | 12 |
| >24 months | 14 | 17 | 20 | 5 | 15 | 35 | 18 | 30 | 10 | 11 | 4 | 33 |
| Unknown | 1 | 3 | 2 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | 3 | 1 |
Table 8.
Duration of treatment with bisphosphonates for those patients with bone metastases/lesions who had treatment stopped owing to end of planned treatment, by malignancy type and by country: data from the detailed questionnaire
| Treatment duration (%) | Total (N = 1806) | France (n = 381) | Germany (n = 516) | Italy (n = 357) | Spain (n = 306) | UK (n = 246) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| ≤6 months | 28 | 20 | 37 | 23 | 14 | 4 | 24 | 23 | 33 | 20 | 50 | 20 |
| 7–12 months | 21 | 22 | 19 | 29 | 17 | 7 | 19 | 16 | 30 | 28 | 26 | 16 |
| 13–24 months | 37 | 38 | 29 | 39 | 55 | 51 | 36 | 31 | 28 | 43 | 9 | 33 |
| >24 months | 14 | 16 | 13 | 4 | 14 | 39 | 18 | 23 | 10 | 9 | 13 | 27 |
| Unknown | 1 | 4 | 2 | 4 | 0 | 0 | 2 | 7 | 0 | 0 | 2 | 4 |
HM, haematological malignancy; ST, solid tumour.
Bisphosphonate treatment was often delayed in all countries except Germany (data not shown). The main justification for delaying bisphosphonate treatment was that bone metastases and lesions were controlled by initial anti‐tumour therapy (ST, 57%; HM, 53%) (Fig. 3). This reason was particularly common in the UK (ST, 86%; HM, 66%). This justification was used fairly consistently in the other countries for patients with ST or multiple myeloma, but was used more frequently for patients with NHL (71%). Across all countries, the most common cancer treatments at diagnosis for patients with ST were hormone therapy (42%), surgery (40%), and chemotherapy (37%). Most patients with HM (77%) received chemotherapy as an initial treatment for their cancer (Table S1).
Figure 3.
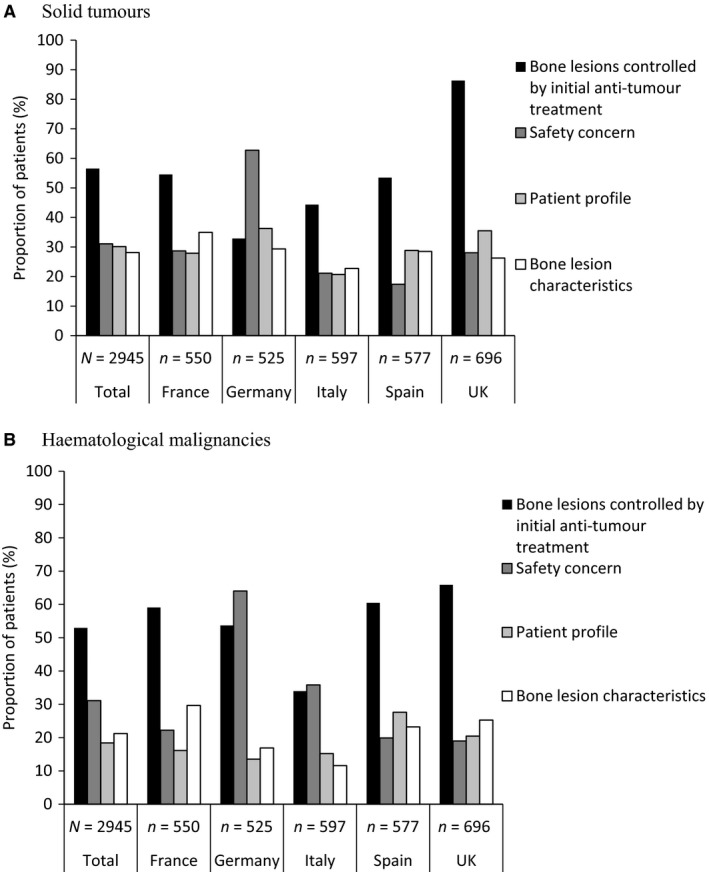
Reasons for delaying bisphosphonate treatment in patients with bone metastases/lesions, by malignancy type and by country in patients with (A) solid tumours and (B) haematological malignancies: data from the detailed questionnaire. Anti‐tumour treatment included radiotherapy, hormonal therapy, chemotherapy and targeted therapy. Safety concerns included existing renal impairment, to avoid renal deterioration and dental health issues. Patient profile included poor performance status and short life expectancy prognosis. Bone lesion characteristics included low risk of fracture/compression, no pain and pain controlled by analgesics. Investigators could give more than one response.
Owing to the risk of side effects associated with bisphosphonates, it is perhaps unsurprising that concern over safety was frequently given as a reason for treatment delay (ST, 31%; HM, 31%). This was the most common reason given in Germany (ST, 63%; HM, 64%). The next most common reasons for treatment delay were patient profile (e.g. poor performance status or short life expectancy) (ST, 30%; HM, 18%) and bone lesion characteristics (e.g. pain controlled with analgesics and the risk of SREs) (ST, 28%; HM, 21%) (Fig. 3). Patient profile was more commonly used as a justification for delaying treatment in patients with lung cancer (50%), bladder cancer (48%) and other ST (47%) than for patients with other types of malignancy.
The most common reasons given by physicians predicting that patients would never receive bisphosphonate treatment were: short life expectancy (ST, 41%; HM, 21%), renal issues (ST, 36%; HM, 39%) and poor benefit–risk ratio (ST, 34%; HM, 31%) (Table 9). These reasons were commonly given in all the countries studied, although their relative frequencies varied. For example, renal complications were the most common reason for not administering bisphosphonates in Germany (ST, 55%; HM, 66%), whereas this reason was recorded much less frequently in the UK (ST, 18%; HM, 22%). More than one‐third of patients who took part in the second part of the audit had renal complications (ST, 31%; HM, 32%) and these proportions were consistent with observations in both Germany and the UK.
Table 9.
Reasons why patients with bone metastases/lesions would never receive bisphosphonates, by malignancy type and by country: data from the detailed questionnaire
| Reason given (%) | Total (N = 1360) | France (n = 250) | Germany (n = 304) | Italy (n = 226) | Spain (n = 216) | UK (n = 364) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| Renal issue | 36 | 39 | 46 | 21 | 55 | 66 | 34 | 59 | 33 | 32 | 18 | 22 |
| Dental health issue | 9 | 10 | 9 | 8 | 20 | 12 | 9 | 15 | 3 | 12 | 2 | 1 |
| Poor benefit–risk ratio | 34 | 31 | 43 | 40 | 29 | 23 | 38 | 21 | 25 | 28 | 36 | 42 |
| Patient refusal | 6 | 3 | 7 | 2 | 10 | 2 | 6 | 8 | 4 | 0 | 5 | 5 |
| Short life expectancy | 41 | 21 | 38 | 13 | 41 | 14 | 32 | 17 | 52 | 42 | 41 | 22 |
| Pain from BM controlled by analgesics/opioids | 14 | 11 | 19 | 21 | 14 | 12 | 8 | 4 | 9 | 5 | 18 | 4 |
| Other | 5 | 17 | 7 | 35 | 3 | 17 | 5 | 18 | 9 | 10 | 4 | 0 |
BM, bone metastases/lesions; HM, haematological malignancy; ST, solid tumour.
Investigators could give more than one response.
Skeletal‐related events
At the timepoint of this survey, many patients had experienced at least one SRE, irrespective of the time of their cancer diagnosis (ST, 22%; HM, 41%) (Table 10). The most common SRE in patients with ST was radiation to bone, and for those with HM it was pathological fracture. Patients who were currently treated or had previously been treated with bisphosphonates were most likely to have experienced an SRE. In those who were expected to receive bisphosphonates in the future, the prevalence of SREs was 10% and 32% among patients with ST and HM respectively. Of those patients who were expected never to receive bisphosphonate treatment (12% of patients with ST and 7% of those with HM), a large proportion had received radiation to bone and/or experienced a pathological fracture.
Table 10.
Skeletal‐related events in patients with bone metastases/lesions by malignancy type and by bisphosphonate treatment status: data from the detailed questionnaire
| All BM patients (N = 8768) | Currently treated (n = 5133) | Previously treated (n = 1699) | Treatment planned (n = 575) | Will never be treated (n = 1361) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST | HM | ST | HM | ST | HM | ST | HM | ST | HM | |
| All SREs (%) | 22 | 41 | 21 | 45 | 25 | 40 | 10 | 32 | 16 | 25 |
| n | 1592 | 646 | 1043 | 386 | 303 | 188 | 51 | 19 | 189 | 47 |
| Radiotherapy to bone | 62 | 28 | 63 | 27 | 63 | 28 | 57 | 20 | 55 | 47 |
| Pathologic fracture | 30 | 62 | 31 | 63 | 29 | 62 | 31 | 74 | 30 | 53 |
| Spinal cord compression | 15 | 19 | 14 | 18 | 16 | 24 | 12 | 16 | 20 | 9 |
| Surgery to bone | 7 | 8 | 6 | 9 | 6 | 7 | 4 | 16 | 12 | 0 |
BM, bone metastases/bone lesions, HM, haematological malignancy; SRE, skeletal‐related event; ST, solid tumour.
Some patients had multiple SREs.
Discussion
This large‐scale chart audit with a robust methodology provides insight into the real‐life management of patients with bone metastases or bone lesions in five European countries. These data suggest that approximately 80% of patients with bone metastases or lesions receive treatment with bisphosphonates during the course of their disease, and although most of these patients were receiving treatment at the time of this survey, there were patients who may benefit from initiating, or continuing treatment.
In general, the findings for patients with ST were consistent with those for patients with HM. Bone metastases and lesions were most likely to be discovered during disease staging at diagnosis, and most patients had multiple metastases or lesions. The major difference between malignancy types was that fewer patients with ST than those with HM were currently being treated, or had treatment planned, with bisphosphonates. This may be due to a combination of patient characteristics, such as poor performance status or life expectancy prognosis, and a lack of consensus in guideline recommendations. Bone pathology is one of the defining features of multiple myeloma (Raje et al. 2014). One study found that 79% of patients with multiple myeloma had bone abnormalities and 67% of patients had lytic bone lesions. In addition, more than half of patients experienced bone pain (Kyle et al. 2003; Raje et al. 2014). Bisphosphonates are therefore recommended in several guidelines to manage pain and control SREs in patients with multiple myeloma (Berenson et al. 2002; Harrouseau et al. 2005). This appears to be reflected in our study where more patients with HM than with ST were receiving bisphosphonate treatment. Bone involvement in breast cancer and prostate cancer is also common, with the reported incidence being as high as 75–90% (Coleman 2001; Parker et al. 2013). Therefore, it was surprising that over one‐third of patients with ST were not receiving bisphosphonate treatment. Bone‐targeted agents are recommended by several guidelines for patients with ST, therefore these results highlight differences between guidelines and real‐life use of bisphosphonates. This also suggests that there are patients who could benefit from, but are not receiving, bisphosphonates.
Approximately, half of the patients in our study received bisphosphonate treatment for 1 year or more. The majority of patients had their bisphosphonate therapy stopped owing to the end of planned treatment and approximately one‐fifth of those individuals received treatment for only 6 months or less. This was more common in the UK, with the majority of patients with ST receiving bisphosphonate treatment for 6 months or less. The optimum duration of bone‐targeted therapy is unknown although pivotal studies for the bisphosphonates were generally conducted over 2 years and clinical benefit was observed throughout this time‐frame (Berenson et al. 2002). Continued treatment for at least 2 years could be beneficial because bone‐targeted therapy delays not only first but also subsequent SREs (Saad et al. 2007a; Stopeck et al. 2010; Fizazi et al. 2011; Henry et al. 2011). A placebo‐controlled study of patients with prostate cancer showed that in the second year of bisphosphonate treatment, the risk of SREs continued to be substantially reduced, with a risk reduction of 53% compared with placebo (Saad et al. 2007a). The same study also demonstrated that treatment significantly reduced the risk of further SREs in patients who have already experienced one event. Furthermore, a significantly reduced risk of SREs has been reported in patients with breast cancer during the second year of bisphosphonate treatment (Aapro et al. 2010).
Perhaps, owing to the lack of information and consensus in the guidelines regarding optimal duration of treatment, effective medication for patients with bone metastases or bone lesions may be being stopped at a point at which benefit could still be derived. At the time of this study, only one guideline recommended continuing bone‐targeted therapy for more than 2 years because of the continued risk of SREs (Aapro et al. 2008). Other guidelines did not advise on optimal treatment duration (Aapro et al. 2008; Cardoso et al. 2010; Mohler et al. 2010), therefore it may be difficult for physicians to make decisions on planned bisphosphonate treatment schedules. More recently, the International Myeloma Network has recommended zoledronic acid should be given continuously to patients with active multiple myeloma until disease progression or until a very good or complete response is achieved (Terpos et al. 2013). Similarly, the European Myeloma Network has recommended continuous treatment with zoledronic acid, and treatment with pamidronate for up to 2 years (Terpos et al. 2015). There is still a need for clarification regarding the optimal duration of bone‐targeted therapy in general for patients with bone metastases resulting from ST because more recently published guidelines have not reached a consensus (Van Poznak et al. 2011; European Association of Urology 2016; European Society for Medical Oncology 2015; National Comprehensive Cancer Network 2016).
In this study, many patients with bone disease secondary to malignancies were expected never to receive bisphosphonate treatment. Short life expectancy was the most common reason cited for not treating patients with bisphosphonates, although over two‐thirds of patients with bone metastases or lesions had a life expectancy of more than 1 year. Therefore, benefit could be derived from treatment with bone‐targeted agents which can reduce the risk of SREs and therefore improve the quality of life of patients (Lipton 2010). More recent guidelines suggest that patients expected to live for 3 months or longer should be considered for bone‐targeted therapy (Coleman et al. 2014a). It remains unknown whether bisphosphonate treatment in patients with asymptomatic multiple myeloma offers any advantage in the prevention of SREs (Terpos et al. 2013, 2014, 2015).
In this survey, it was noted that treatment with bisphosphonates was frequently delayed owing to safety concerns in patients with bone metastases or lesions secondary to ST of HM. These concerns included side effects such as osteonecrosis of the jaw and renal toxicity (Conte & Guarneri 2004; Woo et al. 2006). Osteonecrosis of the jaw has since been identified as being associated with all bone‐targeted agents and while the risk remains low, the European Medicines Agency has recommended the implementation of updates to product information and the introduction of patient reminder cards (EMA 2015a). Interestingly, however, historical data suggest that for most patients, the benefits of treatment outweigh the associated risks (Coleman 2008). In our survey, renal complications were given as a common reason to avoid administering bisphosphonates. Renal morbidity was prevalent in our study, affecting one‐third of patients to some degree. The EU approval in 2010 of the RANK Ligand inhibitor, denosumab, which has no effect on renal function as its metabolism and elimination follow the immunoglobulin clearance pathways (von Moos et al. 2013; EMA 2015b), may lead to more patients receiving and benefiting from bone‐targeted therapies given that renal impairment is particularly relevant in elderly cancer patients (Body et al. 2010; Fizazi et al. 2011). Furthermore, denosumab treatment resulted in a superior reduction in the risk of SREs compared with zoledronic acid in patients with ST and therefore is a more efficacious treatment choice (Henry et al. 2010, 2014; Stopeck et al. 2010; Fizazi et al. 2011).
Although the results of the present study were generally consistent across countries, and across malignancy type, the German data stood out for a number of reasons. First, patients with ST were younger than in the other countries. This could be owing to more widespread cancer screening, leading to diagnosis at a younger age, rather than to differences in the epidemiology of the cancers. This is supported by the finding that bone metastases and lesions were discovered more frequently in German patients through routine screening during follow‐up than during staging at diagnosis. Furthermore, more patients were diagnosed with a solitary bone metastasis or lesion and fewer patients were diagnosed owing to development of pain in Germany than in the other countries, which suggest that these bone metastases and lesions were being detected at an earlier stage. At the time of this study, the German National Health Service had approved the reimbursement of positron emission tomography for disease staging and tumour characterisation in patients with non‐small cell lung cancer (Buck et al. 2010), which may have promoted a different approach to cancer diagnosis in general. Routine bone scanning in asymptomatic patients (not currently recommended by treatment guidelines) could lead to earlier detection of bone metastases and lesions, which is a more proactive attitude to diagnosis and preventive treatment of SREs in Germany than in other countries.
Although we were unable to collect data on denosumab use because the survey was conducted before the agent received marketing authorisation, the results of this patient chart audit are still relevant to current clinical practice because disagreements still exist between guideline recommendations and physicians' opinions relating to optimal bone care for patients with cancer (Payne et al. 2013; Coleman et al. 2014b). Barriers still remain that prevent patients accessing bisphosphonates, including perceived short life expectancy and short treatment duration. Although recent guidelines published after this study was conducted have evolved, for instance, it is now recommend that patients expected to live for 3 months or longer should be considered for bone‐targeted therapy (Coleman et al. 2014a), there are still gaps in the guidelines regarding optimal treatment duration. Improving screening and diagnostic practices for bone metastases and lesions throughout Europe to facilitate early detection (as seen in Germany) may allow earlier access to bone‐targeted agents so that patients with cancer can receive the best standard of care.
Conclusion
The results from this study highlight some notable differences in treatment practices between countries as well as some unexpected findings when the data are considered in the context of international cancer treatment guidelines. It is likely that many of the patients who are expected not to receive bisphosphonates could benefit from this treatment. The number of patients discontinuing treatment after a short time is also of concern, particularly as these agents may prevent not only first SREs but also subsequent events. Therefore, improving access to treatment that reduces the risk of SREs could enhance the quality of life of patients with advanced cancer. This improved access could be achieved through increased awareness of both the overall burden of bone metastases and lesions, and the need to treat patients to prevent SREs, and improvements in cancer treatment guidelines. It is important to breakdown perceived treatment barriers, such as short life expectancy, and to ensure that guidelines provide appropriate advice on duration of treatment. The availability of new bone‐targeted agents, with superior efficacy and no evidence of impact on renal function, may encourage physicians increase their focus on the management of bone involvement from ST and HM.
Conflicts of interest
Thierry Lebret has participated in advisory boards for Novartis and Amgen. Michele Cavo has received honoraria from Amgen. Penella J. Woll has participated in advisory boards for Novartis and Amgen. Caroline Kennedy is an employee of Takeda Global Research & Development Centre (Europe) Ltd, has been an employee of Amgen Limited and holds Amgen Limited Stock. Paul Schoen is an employee of Amgen (Europe) GmbH and holds stock. Christian Jackisch is a member of the Amgen speakers bureau and has received honoraria. Ana Casas and Catherine Deleplace declare that they have no conflict of interest.
Author contributions
Catherine Deleplace, Paul Schoen and Thierry Lebret contributed to the design of this study. Catherine Deleplace and Paul Schoen contributed to the collection of data. Thierry Lebret, Ana Casas, Michele Cavo, Penella J. Woll, Catherine Deleplace, Caroline Kennedy, Paul Schoen and Christian Jackisch critically analysed the data, contributed to the drafting and review of the manuscript, and approved the final version for submission.
Compliance with ethics guidelines
No patient information was collected prospectively; all data were collected through chart survey and anonymised. Therefore, ethics approval and patient consent were not required for this study.
Supporting information
Table S1. Current cancer treatments at diagnosis, by malignancy type and by country: data from the detailed questionnaire.
Acknowledgements
Writing and editorial support was provided by Oxford PharmaGenesis Ltd. Funding for this support was provided by Amgen (Europe) GmbH.
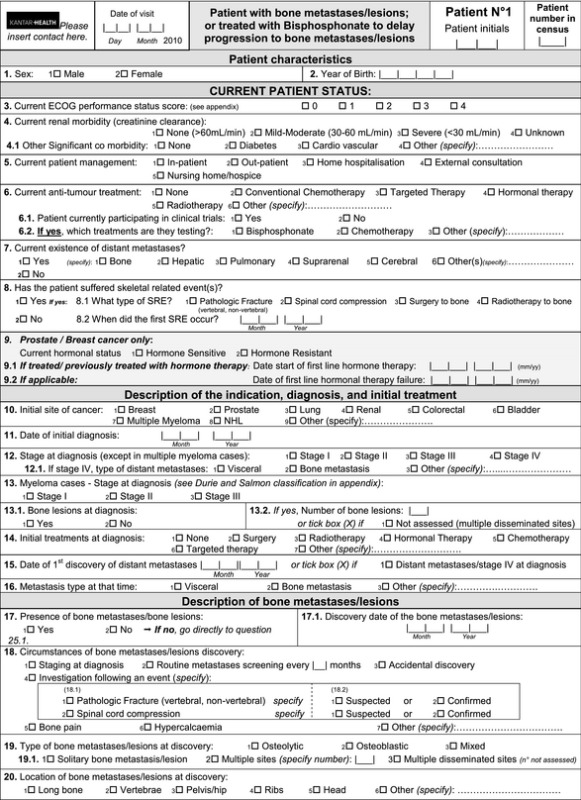
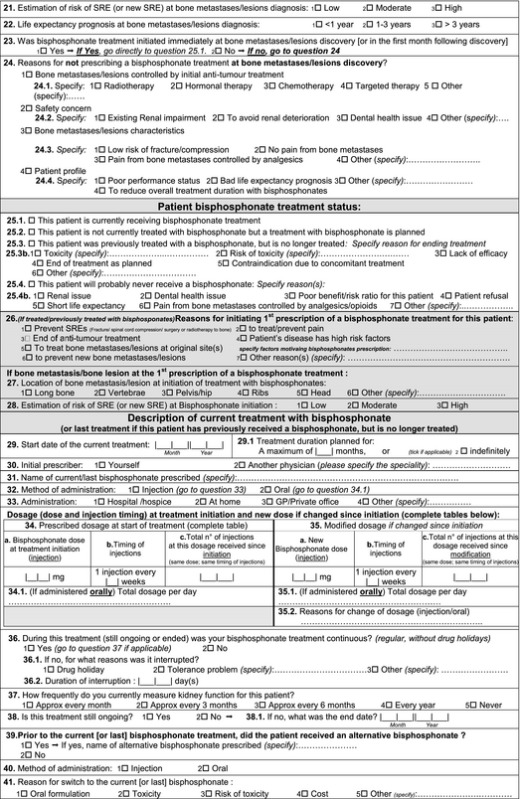
Appendix 1. Simple questionnaire
Appendix 2. Detailed questionnaire
Lebret T., Casas A., Cavo M., Woll P.J., Deleplace C., Kennedy C., Schoen P. & Jackisch C. (2017) European Journal of Cancer Care 26, e12490, doi: 10.1111/ecc.12490 The use of bisphosphonates in the management of bone involvement from solid tumours and haematological malignancies – a European survey
References
- Aapro M., Abrahamsson P.A., Body J.J., Coleman R.E., Colomer R., Costa L., Crino L., Dirix L., Gnant M., Gralow J., Hadji P., Hortobagyi G.N., Jonat W., Lipton A., Monnier A., Paterson A.H., Rizzoli R., Saad F. & Thurlimann B. (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Annals of Oncology 19, 420–432. [DOI] [PubMed] [Google Scholar]
- Aapro M., Saad F. & Costa L. (2010) Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist 15, 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson J.R., Lichtenstein A., Porter L., Dimopoulos M.A., Bordoni R., George S., Lipton A., Keller A., Ballester O., Kovacs M.J., Blacklock H.A., Bell R., Simeone J., Reitsma D.J., Heffernan M., Seaman J. & Knight R.D. (1996) Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. New England Journal of Medicine 334, 488–493. [DOI] [PubMed] [Google Scholar]
- Berenson J.R., Hillner B.E., Kyle R.A., Anderson K., Lipton A., Yee G.C. & Biermann J.S. (2002) American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology 20, 3719–3736. [DOI] [PubMed] [Google Scholar]
- Body J.J. (2003) Effectiveness and cost of bisphosphonate therapy in tumor bone disease. Cancer 97, 859–865. [DOI] [PubMed] [Google Scholar]
- Body J.J., Pereira J., Sleeboom H., Maniadakis N., Terpos E., Acklin Y.P., Finek J., Gunther O., Hechmati G., Mossman T., Costa L., Rogowski W., Nahi H. & Von Moos R. (2015) Health resource utilization associated with skeletal‐related events: results from a retrospective European study. European Journal of Health Economics. doi: 10.1007/s10198‐015‐0716‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body J.J., Lipton A., Gralow J., Steger G.G., Gao G., Yeh H. & Fizazi K. (2010) Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. Journal of Bone and Mineral Research 25, 440–446. [DOI] [PubMed] [Google Scholar]
- Buck A.K., Herrmann K., Stargardt T., Dechow T., Krause B.J. & Schreyogg J. (2010) Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. Journal of Nuclear Medicine 51, 401–412. [DOI] [PubMed] [Google Scholar]
- Cardoso F., Senkus‐Konefka E., Fallowfield L., Costa A. & Castiglione M. (2010) Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 21(Suppl. 5), v15–v19. [DOI] [PubMed] [Google Scholar]
- Coleman R.E. (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews 27, 165–176. [DOI] [PubMed] [Google Scholar]
- Coleman R.E. (2008) Risks and benefits of bisphosphonates. British Journal of Cancer 98, 1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Body J.J., Aapro M., Hadji P. & Herrstedt J. (2014a) Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology 00, 1–14. [DOI] [PubMed] [Google Scholar]
- Coleman R., Body J.J., Aapro M., Hadji P. & Herrstedt J. & E. G. W. Group (2014b) Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology 25(Suppl. 3), iii124–iii137. [DOI] [PubMed] [Google Scholar]
- Conte P. & Guarneri V. (2004) Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 9(Suppl. 4), 28–37. [DOI] [PubMed] [Google Scholar]
- Costa L., Badia X., Chow E., Lipton A. & Wardley A. (2008) Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Supportive Care in Cancer 16, 879–889. [DOI] [PubMed] [Google Scholar]
- Delea T., Mckiernan J., Brandman J., Edelsberg J., Sung J., Raut M. & Oster G. (2006) Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. The Journal of Supportive Oncology 4, 341–347. [PubMed] [Google Scholar]
- Depuy V., Anstrom K.J., Castel L.D., Schulman K.A., Weinfurt K.P. & Saad F. (2007) Effects of skeletal morbidities on longitudinal patient‐reported outcomes and survival in patients with metastatic prostate cancer. Supportive Care in Cancer 15, 869–876. [DOI] [PubMed] [Google Scholar]
- European Association of Urology (2016). Oncology Guidelines: Prostate cancer [Online]. Available at: http://uroweb.org/guideline/prostate-cancer/ (accessed March 2016).
- European Medicines Agency (2015a) PRAC recommends further measures to minimise risk of osteonecrosis of the jaw with bisphosphonate medicine [Online]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/03/WC500184259.pdf.
- European Medicines Agency (2015b) XGEVA® (Denosumab) summary of product characteristics [Online]. EMC. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf.
- European Society for Medical Oncology (2015). ESMO Clinical Practice Guidelines [Online]. European Society of Medical Oncology. Available at: http://www.esmo.org/education-research/esmo-clinical-practice-guidelines.html (accessed March 2016).
- Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., Jiang Q., Tadros S., Dansey R. & Goessl C. (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration‐resistant prostate cancer: a randomised, double‐blind study. Lancet 377, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrouseau J.L., Greil R. & Kloke O. (2005) ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow‐up of multiple myeloma. Annals of Oncology 16(Suppl. 1), 45–47. [DOI] [PubMed] [Google Scholar]
- Hechmati G., Cure S., Gouépo A., Hoefeler H., Lorusso V., Lüftner D., Duran I., Garzon‐Rodriguez C., Ashcroft J., Wei R., Ghelani P. & Bahl A. (2011) Cost of skeletal‐related events in patients with bone metastases to solid tumours based on the health resource utilisation collected in a prospective European multinational observational study. Value in Health 14, A455. [Google Scholar]
- Henry D.H., Von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G.V., Wang J., Jun S., Dansey R.D. & Yeh H. (2010) Delaying skeletal‐related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer. Journal of Clinical Oncology, 2010 ASCO Annual Meeting Proceedings (Post‐Meeting Edition), 28, abstr 9133. [Google Scholar]
- Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan‐Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q., Jun S., Dansey R. & Yeh H. (2011) Randomized, double‐blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of Clinical Oncology 29, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Henry D., Vadhan‐Raj S., Hirsh V., Von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G., Smith G., Feng A., Jun S., Dansey R. & Yeh H. (2014) Delaying skeletal‐related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Supportive Care in Cancer 22, 679–687. [DOI] [PubMed] [Google Scholar]
- Hoefeler H., Duran I., Hechmati G., Garzon Rodriguez C., Lüftner D., Ashcroft J., Bahl A., Atchison C., Wei R., Thomas E. & Lorusso V. (2014) Health resource utilization associated with skeletal‐related events in patients with bone metastases: results from a multinational retrospective – prospective observational study – a cohort from 4 European countries. Journal of Bone Oncology 3, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim T., Flamini E., Fabbri L., Serra P., Mercatali L., Ricci R., Sacanna E., Falasconi M.C., Casadei R., Galassi R., Giannini M., Bazzocchi O., Calzolari F., Nunziatini R., Gaudio M., Maltoni M. & Amadori D. (2009) Multidisciplinary approach to the treatment of bone metastases: Osteo‐Oncology Center, a new organizational model. Tumori 95, 291–297. [DOI] [PubMed] [Google Scholar]
- Ibrahim T., Farolfi A., Mercatali L., Ricci M. & Amadori D. (2013) Metastatic bone disease in the era of bone‐targeted therapy: clinical impact. Tumori 99, 1–9. [DOI] [PubMed] [Google Scholar]
- Kyle R.A., Gertz M.A., Witzig T.E., Lust J.A., Lacy M.Q., Dispenzieri A., Fonseca R., Rajkumar S.V., Offord J.R., Larson D.R., Plevak M.E., Therneau T.M. & Greipp P.R. (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinical Proceedings 78, 21–33. [DOI] [PubMed] [Google Scholar]
- Lage M.J., Barber B.L., Harrison D.J. & Jun S. (2008) The cost of treating skeletal‐related events in patients with prostate cancer. American Journal of Managed Care 14, 317–322. [PubMed] [Google Scholar]
- Lipton A. (2007) Treatment of bone metastases and bone pain with bisphosphonates. Supportive Cancer Therapy 4, 92–100. [DOI] [PubMed] [Google Scholar]
- Lipton A. (2010) Implications of bone metastases and the benefits of bone‐targeted therapy. Seminars in Oncology 37(Suppl. 2), S15–S29. [DOI] [PubMed] [Google Scholar]
- Mohler J., Bahnson R.R., Boston B., Busby J.E., D'amico A., Eastham J.A., Enke C.A., George D., Horwitz E.M., Huben R.P., Kantoff P., Kawachi M., Kuettel M., Lange P.H., Macvicar G., Plimack E. R., Pow‐Sang J.M., Roach M.R., Rohren E., Roth B.J., Shrieve D.C., Smith M.R., Srinivas S., Twardowski P. & Walsh P.C. (2010) NCCN clinical practice guidelines in oncology: prostate cancer. Journal of the National Comprehensive Cancer Network, 8, 162–200. [DOI] [PubMed] [Google Scholar]
- von Moos R., Sternberg C., Body J.J. & Bokemeyer C. (2013) Reducing the burden of bone metastases: current concepts and treatment options. Supportive Care in Cancer 21, 1773–1783. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2016) NCCN Guidelines [Online]. Available at: http://www.nccn.Guidelinesorg/professionals/physician_gls/f_guidelines.asp#site (accessed March 2016).
- Norgaard M., Jensen A.O., Jacobsen J.B., Cetin K., Fryzek J.P. & Sorensen H.T. (2010) Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). Journal of Urology 184, 162–167. [DOI] [PubMed] [Google Scholar]
- Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fossa S.D., Chodacki A., Wiechno P., Logue J., Seke M., Widmark A., Johannessen D.C., Hoskin P., Bottomley D., James N.D., Solberg A., Syndikus I., Kliment J., Wedel S., Boehmer S., Dall'oglio M., Franzen L., Coleman R., Vogelzang N.J., O'bryan‐Tear C.G., Staudacher K., Garcia‐Vargas J., Shan M., Bruland O.S. & Sartor O. (2013) Alpha emitter radium‐223 and survival in metastatic prostate cancer. New England Journal of Medicine 369, 213–223. [DOI] [PubMed] [Google Scholar]
- Payne H., Clarke N., Huddart R., Parker C., Troup J. & Graham J. (2013) Nasty or NICE? Findings from a UK survey to evaluate the impact of the National Institute for Health and Clinical Excellence (NICE) clinical guidelines on the management of prostate cancer. Clinical Oncology (Royal College of Radiologists) 25, 178–189. [DOI] [PubMed] [Google Scholar]
- Pockett R.D., Castellano D., Mcewan P., Oglesby A., Barber B.L. & Chung K. (2010) The hospital burden of disease associated with bone metastases and skeletal‐related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. European Journal of Cancer Care 19, 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje N.S., Yee A.J. & Roodman G.D. (2014) Advances in supportive care for multiple myeloma. Journal of the National Comprehensive Cancer Network 12, 502–511. [DOI] [PubMed] [Google Scholar]
- Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M.A., Coleman R.E., Reitsma D.J., Chen B.L. & Seaman J.J. (2003) Long‐term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double‐blind, multicenter, comparative trial. Cancer 98, 1735–1744. [DOI] [PubMed] [Google Scholar]
- Saad F., Chen Y.M., Gleason D.M. & Chin J. (2007a) Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clinical Genitourinary Cancer 5, 390–396. [DOI] [PubMed] [Google Scholar]
- Saad F., Lipton A., Cook R., Chen Y.M., Smith M. & Coleman R. (2007b) Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 110, 1860–1867. [DOI] [PubMed] [Google Scholar]
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., De Boer R., Lichinitser M., Fujiwara Y., Yardley D., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., & Braun A. O.B.O.T.B.C.S.S. Investigators (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double‐blind study. Journal of Clinical Oncology 28, 5132–5139. [DOI] [PubMed] [Google Scholar]
- Terpos E., Morgan G., Dimopoulos M.A., Drake M.T., Lentzsch S., Raje N., Sezer O., Garcia‐Sanz R., Shimizu K., Turesson I., Reiman T., Jurczyszyn A., Merlini G., Spencer A., Leleu X., Cavo M., Munshi N., Rajkumar S.V., Durie B.G. & Roodman G.D. (2013) International Myeloma Working Group recommendations for the treatment of multiple myeloma‐related bone disease. Journal of Clinical Oncology 31, 2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Berenson J., Raje N. & Roodman G.D. (2014) Management of bone disease in multiple myeloma. Expert Review of Hematology 7, 113–125. [DOI] [PubMed] [Google Scholar]
- Terpos E., Kleber M., Engelhardt M., Zweegman S., Gay F., Kastritis E., van de Donk N.W., Bruno B., Sezer O., Broijl A., Bringhen S., Beksac M., Larocca A., Hajek R., Musto P., Johnsen H.E., Morabito F., Ludwig H., Cavo M., Einsele H., Sonneveld P., Dimopoulos M.A. & Palumbo A. European Myeloma Network (2015) European Myeloma Network guidelines for the management of multiple myeloma‐related complications. Haematologica 100, 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S., Bosserman L.D., Geoghegan C., Hillner B.E., Theriault R.L., Zuckerman D.S., Von Roenn J.H. & American Society of Clinical Oncology (2011) American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone‐modifying agents in metastatic breast cancer. Journal of Clinical Oncology 29, 1221–1227. [DOI] [PubMed] [Google Scholar]
- Weinfurt K.P., Castel L.D., Li Y., Timbie J.W., Glendenning G.A. & Schulman K.A. (2004) Health‐related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Medical Care 42, 164–175. [DOI] [PubMed] [Google Scholar]
- Weinfurt K.P., Li Y., Castel L.D., Saad F., Timbie J.W., Glendenning G.A. & Schulman K.A. (2005) The significance of skeletal‐related events for the health‐related quality of life of patients with metastatic prostate cancer. Annals of Oncology 16, 579–584. [DOI] [PubMed] [Google Scholar]
- Woo S.B., Hellstein J.W. & Kalmar J.R. (2006) Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine 144, 753–761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Current cancer treatments at diagnosis, by malignancy type and by country: data from the detailed questionnaire.


