Abstract
Patulin is a naturally occurring mycotoxin produced by a number of molds and may contaminate a wide variety of food products. In practice, patulin’s main societal relevance concerns apple juice and its products. Multiple advisory bodies, including the U.S. Food and Drug Administration and the World Health Organization, recommend that producers monitor and limit patulin levels in apple juice products. The mechanism of patulin toxicity remains largely unknown. Here we show that patulin induces proteotoxic stress in the yeast S. cerevisiae. The transcription factor Rpn4 controls the abundance of the proteasome, the complex multisubunit protease that destroys proteins, including misfolded proteins. Rpn4 protein is strongly induced by patulin, and Rpn4 levels normalize over time, consistent with homeostatic regulation. A rpn4Δ mutant is highly sensitive to patulin, confirming the physiologic relevance of this response. Rpn4 is known to be regulated both transcriptionally and post-translationally. Patulin induces both pathways of regulation, but the post-transcriptional pathway predominates in controlling Rpn4 protein levels. These results indicate that proteotoxicity represents a major aspect of patulin toxicity. They not only have implications for patulin detoxification but in addition suggest the possibility of some potentially useful patulin applications.
Keywords: patulin, proteotoxicity, Rpn4, proteasome, yeast
1. Introduction
Patulin is a mycotoxin produced by many different molds. It was first isolated from Penicillium patulum, which was later renamed Penicillium griseofulvum (Bennett and Klich, 2003). Its main societal relevance is as a food contaminant, and its associated molds may grow on a wide variety of foods including fruits, grains, and cheeses (U.S. Food and Drug Administration, 2001). Foods that have rotted and have surface damage are at particularly high risk. In practice, however, safety concerns related to patulin are largely limited to apple juice and apple juice products (U.S. Food and Drug Administration, 2001). Significant contaminations of commercial apple juice products by patulin have been documented in various countries (Iwahashi et al., 2006), and several advisory bodies, including the U.S. Food and Drug Administration and the World Health Organization, recommend that producers control patulin levels so as to limit exposure to consumers (U.S. Food and Drug Administration, 2001; World Health Organization, 2001). These recommendations are particularly relevant to young children who may consume higher amounts of apple juice relative to body weight than adults.
Early attempts to develop patulin as an antibiotic were largely abandoned after recognition of patulin’s toxicity (Bennett and Klich, 2003). The molecular basis for patulin’s toxicity remains largely unknown. As a potent electrophile (Fig. 1A), patulin is capable of reacting with a wide range of macromolecules (Fliege and Metzler, 1999; Fliege and Metzler, 2000). DNA damage, for example, may contribute to patulin’s toxicity (Schumacher et al., 2006).
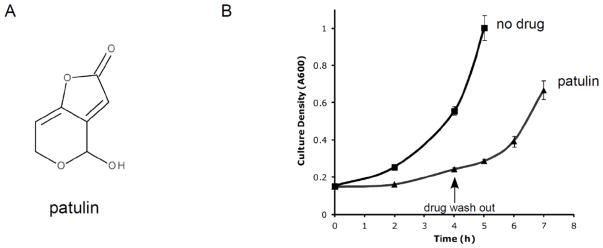
Figure 1. Patulin structure and reversible toxicity.
A) Chemical structure of patulin.
B) Growth of wild-type cells in liquid culture during treatment with patulin (50 μg/mL). After 4 hours of treatment, the drug was washed out. Error bars represent standard deviations from two independent cultures.
Protein misfolding is generally toxic, and may harm cells through multiple mechanisms. A change in protein structure can lead to loss of normal protein function. However, protein misfolding may also cause gain-of-function toxicities by promoting promiscuous protein-protein interactions, which may lead to the formation of insoluble protein aggregates. Protein misfolding is thought to play a key role in a number of human neurodegenerative diseases, including Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Hipp et al., 2014).
The proteasome is a 2.5 MDa multisubunit complex capable of destroying proteins (Matyskiela and Martin, 2013). It houses three distinct proteolytic active sites within a central 700 kDa cylindrical chamber known as the core particle (CP). At either end of the CP is an approximately 900 kDa complex known as the regulatory particle (RP), which orchestrates the various aspects of proteasome function. Most substrates are targeted to the proteasome through covalent modification by the small protein ubiquitin. The RP recognizes the substrate via the ubiquitin tag, unfolds the substrate, removes the ubiquitin molecules, and directs the substrate into the CP for degradation (Finley et al., 2012).
The term proteotoxicity has been used to refer to conditions which compromise or overwhelm the cell’s capacity for protein quality control. Misfolded proteins are a major cause of proteotoxicity, and accordingly, cells have developed complex stress responses to identify and eliminate misfolded proteins. One such proteotoxic stress response is mediated by the transcription factor Rpn4 (Mannhaupt et al., 1999; Xie and Varshavsky, 2001). Rpn4 is capable of controlling proteasome abundance in cells by carrying out the coordinated transcription of all (~35) proteasome genes and a number of proteasome-interacting proteins. It does this by recognizing a promoter element known as the PACE motif (proteasome associated control element; Mannhaupt et al., 1999). Rpn4 is itself a substrate of the proteasome. Under normal conditions, Rpn4 is rapidly destroyed, resulting in low baseline levels (Xie and Varshavsky, 1999; Guerra-Moreno et al., 2015). However, any stress which inhibits or overwhelms proteasome function may result in stabilization of Rpn4 protein. This leads to increased Rpn4 transcriptional activity that promotes new proteasome synthesis until proteasome function is restored to levels adequate to carry out rapid Rpn4 degradation, creating a homeostatic feedback loop (Ju et al., 2004; Wang et al, 2008; Wang et al., 2010). This pathway is functionally conserved in higher organisms where the stress-inducible transcription factor Nrf1 plays a similar role (Radhakrishnan et al., 2010; Steffen et al., 2010).
Here we show that patulin is a potent inducer of the Rpn4 stress response. Cells lacking Rpn4 show increased sensitivity to patulin, indicating the physiologic relevance of this response. Patulin’s regulation of Rpn4 appears to be largely post-transcriptional in nature. These results indicate that proteotoxicity represents a major aspect of patulin toxicity.
2. Materials and Methods
2.1 Yeast strains and chemicals
Yeast were cultured in YPD medium at 30°C unless indicated otherwise. YPD consisted of 1% yeast extract, 2% Bacto-peptone, and 2% dextrose. The wild-type (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and rpn4Δ (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rpn4::KAN) strains have been previously described (Hanna et al., 2014). Patulin, sodium arsenite, α-amanitin, actinomycin D, cycloheximide, and tunicamycin were obtained from Sigma Aldrich. Patulin, actinomycin D, and tunicamycin were prepared in dimethyl sulfoxide (DMSO); sodium arsenite, cycloheximide, and α-amanitin were prepared in water. Chemical structure images were prepared with the Biovia Draw program.
2.2 Generation of the anti-Rpn4 antibody
The full length open reading frame of RPN4 was cloned into pET-45b which provides for an N-terminal 6x-histidine tag. The protein was expressed in E. coli (BL21 DE3) and purified by Nickel-affinity chromatography. This protein was used to generate a rabbit polyclonal antibody (Covance). The antibody was validated using whole cell extracts from wild-type and rpn4Δ strains (Fig. 2A), which confirmed complete absence of the assigned immunoreactive band in the rpn4Δ mutant.
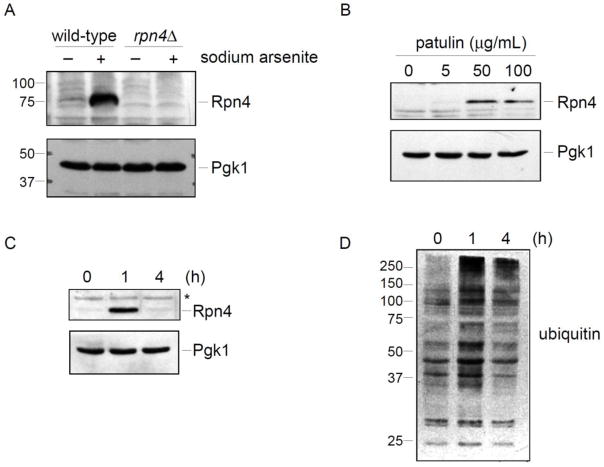
Figure 2. Patulin induces the Rpn4 stress response.
A) Validation of the Rpn4 antibody. Wild-type and rpn4Δ strains were treated with sodium arsenite (1 mM) for one hour. Whole cell extracts were prepared and evaluated by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Pgk1 antibody (lower panel; loading control).
B) Rpn4 protein levels after patulin treatment for one hour at the indicated concentrations. Whole cell extracts from a wild-type strain were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Pgk1 antibody (lower panel; loading control).
C) Temporal dynamics of the Rpn4 response after induction by patulin (50 μg/mL). Whole cell extracts from a wild-type strain were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Pgk1 antibody (lower panel; loading control). Asterisk, non-specific immunoreactive band.
D) Levels of ubiquitin conjugates in whole cell extracts, as determined by SDS-PAGE followed by immunoblot with anti-ubiquitin antibody. The same whole cell extracts were used to prepare the immunoblots in panels C and D. Therefore, the Pgk1 loading control of panel C applies to panel D as well.
2.3 Immunoblot Analysis
Whole cell extracts were prepared from exponential phase cultures using a lithium acetate/sodium hydroxide method which has been previously described in detail (Weisshaar et al., 2017). In the experiment of Figure 5C, whole cell extracts were prepared by resuspending cell pellets in 1X Laemmli loading buffer and boiling for 5 min. Where indicated, actinomycin D (20 μg/mL) or α-amanitin (20 μg/mL) was used to inhibit mRNA transcription. Where indicated, cycloheximide (100 μg/mL) was used to inhibit protein synthesis. Analysis was by standard SDS-polyacrylamide gel electrophoresis (PAGE) followed by immunoblot. The following antibodies were used: anti-Pgk1 (Invitrogen; 459250), anti-ubiquitin (Santa Cruz; SC-8017), anti-Rpn4 (this study), and anti-Rpn8 (Hanna et al., 2007).
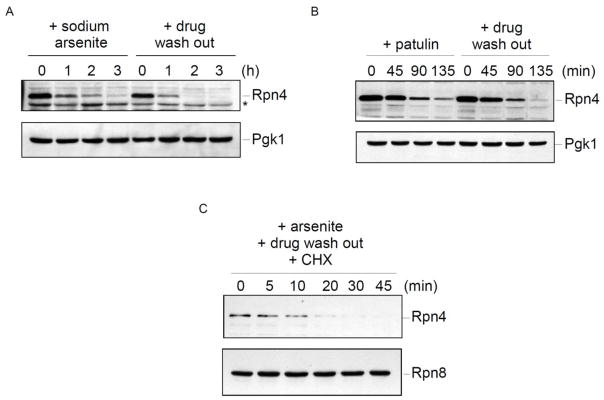
Figure 5. Precise regulation of Rpn4 protein levels in response to stress.
A) Steady-state Rpn4 protein levels after treatment with sodium arsenite (1 mM). The zero time point reflects Rpn4 induction after one hour of drug treatment. Sodium arsenite was then maintained in the culture or washed out, and Rpn4 levels were followed over time. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Pgk1 antibody (lower panel; loading control).
B) Steady-state Rpn4 protein levels after treatment with patulin (50 μg/mL). The zero time point reflects Rpn4 induction after one hour of drug treatment. Extracts were prepared and analyzed as in panel A.
C) Cycloheximide chase analysis of Rpn4 turnover. Rpn4 protein was induced by treatment with sodium arsenite (1 mM) for one hour. The drug was then washed out and cycloheximide (100 μg/mL) was added to inhibit new protein synthesis. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Rpn8 antibody (lower panel; loading control).
2.4 Phenotypic analysis
Overnight cultures were normalized by optical density and spotted in three-fold serial dilutions onto YPD plates lacking or containing patulin (5 μg/mL) and incubated at 37°C for the indicated times. The increased temperature augments patulin toxicity.
2.5 RT-PCR
Reverse transcription-PCR was performed as described previously (Guerra-Moreno et al., 2016; Weisshaar et al., 2017). The following oligonucleotides were used to amplify RPN4: 5-CTAATCCAGGTCACAGTCAG-3 and 5-GGTACATACGGGTCTGATAC-3. The following oligonucleotides were use to amplify ACT1: 5-CTGGTATGTTCTAGCGCTTG-3 and 5-GATACCTTGGTGTCTTGGTC-3. To monitor HAC1 splicing, the following oligonucleotides were used: 5-CACTCGTCGTCTGATACG-3 and 5-CATTCAATTCAAATGAATTCAAACCTG-3. As a positive control for HAC1 splicing, cells were treated with tunicamycin (5 μg/mL) for 1 hour.
3. Results
3.1. Similarities between patulin and trivalent arsenic
We recently carried out a proteomic analysis of the cellular response to trivalent arsenic in the yeast S. cerevisiae (Guerra-Moreno et al., 2015). This analysis, which detected nearly 4,600 proteins (out of a predicted 6,000 in this organism), indicated a comprehensive remodeling of the proteome in response to this drug. Central to the cellular response to arsenic were broad changes in pathways related to protein homeostasis, or proteostasis, including regulation of both protein synthesis and protein degradation. In particular we observed an approximately 20-fold upregulation of Rpn4 at the protein level at one hour after arsenic treatment, and this was accompanied by an increase in the protein levels of most proteasome subunits (Guerra-Moreno et al., 2015; Guerra-Moreno et al., 2016). This upregulation of Rpn4 was consistent with a response to proteotoxic stress, and appeared to be efficacious inasmuch as Rpn4 levels, after initially rising, tended to normalize over time.
The cellular response to patulin toxicity in S. cerevisiae has been previously studied using microarray analysis of gene expression (Iwahashi et al., 2006). We noticed a number of similarities between our proteomic analysis of arsenic toxicity and this gene expression analysis of patulin toxicity. First, we looked at the 20 most upregulated genes in response to patulin (Iwahashi et al., 2006). Of these, the corresponding protein was detected in our proteomic analysis of arsenic toxicity in 14 of the cases. Of these 14, ten were among the 100 most upregulated proteins in the proteome. Second, and perhaps more interesting, a large number of proteasome subunit genes was upregulated at the RNA level in response to patulin (Iwahashi et al., 2006). This suggested that cells exposed to patulin might be under proteotoxic stress, triggering the Rpn4 stress response. We set out to test this hypothesis in this work.
3.2. Patulin toxicity is reversible
We first sought to establish conditions under which patulin toxicity was reversible to ensure the physiologic relevance of our studies. We treated logarithmically growing yeast cultures with patulin at a concentration of 50 μg/mL. This caused a significant growth defect, with a doubling time of approximately 5 hours (Fig. 1B). After 4 hours of patulin treatment, we washed out the drug. If the cells had died or had been irreparably damaged, they would have been unable to resume normal growth. By contrast, we saw that within two hours of drug wash-out, the cells resumed growth with a normal doubling time of approximately 1.5 hours (Fig. 1B). Thus, any cellular effects observed under these conditions are likely to reflect a physiologically relevant stress response, and not merely non-specific effects related to cell death.
3.3. Patulin is a potent inducer of the Rpn4 stress response
Under conditions that inhibit or overwhelm protein degradation by the proteasome, Rpn4 is upregulated at the protein level. This results in the coordinated transcription of proteasome genes, resulting in the generation of new proteasome complexes (Mannhaupt et al., 1999). Rpn4 is itself a substrate of the proteasome (Xie and Varshavsky, 2001), and thus new proteasome biogenesis continues until sufficient proteasome capacity exists to restore normal rates of Rpn4 degradation, normalizing the proteotoxic stress response.
We previously showed by proteomic analysis that Rpn4 is strongly upregulated by trivalent arsenic (Guerra-Moreno et al., 2015). To more easily measure Rpn4 protein levels, we prepared an antibody against full length recombinant Rpn4 that had been expressed in and purified from bacteria. In the absence of stress, Rpn4 protein levels were low, being just at or below the threshold of detection (Fig. 2A). However, after arsenic treatment, Rpn4 levels rose dramatically (Fig. 2A), consistent with the prior proteomic analysis (Guerra-Moreno et al., 2015). The induced immunoreactive band was completely absent in a rpn4Δ mutant, confirming the accuracy of the band assignment (Fig. 2A). Rpn4 protein migrated slightly above its predicted molecular weight of 60 kDa. The reason for this remains unclear although the protein does show highly charged areas which can result in electrophoretic anomalies.
We next looked at induction of the Rpn4 stress response by patulin. Rpn4 was strongly induced at a concentration of 50 μg/mL (Fig. 2B). This likely represented near maximal induction of the response as Rpn4 levels were not further increased at 100 μg/mL. At a lower concentration of 5 μg/mL, Rpn4 showed a much lesser but still detectable increase in Rpn4 levels. Thus, patulin induces the Rpn4 response in a dose-dependent manner.
We looked at the temporal nature of Rpn4 induction by patulin. Although Rpn4 levels were significantly induced at 1 hour after treatment, by 4 hours Rpn4 levels had returned to near baseline (Fig. 2C). This transient induction of Rpn4 protein levels is consistent with its known homeostatic regulation, and again was quite similar to what we had previously observed with arsenic (Guerra-Moreno et al., 2015). Ubiquitin-protein conjugates represent major degradative substrates of the proteasome, and as such ubiquitin conjugates tend to accumulate in response to proteotoxic stress. We looked at total ubiquitin immunoreactive material in response to patulin treatment under the same conditions. We observed an increase in total ubiquitin immunoreactive material, especially in the high molecular weight range, at one hour after patulin treatment (Fig 2D). After this initial spike, the levels of ubiquitin conjugates tended to decrease over time, similar to the dynamics observed with Rpn4 and again consistent with a homeostatic stress response (Fig. 2D).
The preceding data suggest an important role for Rpn4 and protein degradation in protecting cells against patulin toxicity. To test this model directly, we treated cells with patulin and measured survival by a phenotypic plating assay. As shown in Figure 3, the rpn4Δ mutant showed a dramatic sensitivity to patulin treatment.
Figure 3. Impaired growth of the rpn4Δ mutant after patulin treatment.
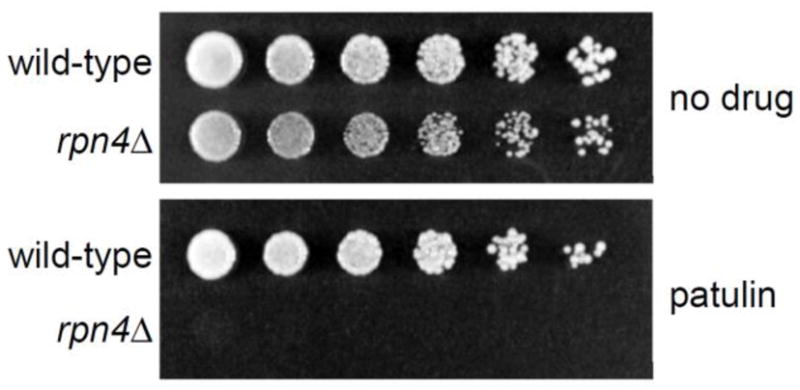
Wild-type and rpn4Δ cells were spotted in three-fold serial dilutions on plates lacking or containing patulin (5 μg/mL) and cultured at 37°C for 4 days.
3.4 Rpn4 upregulation by patulin is largely mediated by post-transcriptional mechanisms
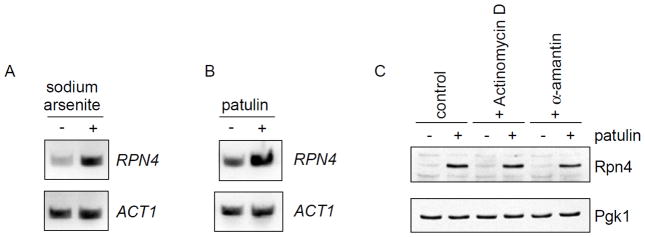
As described above, Rpn4 protein levels can be regulated by protein degradation and the rate of degradation is thought to reflect the cell’s degradative capacity (Xie and Varshavsky, 2001). Rpn4 is also known to be regulated at the transcriptional level. Multiple transcription factors including Yap1, Pdr1/3, and Hsf1 are known to regulate RPN4 transcription (Owsianik et al., 2002; Hahn et al., 2006; Salin et al., 2008), although the physiologic significance of this transcriptional regulation is less well established. We used RT-PCR to determine whether RPN4 was transcriptionally induced in response to stress. We started by confirming the induction of RPN4 transcription by arsenic (Fig. 4A), which was previously demonstrated by microarray analysis (Haugen et al., 2004). Cells showed a comparable induction of RPN4 transcription in response to patulin (Fig. 4B). We wanted to gauge the relative contributions of transcriptional and post-transcriptional regulation of Rpn4 in response to patulin toxicity. To eliminate any transcriptional contribution, we employed two structurally and mechanistically distinct inhibitors of transcription, actinomycin D and α-amanitin, at concentrations known to completely or nearly completely block mRNA transcription (Schultz and Hall, 1976; Bensaude, 2011). Neither compound had any effect on Rpn4 protein accumulation in response to patulin treatment (Fig. 4C). These results suggest that induction of the Rpn4 response upon patulin treatment is largely mediated by post-transcriptional means. Rpn4 is known to be an efficient substrate of the proteasome with a half-life under non-stress conditions on the order of minutes (Xie and Varshavsky, 2001). Thus, post-translational degradation appears to be the major determinant of Rpn4 induction in response to patulin.
Figure 4. Induction of Rpn4 protein levels by patulin appears largely post-transcriptional.
A–B) Induction of RPN4 transcription, as determined by RT-PCR, after treatment with sodium arsenite (1 mM) or patulin (50 μg/mL) for one hour. ACT1 serves as a control.
C) Rpn4 protein levels after treatment with patulin (30 μg/mL) in the absence or presence of the transcriptional inhibitors actinomycin D (20 μg/mL) or α-amanitin (20 μg/mL). Whole cell extracts from a wild-type strain were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Rpn4 antibody (upper panel) or anti-Pgk1 antibody (lower panel; loading control).
3.5 Precise regulation of Rpn4 protein levels in response to proteotoxic stress
After induction by proteotoxic stress, Rpn4 protein levels appear to normalize over time (Fig. 2C; Guerra-Moreno et al., 2015). This suggests that the cellular stress response is efficacious and that as proteotoxicity is reduced, less Rpn4 protein is needed. To look at this in further detail, we induced Rpn4 by treating cells with sodium arsenite for one hour. We then followed the steady-state abundance of Rpn4 over time (Fig. 5A). There was a gradual decrease over a three-hour time period. In a parallel experiment, we washed out the drug after the initial one hour treatment. This would be expected to decrease proteotoxic stress as there would be less drug available for import during the time course. Strikingly, Rpn4 protein levels normalized more quickly under these conditions (Fig. 5A). These results suggest that Rpn4 levels may be quite sensitive to the level of proteotoxic stress, and can be regulated in a precise manner.
We repeated this experiment with patulin (Fig. 5B). Again, the rate of Rpn4 normalization over time was faster under conditions where the drug had been washed out. However, the effect did appear more robust with sodium arsenite treatment. Yeast possess a dedicated arsenic efflux system (Maciaszczyk-Dziubinska et al., 2011) which likely contributes to arsenic detoxification, particularly after the drug has been washed out. It is unknown whether a similar efflux system exists for patulin.
Previous measurements of the turnover of Flag-tagged Rpn4 indicated a very short half-life, on the order of several minutes (Xie and Varshavsky, 2001). We were unable to measure the rate of Rpn4 turnover under non-stress conditions owing to the very low baseline levels of Rpn4 (Fig. 2A–B). Instead, we induced Rpn4 protein levels by treatment with sodium arsenite, washed out the drug, and then treated cells with cycloheximide to inhibit new protein synthesis. As shown in Figure 5C, Rpn4 showed very rapid degradation, with a half-life on the order of five to ten minutes. Together, these results suggest that Rpn4 protein levels are finely tunable in response to varying degrees of proteotoxic stress, and support the idea that, at least under the stressors studied here, they may be principally regulated at the post-transcriptional level.
3.6 Patulin does not induce the unfolded protein response
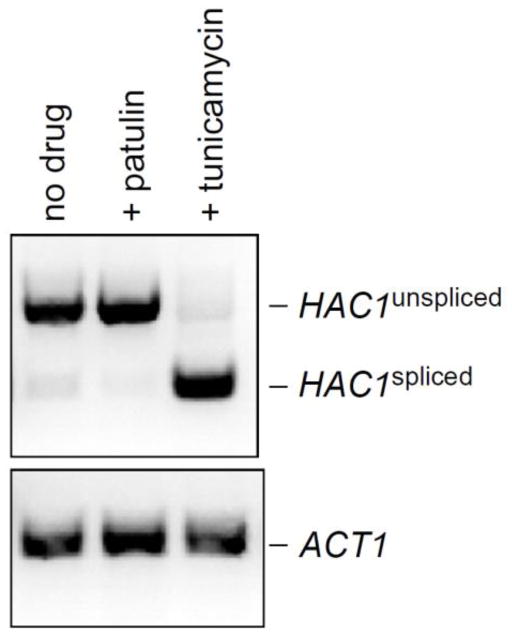
In addition to the Rpn4 response, cells harbor additional proteotoxic stress responses. The unfolded protein response (UPR) detects protein misfolding in the endoplasmic reticulum (ER) and coordinates a comprehensive cellular response to improve ER proteostasis (Walter and Ron, 2011). A key aspect of the UPR involves activation of the transcription factor Hac1 by the ER membrane protein Ire1. By an unusual regulatory process, Ire1 promotes the splicing of HAC1 mRNA (Cox and Walter, 1996) resulting in accumulation of Hac1 protein which then coordinates a broad transcriptional program whose targets include ER chaperones, components of the ER-associated degradation (ERAD) pathway, secretory genes, phospholipid synthesis genes, and many others (Travers et al., 2000). Splicing of HAC1 thus serves as a sensitive measure of UPR induction.
To determine whether patulin was capable of inducing the UPR, we monitored HAC1 splicing by RT-PCR. As a positive control, we used tunicamycin, an inhibitor of ER glycosylation and a known inducer of the UPR. As shown in Figure 6, tunicamycin strongly induced HAC1 splicing, as expected. By contrast, no induction of HAC1 splicing was observed in response to patulin treatment (Fig. 6).
Figure 6. Patulin fails to induce the unfolded protein response.
HAC1 splicing, which is a key aspect of the unfolded protein response, was measured by RT-PCR after treatment with patulin (30 μg/mL) for one hour. Tunicamycin, a known inducer of the unfolded protein response, was used as a positive control. ACT1 (lower panel) serves as a control.
4. Discussion
Here we have used yeast to better understand the toxicity of the naturally occurring mycotoxin patulin. Our data indicate that proteotoxicity is a major aspect of patulin’s effect on cells. In particular, patulin appears to be a potent inducer of the Rpn4 stress response. This response is best known for controlling the abundance of the proteasome in response to changing cellular conditions (Mannhaupt et al., 1999; Xie and Varshavsky, 2001; Ju et al., 2004; Hanna and Finley, 2007). Rpn4 protein levels are thought to reflect the functional degradative capacity of the cell’s proteasomes. Thus, any stress which either inhibits proteasome function or increases demand for proteasome function could stimulate Rpn4 accumulation. This raises the question of why patulin induces Rpn4 so potently. Previous work has shown that patulin is capable of covalently modifying proteins (Fliege and Metzler, 1999). Patulin showed strong affinity for free thiol groups, but was also capable of modifying lysine and histidine side chains, as well α-amino groups (Fliege and Metzler, 1999). Such modification may be sufficient to induce protein misfolding. In some cases, patulin was even capable of intra- and intermolecular crosslinking of proteins. Thus, induction of protein misfolding by patulin could increase demand for proteasome-mediated protein degradation, resulting in stimulation of the Rpn4 stress response. Patulin’s potential spectrum of substrates would be anticipated to be quite broad given patulin’s relatively non-specific mode of modifying proteins.
Like patulin, trivalent arsenic is a potent inducer of the Rpn4 response (Guerra-Moreno et al., 2015). Moreover, trivalent arsenic is also known to covalently modify proteins (Zhang et al., 2010). Thus, we suspect the two drugs may share at least some aspects of their toxicity and mechanism of action. In addition to its role as an environmental toxin, trivalent arsenic is also a highly effective FDA approved therapy for acute promyelocytic leukemia and has helped transform this previously fatal disease into one with cure rates exceeding 90% (Rice and de The, 2014). This leukemia is caused by a cytogenetic translocation between chromosomes 15 and 17 which results in the novel fusion protein PML-RARα. Trivalent arsenic covalently binds this oncogenic fusion protein, promoting its degradation by proteasome (Zhang et al., 2010).
At present, patulin’s main importance to society is as a naturally occurring toxin that may contaminate foodstuffs, especially apple juice products. At a minimum, our data suggest patulin may also be a useful research tool to induce proteotoxicity. In addition, patulin might have clinically useful properties. Early work to develop patulin as an antibiotic failed on account of patulin’s toxicity (Bennett and Klich, 2003). However, there may be disease contexts, for example certain cancers, in which induction of proteotoxicity may be therapeutic. Further efforts to better understand patulin’s cellular and molecular effects could be helpful in these contexts.
Highlights.
The mycotoxin patulin induces the Rpn4 proteotoxic stress response in yeast.
Induction of Rpn4 protein appears to occur mainly by post-transcriptional mechanisms.
Rpn4 protein levels are precisely regulated in response to proteotoxic stress.
Acknowledgments
This work was supported by NIH grant DP5-OD019800. The authors thank members of the Hanna lab for comments on the manuscript. The authors report no financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Finley D, Ulrich HD, Sommer T, Kaiser P. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics. 2012;192:319–60. doi: 10.1534/genetics.112.140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R, Metzler M. The mycotoxin patulin induces intra- and intermolecular protein crosslinks in vitro involving cysteine, lysine, and histidine side chains, and alpha-amino groups. Chem Biol Interact. 1999;123:85–103. doi: 10.1016/s0009-2797(99)00123-4. [DOI] [PubMed] [Google Scholar]
- Fliege R, Metzler M. Electrophilic properties of patulin. N-acetylcysteine and glutathione adducts. Chem Res Toxicol. 2000;13:373–81. doi: 10.1021/tx9901480. [DOI] [PubMed] [Google Scholar]
- Guerra-Moreno A, Isasa M, Bhanu MK, Waterman DP, Eapen VV, Gygi SP, Hanna J. Proteomic Analysis Identifies Ribosome Reduction as an Effective Proteotoxic Stress Response. J Biol Chem. 2015;290:29695–706. doi: 10.1074/jbc.M115.684969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Moreno A, Hanna J. Tmc1 Is a Dynamically Regulated Effector of the Rpn4 Proteotoxic Stress Response. J Biol Chem. 2016;291:14788–95. doi: 10.1074/jbc.M116.726398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Neef DW, Thiele DJ. A stress regulatory network for co-ordinated activation of proteasome expression mediated by yeast heat shock transcription factor. Mol Microbiol. 2006;60:240–51. doi: 10.1111/j.1365-2958.2006.05097.x. [DOI] [PubMed] [Google Scholar]
- Hanna J, Finley D. A proteasome for all occasions. FEBS Lett. 2007;581:2854–61. doi: 10.1016/j.febslet.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Meides A, Zhang DP, Finley D. A ubiquitin stress response induces altered proteasome composition. Cell. 2007;129:747–59. doi: 10.1016/j.cell.2007.03.042. [DOI] [PubMed] [Google Scholar]
- Hanna J, Waterman D, Isasa M, Elsasser S, Shi Y, Gygi S, Finley D. Cuz1/Ynl155w, a zinc-dependent ubiquitin-binding protein, protects cells from metalloid-induced proteotoxicity. J Biol Chem. 2014;289:1876–85. doi: 10.1074/jbc.M113.534032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen AC, Kelley R, Collins JB, Tucker CJ, Deng C, Afshari CA, Brown JM, Ideker T, Van Houten B. Integrating phenotypic and expression profiles to map arsenic-response networks. Genome Biol. 2004;5:R95. doi: 10.1186/gb-2004-5-12-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24:506–14. doi: 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Iwahashi Y, Hosoda H, Park JH, Lee JH, Suzuki Y, Kitagawa E, Murata SM, Jwa NS, Gu MB, Iwahashi H. Mechanisms of patulin toxicity under conditions that inhibit yeast growth. J Agric Food Chem. 2006;54:1936–42. doi: 10.1021/jf052264g. [DOI] [PubMed] [Google Scholar]
- Ju D, Wang L, Mao X, Xie Y. Homeostatic regulation of the proteasome via an Rpn4-dependent feedback circuit. Biochem Biophys Res Commun. 2004;321:51–7. doi: 10.1016/j.bbrc.2004.06.105. [DOI] [PubMed] [Google Scholar]
- Mannhaupt G, Schnall R, Karpov V, Vetter I, Feldmann H. Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 1999;450:27–34. doi: 10.1016/s0014-5793(99)00467-6. [DOI] [PubMed] [Google Scholar]
- Maciaszczyk-Dziubinska E, Migocka M, Wysocki R. Acr3p is a plasma membrane antiporter that catalyzes As(III)/H(+) and Sb(III)/H(+) exchange in Saccharomyces cerevisiae. Biochim Biophys Acta. 2011;1808:1855–9. doi: 10.1016/j.bbamem.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Matyskiela ME, Martin A. Design principles of a universal protein degradation machine. J Mol Biol. 2013;425:199–213. doi: 10.1016/j.jmb.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Balzil L, Ghislain M. Control of 26S proteasome expression by transcription factors regulating multidrug resistance in Saccharomyces cerevisiae. Mol Microbiol. 2002;43:1295–308. doi: 10.1046/j.1365-2958.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KL, de Thé H. The acute promyelocytic leukaemia success story: curing leukaemia through targeted therapies. J Intern Med. 2014;276:61–70. doi: 10.1111/joim.12208. [DOI] [PubMed] [Google Scholar]
- Salin H, Fardeau V, Piccini E, Lelandais G, Tanty V, Lemoine S, Jacq C, Devaux F. Structure and properties of transcriptional networks driving selenite stress response in yeasts. BMC Genomics. 2008;9:333. doi: 10.1186/1471-2164-9-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LD, Hall BD. Transcription in yeast: alpha-amanitin sensitivity and other properties which distinguish between RNA polymerases I and III. Proc Natl Acad Sci U S A. 1976;73:1029–33. doi: 10.1073/pnas.73.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher DM, Müller C, Metzler M, Lehmann L. DNA-DNA cross-links contribute to the mutagenic potential of the mycotoxin patulin. Toxicol Lett. 2006;166:268–75. doi: 10.1016/j.toxlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Steffen J, Seeger M, Koch A, Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–58. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–58. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration. [accessed 02/02/2017];Patulin in Apple Juice, Apple Juice Concentrates and Apple Juice Products. 2001 http://www.fda.gov/Food/FoodborneIllnessContaminants/NaturalToxins/ucm212520.htm.
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu H, Ju D, Xie Y. Disruption of Rpn4-induced proteasome expression in Saccharomyces cerevisiae reduces cell viability under stressed conditions. Genetics. 2008;180:1945–53. doi: 10.1534/genetics.108.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu H, Ha SW, Ju D, Xie Y. Proteasomal degradation of Rpn4 in Saccharomyces cerevisiae is critical for cell viability under stressed conditions. Genetics. 2010;184:335–42. doi: 10.1534/genetics.109.112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar N, Welsch H, Guerra-Moreno A, Hanna J. Phospholipase Lpl1 Links Lipid Droplet Function with Quality Control Protein Degradation. Mol Biol Cell. 2017;28:716–725. doi: 10.1091/mbc.E16-10-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization: Thirty-fifth Report of the Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additive and Contaminants. World Health Organization; Geneva: 1990. [PubMed] [Google Scholar]
- Xie Y, Varshavsky A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc Natl Acad Sci U S A. 2001;98:3056–61. doi: 10.1073/pnas.071022298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de Thé H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328:240–3. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]