Abstract
There is increased awareness that patients with lung diseases develop muscle dysfunction. Muscle dysfunction is a major contributor to a decreased quality of life in patients with chronic pulmonary diseases. Furthermore, muscle dysfunction exacerbates lung disease outcome, as a decrease in muscle mass and function are associated with increased morbidity, often long after critical illness or lung disease has been resolved. As we are learning more about the role of metabolism in health and disease, we are appreciating more the direct role of metabolism in skeletal muscle homeostasis. Altered metabolism is associated with numerous skeletal muscle pathologies and, conversely, skeletal muscle diseases are associated with significant changes in metabolic pathways. In this review, we highlight the role of metabolism in the regulation of skeletal muscle homeostasis. Understanding the metabolic pathways that underlie skeletal muscle wasting is of significant clinical interest for critically ill patients as well as patients with chronic lung disease, in which proper skeletal muscle function is essential to disease outcome.
Keywords: skeletal muscle homeostasis, satellite cells, chronic obstructive pulmonary disease, acute respiratory distress syndrome, metabolic maladaptations
Skeletal muscle has many important biological functions, including insulating the internal organs, maintaining core body temperature, and supporting movement. Skeletal muscle makes up a significant portion of total human body mass and is a high-energy-consuming organ, both, at rest and during exercise (1). Healthy skeletal muscles display a tightly regulated metabolic homeostasis. Altered metabolism is associated with skeletal muscle pathologies seen in patients with diabetes, obesity, Pompe’s disease, pulmonary arterial hypertension, and cancer, among others (2–6). Skeletal muscle diseases, such as cachexia, sarcopenia, and muscular dystrophies, are associated with changes in metabolism (2). Skeletal muscle alterations and skeletal muscle wasting in patients with lung disease contribute to a poor clinical outcome and are associated with increased morbidity and mortality (7–9). Recently, various reports have pointed to the importance of metabolism in regulating the skeletal muscle stem cell niche, the satellite cells. The satellite cells regulate skeletal muscle repair and homeostasis. The aim of this review is to discuss the role of metabolism and skeletal muscle homeostasis in health and disease, and, specifically, lung disease–associated muscle wasting, as an important contributor to morbidity and mortality.
The Energy Demands of Skeletal Muscle in Health and Disease
Skeletal Muscle Energetics
Given its important role in body movement, skeletal muscle requires high rates of cellular metabolism and energy production. Cellular ATP is derived from several breakdown pathways: carbohydrates via glycolysis, fats via the fatty acid oxidation, and proteins via proteolysis, all leading to pyruvate and/or acetyl coenzyme A (CoA), which, in the presence of oxygen, are used in the mitochondria to generate ATP via oxidative phosphorylation (10). Skeletal muscle relies on each of these energy-generating processes, depending on activity status, substrate, oxygen demand, and need for energy (10). It is important to note that, beyond ATP production, mitochondria are necessary for generating metabolites that are essential building blocks for macromolecule synthesis, including lipids, proteins, and sugars. Furthermore, emerging data demonstrate that mitochondria participate as signaling organelles by releasing reactive oxygen species (ROS) to oxidize cysteine residues in proteins to alter their function, as well as metabolites, such as citrate, that generate acetyl-CoA levels for protein acetylation in the cytosol and nucleus (11, 12). The bioenergetic, biosynthetic, and signaling roles of mitochondria are under the control of many processes, including fission and fusion of mitochondria (i.e., mitochondrial dynamics and quality control of mitochondria, such as the balance between mitochondrial biogenesis and mitophagy) (13). Mitochondria dynamics are discussed in greater detail in later sections, as contributors to skeletal muscle maladaptations in patients with lung disease. Worth discussing here is the role of autophagy in skeletal muscle mitochondrial function and skeletal muscle repair. The importance of autophagy in skeletal muscle repair has been previously shown in reports demonstrating that surviving myofibers in a strenuous exercise model in mice are positive for autophatic vacuoles, which are prominent 2–7 days after strenuous exercise, commonly recognized as the repair phase of skeletal muscle repair after injury (14). Furthermore, a blunted autophagy response by 3-methyladine treatment significantly impaired the repair of skeletal muscle in C57BL/6 mice exposed to myotoxic injury (15). Specifically, expression of several autophagy-related proteins, such as unc-51 like autophagy activating kinase 1, was significantly greater in injured than in uninjured muscles, and significant recovery of muscle strength and mitochondrial function were evident 14 days after injury in injured, control mice compared with injured mice in which autophagy was impaired (15). These data suggest that an autophagic response is important in skeletal muscle mitochondrial function and skeletal muscle repair after injury. In patients with chronic obstructive pulmonary disease (COPD) and lung cancer cachectic mice autophagy markers are significantly elevated (16, 17). Future studies are needed to determine whether skeletal muscle–specific defects in the autophagic response in lung diseases worsen disease outcomes.
Skeletal Muscle Composition and Energy Demands
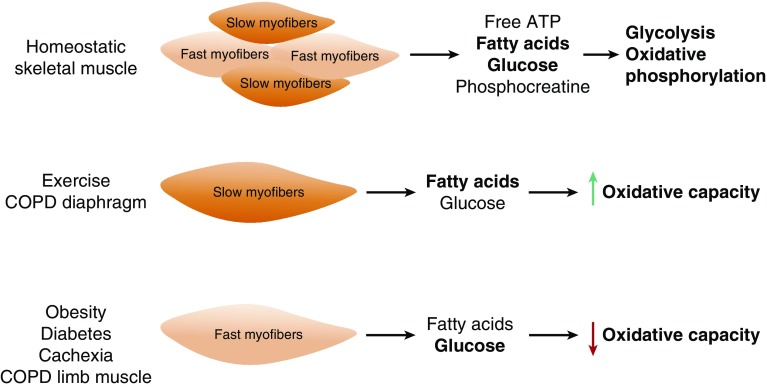
Skeletal muscle is composed of slow- and fast-twitch fibers that differ in the composition of contractile proteins, oxidative capacity, and substrate used for ATP production (Figure 1). Slow-twitch fibers are more resistant to fatigue, have higher oxidative capacity, and use fatty acids as their source for ATP production. Fast-twitch fibers fatigue easily, have lower oxidative capacity, and use glucose as their energy source via anaerobic glycolysis, an inefficient ATP-generating pathway when the O2 supply is limited or impaired (two molecules of ATP versus 32 molcules of ATP generated by aerobic glycolysis coupled to oxidative phosphorylation). Therefore, skeletal muscle fiber composition greatly impacts its metabolic program. Skeletal muscle fiber composition is plastic, and muscle fibers can switch from one fiber type to another, depending on physical activity status/demand. For example, endurance or aerobic exercise imposes a higher physical and metabolic demand on skeletal muscle, which, in turn, results in a switch from fast- to slow-twitch skeletal muscle fiber phenotype (1). Obesity and diabetes, where individuals have a higher caloric intake without an increase in energy demand, have been shown to lead to higher fast-twitch skeletal muscle fiber composition (1, 18). Fiber type switching is also observed in the diaphragm of patients with COPD, where a fast-to-slow fiber switch phenotype is associated with a significantly decreased diaphragmatic force (19). Diaphragm single-fiber maximal specific force measurements revealed a lower force in the fibers isolated from patients with COPD compared with that of patients with normal pulmonary function test. Furthermore, type 1 fibers from patients with COPD produced significantly lower specific force compared with type 2 fibers, suggesting not only a switch in fiber type, but adaptations within a fiber type as well (19). The increase in slow fibers in the diaphragm of patients with COPD is thought to be an adaptive response that increases resistance to fatigue (20); however, this remains a controversial topic and an attractive area of research. Differently from the diaphragm muscle of patients with COPD, a slow-to-fast muscle fiber switch is more commonly observed in the limb muscles of patients with COPDd and Figure 1 (21, 22). Although both respiratory and limb muscles are affected in patients with COPD, the limb muscles show greater reduced function (23). Given the varying degree of loss of function in the limb muscles of patients with COPD, analyses of both upper- and lower-limb muscles have been suggested when making predictions about the progression of skeletal muscle wasting in patients with COPD (24).
Figure 1.
Skeletal muscle fiber type and metabolic demands in health and disease. Skeletal muscle is comprised of both slow- and fast-twitch muscle fibers, and relies on glycolysis and oxidative phosphorylation to meet its energy demands. During endurance exercise and in the diaphragm of patients with chronic obstructive pulmonary disease (COPD), there is a switch toward a slow-twitch myofiber phenotype and increased fatty acid oxidation. Alternatively, the skeletal muscle of sedentary individuals, obese patients, those with diabetes or cachexia, and in the limb muscles of patients with COPD are comprised primarily of fast-twitch myofibers, which exhibit lowered oxidative capacity.
Metabolic Maladaptations and Disease States
Metabolic maladaptations can result in a pathological state, and, often, the skeletal muscle is negatively affected. It has been reported that skeletal muscle mitochondrial alterations (decreased pyruvate oxidation and increased ROS) in postoperative patients result in insulin resistance (25). In addition, approximately 50% of patients with cancer develop cachexia, a severe muscle-wasting syndrome associated with weakness and fatigue (26–28). Indeed, C2C12 myoblasts exposed to Lewis lung carcinoma–conditioned media displayed decreased oxygen consumption (including ATP-coupled oxygen consumption) and a transient increase in ROS (without changes in the content of mitochondrial complexes I, III, IV, or V) (27). Several reports have underscored the role of mitochondrial dysfunction in cachexia as a contributor to muscle wasting (29–31). The various molecular and metabolic mechanisms underlying skeletal muscle wasting in cancer-mediated cachexia were recently reviewed, highlighting the cross-talk among signals, such as atrophy, reduced protein synthesis, increased degradation, and the respective feedback regulation (28). In addition, transcriptome and cytokine profiling of human cancer-induced cachexia models in mice revealed that cancer cells secrete inflammatory cytokines, leading to increased fatty acid metabolism and p38 stress–response in skeletal muscle (32). Indeed, metabolomics profiling analyses confirmed that cachectic conditioned media induced lipolysis and fatty acid oxidation in human myotubes, an effect that was fully reversed by the pharmacological blockade of fatty acid utilization by the specific fatty acid import inhibitor etomoxir (32).
Adenosine Monophosphate-Activated Protein Kinase and Metabolism
We have recently reported that activation of adenosine monophosphate-activated protein kinase (AMPK), a master regulator of metabolism, leads to the up-regulation of the muscle-specific E-3 ubiquitin ligase, muscle-specific Ring finger protein 1, as a result of high CO2 exposure, resulting in decreased C2C12 myotube cross-sectional area and skeletal muscle dysfunction in mice (33). AMPK activity is essential in regulating the energy available to muscle fibers by specifically regulating fatty acid oxidation pathways (34). Activation of AMPK by phosphorylation of threonine 172 on its catalytic domain leads to a series of downstream signaling events, including the phosphorylation and inhibition of the downstream target, acetyl-CoA carboxylase. Acetyl-CoA carboxylase inhibition, in turn, reduces malonyl-CoA synthesis, a signal that activates carnitine palmitoyl transferase and increases mitochondrial import of long-chain acyl-CoA fatty acids in skeletal muscle, where fatty acids can be readily used to generate ATP via oxidative phosphorylation (34). The molecular pathways through which AMPK affects mitochondrial energetics and oxidative phosphorylation are highlighted clearly in an extensive review by Kahn and colleagues (34).
AMPK and In Vitro Skeletal Muscle Differentiation
AMPK activation directly affects skeletal muscle homeostasis by impairing differentiation. Activation of AMPK inhibits murine C2C12 myoblast differentiation in vitro (35). 5-Aminoimidazole-4-carboxamide ribonucleotide-mediated activation of AMPK in C2C12 myoblasts impairs myotube formation as early as 24 hours after exposure to low serum, an important differentiation signal. Accordingly, 5-aminoimidazole-4-carboxamide ribonucleotide-mediated activation of AMPK in C2C12 myoblasts resulted in significantly reduced levels of both embryonic myosin heavy chain and myogenin, two well known skeletal muscle differentiation markers (35). New metabolic targets for the control of myogenic differentiation in skeletal muscle were recently identified using an RNA interference screen of 50 known metabolic mediators of carbon catabolism (36). This study revealed that knockdown of three metabolic enzymes—phosphoglycerate kinase 1, hexose-6-phosphate dehydrogenase, and ATP citrate lyase (Acl or Acly)—induced differentiation in murine C2C12 myoblasts (36). Thus, metabolic dysregulation in skeletal muscle can have biological consequences on skeletal muscle homeostasis and function.
Satellite Cells and Skeletal Muscle Homeostasis
Skeletal Muscle Repair
Skeletal muscle is a highly dynamic tissue with ongoing cycles of injury and repair (37). In homeostatic muscle, both injury and repair happen simultaneously and repeatedly. In healthy muscle, homeostasis relies on the activity of the satellite cells, the stem cells of skeletal muscle. Quiescent skeletal muscle satellite cells are found below the basal lamina. After skeletal muscle insult or injury, the satellite cells can re-enter the cell cycle, become activated myoblasts, and fuse with one another to form immature myotubes or fuse directly to the site of muscle damage and help repair the injured muscle (38, 39). During the repair process, activated satellite cells give rise not only to myoblasts, but to a daughter satellite cell as well, replenishing the satellite cell pool (38, 39).
Metabolic Status and Muscle Repair
Muscle satellite cells must continuously reprogram their energy demands depending on their activity state and caloric intake has been shown to affect satellite cell mitochondrial mass and oxidative capacity, and satellite cell number (10, 40). Caloric restriction was associated with a significant increase in the transplant efficiency of the satellite cells and enhanced tibialis anterior muscle repair after freeze injury (40). A glycolytic or oxidative metabolic status dictates satellite cell myogenic activity (41). Specifically, a forced shift to oxidative metabolism by replacing glucose with galactose in the C2C12 growth medium results in increased nicotinamide adenine dinucleotide (NAD+)/NAD+ (reduced) levels and significantly reduced myogenic differentiation protein (MyoD) levels (41). Increased NAD+ levels by nicotinamide riboside also prevented senescence in the Mdx mouse model of muscular dystrophy and significantly improved muscle repair after cardiotoxin injection, indicating a direct role for metabolic pathway modulation in skeletal muscle repair (42). Genetically engineered mice that lack the Nampt gene, a critical enzyme in the NAD pathway, respond remarkably to nicotinamide riboside treatment in vivo, indicated by improvement of both muscle mass and muscle function (43). In addition, short-hairpin RNA–mediated reduction in sirtuin 1 (SIRT1) levels increased global acetylation of lysine 16 of histone 4 (a direct SIRT1 substrate) and restored MyoD levels in C2C12 myoblasts grown in galactose medium, pointing to an important role for muscle metabolism in regulating epigenetic changes and muscle repair (41). Histone deacetylase (HDAC) 4 expression increases in the tibialis anterior muscle that had been injured by cardiotoxin (44), and specific deletion of HDAC4 in satellite cells results in decreased expression of the satellite cell transcription factor, paired box protein 7, decreased satellite cell proliferation and impaired muscle regeneration (44). HDAC1-deficient dogs and HDAC−/− mice exhibit reduced skeletal muscle mass, significantly smaller myofiber cross-sectional area, and significantly impaired myoblast fusion compared with controls (45). SIRT1 has also been shown to regulate skeletal muscle differentiation by affecting other targets, such as proliferator-activated receptor γ coactivator α1, MyoD, and forkhead box O1/3a (46, 47). In critically ill patients, transcriptome analyses revealed an enrichment of dysregulated skeletal muscle repair genes in the modules associated with muscle weakness and atrophy, in both early and late intensive care unit (ICU)–acquired weakness (48). The role of epigenetic changes in aging satellite cells has been shown to be a major contributor to impaired regeneration in aged muscle as well (1–5, 7, 9, 10, 18, 25–31, 33, 35–41, 46, 47, 49–57), and only recently has this topic attracted due attention, as the World population continues to grow older and sarcopenia presents many socioeconomic challenges.
Recent studies using carbon tracing and proteomic analyses reveal that lipid-based metabolism contributes to increased histone acetylation in immortalized murine hepatocytes, where fatty acid oxidation results in nearly all (90%) of lysine acetylation in histones (58), further pointing to a critical role for metabolism-driven epigenetic changes. Lipid accumulation in the diaphragm of mechanically ventilated brain-dead patients and down-regulation of AMPK not only help explain exacerbation of diaphragm function due to oxidative stress (59), but it also points out the possible role of altered metabolism in negatively affecting skeletal muscle gene function. In the same study, the induction of hyperlipidemia worsened diaphragmatic oxidative stress in mice placed on mechanical ventilation, whereas transgenic overexpression of a mitochondria-localized antioxidant (peroxiredoxin-3) significantly protected against ventilator-induced diaphragmatic dysfunction (59). Fatty acid–based metabolism, hence, presents a potential therapeutic intervention area for proper skeletal muscle function.
Skeletal Muscle Wasting in Lung Disease
Skeletal Muscle Metabolism and Lung Disease
Skeletal muscle wasting in patients with lung diseases, including COPD, acute respiratory distress syndrome, and cystic fibrosis, contributes to a worse outcomes and it is associated with increased morbidity (7). Critically ill patients continue to exhibit weakness, fatigue, reduced exercise capacity (decreased 6-minute walk distance), and loose muscle mass even after ICU discharge and after lung recovery (8). Patients with lung diseases suffer increased work of breathing, and a large fraction of this increased work is performed by the diaphragm muscle. Diaphragm dysfunction has been reported in patients with COPD (60, 61). Quadriceps muscle dysfunction, characterized by reduced force and reduced exercise tolerance, is another hallmark of COPD disease manifestation, even at early disease stages (50–52). Bioenergy maladaptations characterize diaphragmatic muscle fibers of patients with COPD, where a significantly higher mitochondrial capacity is observed in all the diaphragm fiber types of patients with COPD compared with control subjects (62). Furthermore, mitochondria of patients with COPD display significant alterations in other muscle groups as well, including the vastus lateralis and external intercostalis muscles (53, 63). Specifically, oxygen consumption measurements in mitochondria isolated from the vastus lateralis and external intercostalis muscles of patients with COPD revealed reduced basal mitochondrial oxygen consumption and reduced ATP production and increased ROS in both muscle groups as compared with control subjects (53). Gene expression profiling of vastus lateralis muscle biopsies from patients with stable COPD and patients with acute exacerbation of COPD revealed roughly 2,000 differentially expressed genes between the two groups, and further gene ontology analysis revealed that several dysregulated transcripts belonged to pathways involved in oxidative phosphorylation (54). Skeletal muscle dysfunction also plays a role in the pathophysiology of acute respiratory distress syndrome, and is associated with prolonged mechanical ventilation, weaning failure, and a markedly elevated risk of returning to ICU after discharge, creating a vicious cycle that leads not only to increased hospital stay and impaired quality of life, but also increased morbidity and mortality (9, 55). Muscle fiber analyses from biopsies of critically ill patients placed on mechanical ventilation reveal significant diaphragm muscle fiber atrophy and reduced contractile force compared with control subjects (64). Mitochondrial antioxidant SS-31 treatment in rats exposed to mechanical ventilation protects the diaphragm from mechanical ventilation–induced weakness (65). Decreased quadriceps type I fiber cross-sectional area and reduced exercise capacity were also observed in patients with idiopathic pulmonary arterial hypertension (PAH) (6). In addition, mRNA expression analyses revealed that patients with PAH did not have any alternation in transcripts related to mitochondrial biogenesis or mitochondrial fission; rather, the PAH muscle exhibited significantly reduced levels of the mitochondrial fusion protein, mitofusin2 (6). Hence, mitochondrial maturation might be a contributing factor to underlying altered metabolism in disease states.
Understanding the metabolic regulation of skeletal muscle repair is of significant clinical interest for patients with lung diseases, and could help with the design of novel therapeutic pathways. A proposed model for the modulation of metabolism with respect to skeletal muscle homeostasis is presented in Figure 2. In addition to the traditional pharmacological interventions for patients with COPD and other lung diseases, attention should be redirected toward understanding the imbalance in metabolic state of the cells during critical illness, where metabolism could act as a master integrator of both extracellular and intracellular stimuli in skeletal muscle. Furthermore, understanding the role of metabolism in skeletal muscle of lung diseases, such as bacterial and viral infection, could help identify other disease contributing factors, besides the well accepted aging-related changes in the skeletal muscle of these lung disease models (66). Conversely, longitudinal studies elucidating the impact of aging on metabolic and regenerative potential of skeletal muscle are needed. Of great importance remains the role of metabolism in epigenetic imprinting of skeletal muscle, where maladaptive responses can drastically change the course of disease progression for critically ill patients. Although most of these therapeutic interventions warrant further basic science research, implementation of exercise-based therapies, especially during the ICU stay, might have a beneficial and long-term effect for disease outcome in patients with lung diseases.
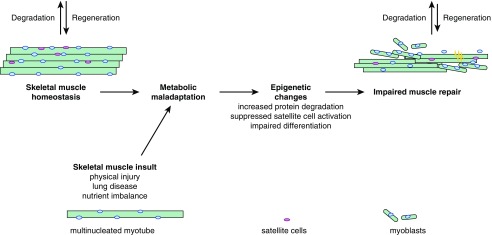
Figure 2.
Metabolic maladaptations result in impaired skeletal muscle repair. Shown are homeostatic skeletal muscle and impaired skeletal muscle repair after injury and/or metabolic maladaptation.
Footnotes
This work was supported by National Institutes of Health grants HL-085534, AG049665, and T32HL076139.
Author Contributions: All authors contributed to the development and writing of the manuscript and have approved the final version.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0355TR on January 13, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015;21:237–248. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelini C, Semplicini C. Metabolic myopathies: the challenge of new treatments. Curr Opin Pharmacol. 2010;10:338–345. doi: 10.1016/j.coph.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Raben N, Wong A, Ralston E, Myerowitz R. Autophagy and mitochondria in Pompe disease: nothing is so new as what has long been forgotten. Am J Med Genet C Semin Med Genet. 2012;160C:13–21. doi: 10.1002/ajmg.c.31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandel NS. Mitochondria and cancer. Cancer Metab. 2014;2:8. doi: 10.1186/2049-3002-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batt J, Ahmed SS, Correa J, Bain A, Granton J. Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2014;50:74–86. doi: 10.1165/rcmb.2012-0506OC. [DOI] [PubMed] [Google Scholar]
- 7.Barreiro E, Sznajder JI, Nader GA, Budinger GR. Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med. 2015;191:616–619. doi: 10.1164/rccm.201412-2189OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 10.Koopman R, Ly CH, Ryall JG. A metabolic link to skeletal muscle wasting and regeneration. Front Physiol. 2014;5:32. doi: 10.3389/fphys.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salminen A, Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:97–106. doi: 10.1007/BF02889856. [DOI] [PubMed] [Google Scholar]
- 15.Nichenko AS, Southern WM, Atuan M, Luan J, Peissig KB, Foltz SJ, Beedle AM, Warren GL, Call JA. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am J Physiol Cell Physiol. 2016;311:C190–C200. doi: 10.1152/ajpcell.00066.2016. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Gosker HR, Schols AM, Kapchinsky S, Bourbeau J, Sandri M, Jagoe RT, Debigaré R, Maltais F, Taivassalo T, et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1313–1320. doi: 10.1164/rccm.201304-0732OC. [DOI] [PubMed] [Google Scholar]
- 17.Chacon-Cabrera A, Fermoselle C, Urtreger AJ, Mateu-Jimenez M, Diament MJ, de Kier Joffé ED, Sandri M, Barreiro E. Pharmacological strategies in lung cancer–induced cachexia: effects on muscle proteolysis, autophagy, structure, and weakness. J Cell Physiol. 2014;229:1660–1672. doi: 10.1002/jcp.24611. [DOI] [PubMed] [Google Scholar]
- 18.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. PGC-1α–responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 19.Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168:706–713. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- 20.Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg A, Törnberg A, Wadell K. Correlation between limb muscle endurance, strength, and functional capacity in people with chronic obstructive pulmonary disease. Physiother Can. 2016;68:46–53. doi: 10.3138/ptc.2014-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:637–658. doi: 10.2147/copd.s4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreiro E, Bustamante V, Cejudo P, Gáldiz JB, Gea J, de Lucas P, Martínez-Llorens J, Ortega F, Puente-Maestu L, Roca J, et al. SEPAR. Guidelines for the evaluation and treatment of muscle dysfunction in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:384–395. doi: 10.1016/j.arbres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg A, Saey D, Maltais F. Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:1269–1277. doi: 10.1513/AnnalsATS.201505-278PS. [DOI] [PubMed] [Google Scholar]
- 25.Hagve M, Gjessing PF, Fuskevåg OM, Larsen TS, Irtun Ø. Skeletal muscle mitochondria exhibit decreased pyruvate oxidation capacity and increased ROS emission during surgery-induced acute insulin resistance. Am J Physiol Endocrinol Metab. 2015;308:E613–E620. doi: 10.1152/ajpendo.00459.2014. [DOI] [PubMed] [Google Scholar]
- 26.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 27.McLean JB, Moylan JS, Andrade FH. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front Physiol. 2014;5:503. doi: 10.3389/fphys.2014.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Julienne CM, Dumas JF, Goupille C, Pinault M, Berri C, Collin A, Tesseraud S, Couet C, Servais S. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J Cachexia Sarcopenia Muscle. 2012;3:265–275. doi: 10.1007/s13539-012-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumas JF, Peyta L, Couet C, Servais S. Implication of liver cardiolipins in mitochondrial energy metabolism disorder in cancer cachexia. Biochimie. 2013;95:27–32. doi: 10.1016/j.biochi.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Tzika AA, Fontes-Oliveira CC, Shestov AA, Constantinou C, Psychogios N, Righi V, Mintzopoulos D, Busquets S, Lopez-Soriano FJ, Milot S, et al. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol. 2013;43:886–894. doi: 10.3892/ijo.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med. 2016;22:666–671. doi: 10.1038/nm.4093. [DOI] [PubMed] [Google Scholar]
- 33.Jaitovich A, Angulo M, Lecuona E, Dada LA, Welch LC, Cheng Y, Gusarova G, Ceco E, Liu C, Shigemura M, et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1) J Biol Chem. 2015;290:9183–9194. doi: 10.1074/jbc.M114.625715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Miyake M, Takahashi H, Kitagawa E, Watanabe H, Sakurada T, Aso H, Yamaguchi T. AMPK activation by AICAR inhibits myogenic differentiation and myostatin expression in cattle. Cell Tissue Res. 2012;349:615–623. doi: 10.1007/s00441-012-1422-8. [DOI] [PubMed] [Google Scholar]
- 36.Bracha AL, Ramanathan A, Huang S, Ingber DE, Schreiber SL. Carbon metabolism–mediated myogenic differentiation. Nat Chem Biol. 2010;6:202–204. doi: 10.1038/nchembio.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceco E, McNally EM. Modifying muscular dystrophy through transforming growth factor-β. FEBS J. 2013;280:4198–4209. doi: 10.1111/febs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161–181. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 39.Olguín HC, Pisconti A. Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions. J Cell Mol Med. 2012;16:1013–1025. doi: 10.1111/j.1582-4934.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, et al. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 43.Frederick DW, Loro E, Liu L, Davila A, Jr, Chellappa K, Silverman IM, Quinn WJ, III, Gosai SJ, Tichy ED, Davis JG, et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi MC, Ryu S, Hao R, Wang B, Kapur M, Fan CM, Yao TP. HDAC4 promotes Pax7-dependent satellite cell activation and muscle regeneration. EMBO Rep. 2014;15:1175–1183. doi: 10.15252/embr.201439195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blondelle J, Ohno Y, Gache V, Guyot S, Storck S, Blanchard-Gutton N, Barthélémy I, Walmsley G, Rahier A, Gadin S, et al. HACD1, a regulator of membrane composition and fluidity, promotes myoblast fusion and skeletal muscle growth. J Mol Cell Biol. 2015;7:429–440. doi: 10.1093/jmcb/mjv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 47.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh CJ, Batt J, Herridge MS, Mathur S, Bader GD, Hu P, Dos Santos CC. Transcriptomic analysis reveals abnormal muscle repair and remodeling in survivors of critical illness with sustained weakness. Sci Rep. 2016;6:29334. doi: 10.1038/srep29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 50.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 52.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puente-Maestu L, Pérez-Parra J, Godoy R, Moreno N, Tejedor A, González-Aragoneses F, Bravo JL, Alvarez FV, Camaño S, Agustí A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33:1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 54.Crul T, Testelmans D, Spruit MA, Troosters T, Gosselink R, Geeraerts I, Decramer M, Gayan-Ramirez G. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem. 2010;25:491–500. doi: 10.1159/000303054. [DOI] [PubMed] [Google Scholar]
- 55.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness JAMA 20133101591–1600.[Published erratum appears in JAMA 311:625.] [DOI] [PubMed] [Google Scholar]
- 56.Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, et al. Blue journal conference: aging and susceptibility to lung disease. Am J Respir Crit Care Med. 2015;191:261–269. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonnell E, Crown SB, Fox DB, Kitir B, Ilkayeva OR, Olsen CA, Grimsrud PA, Hirschey MD. Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Reports. 2016;17:1463–1472. doi: 10.1016/j.celrep.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med. 2012;186:1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- 60.Doucet M, Russell AP, Léger B, Debigaré R, Joanisse DR, Caron MA, LeBlanc P, Maltais F. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 61.Mador MJ, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Res. 2001;2:216–224. doi: 10.1186/rr60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, Dudley G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol (1985) 2002;92:1205–1213. doi: 10.1152/japplphysiol.00116.2001. [DOI] [PubMed] [Google Scholar]
- 63.Barreiro E, Sznajder JI. Epigenetic regulation of muscle phenotype and adaptation: a potential role in COPD muscle dysfunction. J Appl Physiol (1985) 2013;114:1263–1272. doi: 10.1152/japplphysiol.01027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191:1126–1138. doi: 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation–induced diaphragm weakness. Crit Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartley JM, Pan SJ, Keilich SR, Hopkins JW, Al-Naggar IM, Kuchel GA, Haynes L. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY) 2016;8:620–635. doi: 10.18632/aging.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]