Abstract
Generating mature, differentiated, adult lung cells from pluripotent cells, such as induced pluripotent stem cells and embryonic stem cells, offers the hope of both generating disease-specific in vitro models and creating definitive and personalized therapies for a host of debilitating lung parenchymal and airway diseases. With the goal of advancing lung-regenerative medicine, several groups have developed and reported on protocols using defined media, coculture with mesenchymal components, or sequential treatments mimicking lung development, to obtain distal lung epithelial cells from stem cell precursors. However, there remains significant controversy about the degree of differentiation of these cells compared with their primary counterparts, coupled with a lack of consistency or uniformity in assessing the resultant phenotypes. Given the inevitable, exponential expansion of these approaches and the probable, but yet-to-emerge second and higher generation techniques to create such assets, we were prompted to pose the question, what makes a lung epithelial cell a lung epithelial cell? More specifically for this Perspective, we also posed the question, what are the minimum features that constitute an alveolar type (AT) 2 epithelial cell? In addressing this, we summarize a body of work spanning nearly five decades, amassed by a series of “lung epithelial cell biology pioneers,” which carefully describes well characterized molecular, functional, and morphological features critical for discriminately assessing an AT2 phenotype. Armed with this, we propose a series of core criteria to assist the field in confirming that cells obtained following a differentiation protocol are indeed mature and functional AT2 epithelial cells.
Keywords: type II pneumocyte, induced pleuripotent stem cell, embryonic stem cell, surfactant, lamellar body
The Promise of Regenerative Medicine for Understanding and Treating Lung Disease
Chronic lung disease is now recognized as a major cause of global morbidity and mortality (http://www.who.int/respiratory/en/). Impaired restitution of dying alveolar epithelial and endothelial cells through either self-renewal or via contributions from endogenous lung progenitor cells results in a loss of gas exchange surface area and/or barrier function, as well as the potential promotion of expansion of mesenchymal compartments (1). This acquired “stem/progenitor cell failure” from a loss of key cell populations and the resulting aberrant injury/repair represent a central tenet in pathogenesis of degenerative parenchymal lung diseases, such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis, as well as in the development and propagation of acute lung injury from a variety of etiologies (2–8). As there is a limitation of present therapies to reverse or correct such losses, regenerative medicine, with the hope of replacing damaged lung with healthy cells or tissue, represents a new paradigm for the definitive management of parenchymal pulmonary diseases.
The alveolar type (AT) 2 pneumocyte (hereafter AT2 cell) is an endodermally derived, multifunctional, polarized epithelial cell spatially restricted to the distal mammalian adult lung (9). Since the first report of the successful isolation of a surfactant-producing epithelial cell from the lungs of rodents by Kikkawa and Yoneda in 1974 (10), a wealth of data using primary cultured AT2 cells isolated from a variety of species has assigned important biosynthetic, secretory, metabolic, host defense, and repair/regenerative functions to this population, rendering this cell critical to the maintenance of alveolar homeostasis (11, 12). In addition, AT2 dysfunction or dropout has been implicated in the pathogenesis of a variety of parenchymal lung diseases, including idiopathic pulmonary fibrosis, Hermansky-Pudlak syndrome–related pulmonary fibrosis, and chronic obstructive pulmonary disease, among others (1, 8, 13–17). However, despite improvements in cell yields and purities over the past 40 years, major barriers to better understanding the role of the AT2 cell in health and disease have persisted from both the inability to culture and maintain primary AT2 cells in a well differentiated state, as well as the discouraging reality that stable cell lines (including A549, L2, H441, RLE-6N, and MLE12/15) fail to faithfully recapitulate the morphologic, functional, molecular, proteomic, and/or lipidomic markers of the AT2 phenotype (12, 18). Now, with the rapid emergence of stem cell biology and the use of reprogrammed pluripotent progenitor populations, eternal hope has sprung once again that, a suitable alternative to the use of primary cultured AT2 cells can be found.
Lung Stem Cell Biology and the AT2 Cell: a Clear and Present Conundrum
The promise and power of multipotent adult stem cells, embryonic stem cells (ESCs), and, more recently, induced pluripotent stem cell (iPSC) –based technologies for regenerative and personalized medicine cannot be understated. The first studies that suggested that multipotent adult stem cells could differentiate into lung epithelium involved attempts to use in vivo differentiation of injected bone marrow cells to generate populations of either distal lung epithelial precursors or potentially fully differentiated distal lung epithelial cell lineages, both AT2 (19) and AT1 (20). However, these early studies and subsequent reports that leveraged more robust imaging (e.g., confocal microscopy) and labeling protocols determined that both engraftment and in vivo differentiation of the injected cells was extremely low and required the concomitant induction of lung injury using exogenous agents, such as bleomycin or Pseudomonas infection (21–24). Recently, endogenous, multipotent lung stem cells have been identified in the distal airways (25, 26). Dubbed either lineage-negative epithelial stem progenitor cells (25) or distal airway stem cells (p63/Krt5) (26), these basally located cells can be isolated as a single rare population distinct from the more prevalent AT2 cells or bronchiolar cells. When transferred to influenza-challenged recipient lungs, lineage-negative epithelial stem progenitor cells or distal airway stem cells (p63/Krt5) can repopulate the areas of severest injury. Although capable of differentiating to several cell types in vivo (including AT1, AT2, and bronchiolar cells), the utility of these cells for in vitro disease modeling or regenerative therapy remains to be defined.
A second wave of stem cell–based techniques to generate lung epithelia has involved in vitro differentiation protocols applied to animal-derived ESCs and a limited number of human ESC (hESC) lines (27–29). The initial proof-of-concept studies performed in the early part of this century demonstrated that, under specific conditions, these primordial precursor populations could be driven to adopt some phenotypic features associated with more terminally differentiated cells normally derived during the course of the fetal development program from all three major definitive embryonic germ layers (ectoderm, mesoderm, and endoderm), including distal airway epithelia. Wang and colleagues (27) used transfection of an undifferentiated hESC line (ES 9.2) with a human surfactant protein (SP-C) promoter–neomycin transgene (3′-hprt-SPCP.NEO) to permit isolation of clonal populations of lung epithelial cells expressing mRNA transcripts of SP-C, cystic fibrosis transmembrane conductance regulator, and α1-antitrypsin, as well as proSP-C and surfactant protein A (SP-A) proteins by immunofluorescence. These cells also contained some ultrastructural evidence of lamellar body (LB) formation. Subsequently, a second group has shown that undifferentiated hESCs grown on porous membranes and sequentially cultured in a defined differentiation medium at air–liquid interface were capable of generating a cell population demonstrating quantitative increases in mRNA (by real-time quantitative PCR) and protein expression (immunohistochemistry [IHC]) of distal lung epithelial markers, Nkx2-1, CC16, SP-C, and aquaporin (AQP) 5 (28). More recently, purification and directed differentiation of primordial lung and thyroid progenitors derived using Nkx2–1GFP knockin reporter mouse embryonic stem cells (ESCs) has been described (29).
Limited in scale-up by a number of barriers (including political, ethical, and pragmatic), the early successes with hESCs have been largely supplanted by a wave of newer iPSC-based methodologies. First described by Takahashi and colleagues (30), pluripotent iPSCs induced from a variety of somatic cells from human and animal donor sources (including skin fibroblasts and peripheral blood mononuclear cells) via overexpression of Oct4, Klf4, Sox2, and cMyc (colloquially referred to as “Yamanaka factors”), have further revolutionized the field of stem cell biology. These reprogrammed cells have opened up the promise of developing disease/patient–specific in vitro models to enhance understanding of disease pathogenesis, to promote discovery of personalized therapeutic portfolios, and to provide a source of organ-specific cellular components for future tissue engineering and organ refurbishing strategies.
Although these iPSC approaches have afforded remarkable progress in a number of nonpulmonary fields with proof-of-concept reports of generation of patient-specific and/or disease-specific cardiomyocytes, hepatocytes, and other lineages (31–33), the promise of iPSCs for lung biology has remained somewhat unfulfilled. In the lung, most approaches have entailed taking reprogrammed primordial iPSCs from early embryonic stages through to definitive endoderm, anterior endoderm, and subsequent differentiation toward a distal respiratory epithelial phenotype through in vitro sequential exposure to/incubation with combinations of exogenous mediators (growth factors, hormones, and inhibitors) that recapitulate developmental pathways. Using this approach, Ghaedi and colleagues (34) obtained a CD54-positive epithelial cell population from human iPSCs, which expressed molecular markers associated with an AT2 phenotype (proSP-C, proSP-B, SP-A), but bore a limited ultrastructural resemblance to a fully differentiated AT2. Using a similar in vitro strategy, Huang and colleagues (35) generated an iPSC-derived lung epithelium expressing both AT2 (proSP-C, SP-A) and AT1 (AQP5) markers that ultrastructurally appeared similar to human fetal lung epithelium. This preparation was also capable of demonstrating endocytosis of a boron-dipyrromethene (BODIPY) dye-labeled SP-B preparation, a known, but not specific, AT2 functional attribute (36, 37). In a third report, a method to generate random integration-free human iPSCs in which exogenous reprogramming factor transgenes can be subsequently excised, has been described (38). In contrast to the use of prolonged culture strategies, the generated iPSCs have been reported to be capable of rapid differentiation to AT2 cells when cultured on Matrigel for 14 days; however, the molecular and functional endpoints used to assess the AT2 phenotype were incompletely described.
Despite the enthusiasm associated with these approaches touting the successful engineering of differentiated distal lung cell populations from definitive endoderm progenitors (34, 35, 38), as indicated previously here, the resultant iPSC-derived AT2-like epithelia in vitro represent a cautionary tale. When placed into the context of the substantial body of data generated from many investigators using primary AT2 cells isolated from a variety of species, in vivo proof-of-concept studies using transgenic mouse models, and translational correlations with human specimens, the phenotypic characterization of many of these promising reagents indicates there is still work to be done. Given this current disconnect between the two fields (established AT2 cell biology versus emerging iPSC-derived lung epithelia) and the directions that the rapidly advancing field of iPSC-based in vitro modeling and therapeutics is heading, it seems prudent to attempt to establish a clearer consensus of the Holy Grail of what makes an AT2 cell an AT2 cell.
Quacking Like a Duck: What Is an AT2 Cell?
The alveolar sacs of the distal lung are lined by a coupled mosaic of two major epithelial subtypes: the AT1 and AT2 cell. AT1 cells, which represent 8% of total lung cells, are complex branched cells with multiple cytoplasmic plates that are relatively devoid of organelles (39). This structure permits this numerically modest cell population to provide a major contribution to the gas exchange surface in the alveolus. On the other hand, while contributing only 7% of the alveolar surface area, but comprising 16% of the total parenchymal cell number (39), AT2 cells represent metabolically and functionally complex differentiated epithelia that can be readily isolated from the lung and studied in vitro (12). The majority of early published studies of AT2 cell functions were performed with AT2 cells obtained from adult rat sources using improved methods of purification and culture pioneered by Dobbs, Williams, Mason, and others (40–44). Although less frequently used, these methods have been adopted and modified for mouse, guinea pig, rabbit, and bovine sources. In recent years, the ability to sort cells using monoclonal antibodies against AT2 surface markers (45–47) has increased the use of adult human AT2 cells, which has complemented prior work by Ballard’s group (48, 49), which successfully reported the isolation of AT2 cells from cultured human fetal lung explants.
When studied in vitro, AT2 cells from all these sources represent an experimental challenge, as primary isolates maintained on tissue culture plastic in the presence of serum, typically with 2–7 days of plating, undergo morphological and biochemical changes associated with a loss of AT2 phenotype, including SP mRNAs and LBs (i.e., “dedifferentiation”) (50–52). In some cases a coincidental switch to expression of more AT1-like markers (e.g., T1α [aka, podoplanin], AQP5, homeodomain-only protein [also known as Hopx]) has been found (i.e., “transdifferentiation”) (41, 47, 51, 53–56). Various efforts, including the use of hormones (dexamethasone), cAMP, growth factors (e.g., keratinocyte growth factor), and three-dimensional culture (Matrigel substrata or organoids) have been shown to delay, but not prevent, these phenotypic changes (48, 49, 57, 58).
Despite these caveats associated with culture-induced phenotypic changes, this model system of primary AT2 cells has been successfully employed for many years to characterize various AT2 functions and, in particular, surfactant metabolism. AT2 cells have been shown to synthesize, secrete, and recycle all components of pulmonary surfactant, the biochemically heterogeneous complex composed of primarily lipids and protein, which function collectively to augment surface tension at the air–liquid interface along the epithelial lining layer (52, 59). AT2 cells synthesize all subclasses of surfactant lipids (principally phosphatidylcholine [PC] with one [lyso-PC] or two [diplmitoyl (DP)PC] palmitic acid side chains) either de novo from precursors, such as palmitate and choline, or via an acylation/reacylation pathway that reuses lipid recycled from the distal airspace (60). In addition to these sources of lipid, within the alveolar niche, lipofibroblasts, which are lipid droplet–containing interstitial fibroblasts located within close proximity to AT2 cells, contribute to the production of pulmonary surfactant by assimilating neutral lipids and transferring them to AT2 cells for final processing of the surfactant (47, 61).
Beyond lipid, biochemical analysis of surfactant has identified four unique protein components, designated SPs: SP-A, SP-B, SP-C, and SP-D (reviewed in Refs. 62–65). AT2 cells again are the principal source of all four proteins expressing mRNA signatures for each. However, in the adult, only the expression of SP-C is restricted exclusively to AT2 cells (66, 67), with mRNA for SP-A, SP-B, and SP-D detected in nonciliated airway epithelium (68–71) and rarely in extrapulmonary sites (68). Furthermore, only AT2 cells are capable of the complete biosynthesis of SP-B and -C from proprotein precursors (62, 63, 72–78).
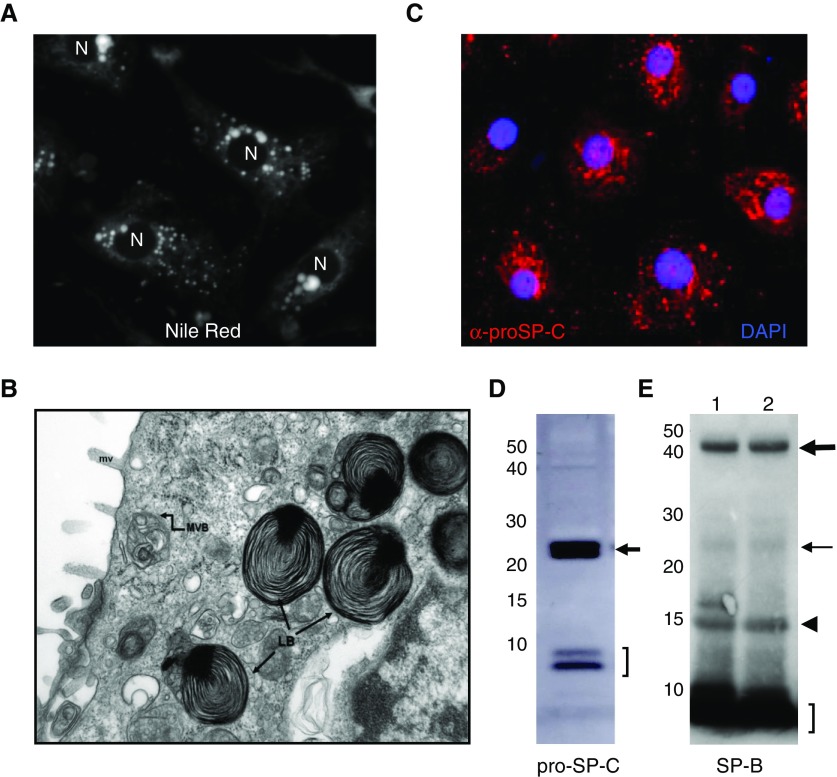
When compared with neuroendocrine cells and many secretory epithelia (exocrine and endocrine) in other tissues, both functionally and morphologically, the AT2 cell is somewhat unique, given the diversity of cellular cargo that must be managed (phospholipids, hydrophobic proteins, multimeric hydrophilic proteins, cytokines). In addition, at the ultrastructural level, AT2 cells lack a classic dense secretory granule, but instead, transmission electron microscopy (TEM) reveals the presence of cytoplasmic, osmiophilic, lamellated organelles (LBs), long recognized as the storage organelle from which surfactant is released into the alveolar lumen (79) (Figure 1B). The presence of lipid within LBs can be easily and rapidly detected using Nile red staining and fluorescence microscopy (Figure 1A). Biochemically, based on a substantial body of work, the basic biology of the lamellar body appears to significantly overlap with that of a number of other lysosome-related organelles in that they are acidic, express lysosomal markers (CD63, lysosomal associated membrane protein [LAMP]-1), but diverges, as they contain surfactant lipids, SP-B, and SP-C, as well as a portion of the intracellular pool of SP-A (80). LBs release surfactant into the alveolus via regulated exocytosis regulated by a variety of signaling pathways (reviewed in Ref. 81).
Figure 1.
Examples of methodologies used to define alveolar type (AT) 2 cell phenotypic features. (A) Human AT2 cells prepared from differentiated human fetal explants after 4 days in culture stained for lipid using Nile red to identify lamellar bodies (LBs). N, nuclei. (B) Transmission electron micrograph of human AT2 cell from normal adult lung showing well formed LBs (LB→) containing a dense core granule. In addition, multivesicular bodies (MVBs) and microvilli (mv) are also identified. (C) Immunohistochemistry of human AT2 cell monolayer prepared as in A and stained for pro–surfactant protein (SP) C using anti–NPROSP-C (red) and 4′,6-diamidino-2-phenylindole (DAPI; nuclei) showing punctate cytosolic staining indicative of post–Golgi trafficking of proSP-C to LBs. (D) Western blotting with proSP-C antisera of freshly isolated mouse AT2 showing the 21-kD proSP-C primary translation product (arrow) and low–molecular weight processed intermediates (bracket). (E) Western blotting of two separate preparations of human AT2 cells prepared as in A and C using SP-B antisera. The proSP-B primary translation product (thick arrow), 25-kD intermediates (thin arrow), and mature SP-B (bracket) are identified. In addition, dimeric mature SP-B (arrowhead) also appears.
In addition to biosynthesis, AT2 cells participate in the uptake, catabolism, and reuse of surfactant (82–84). Protein and lipid components are endocytosed via clathrin-dependent and -independent mechanisms, routed through the central vacuolar system (early endosomes, late endosomes/multivesicular bodies) and sorted to lysosomes for degradation or to LB for resecretion (85, 86). Beyond playing a major role in surfactant homeostasis in the distal lung, there have been a number of other functional, molecular, and metabolic activities associated with an AT2 phenotype. These include, but are not limited to, a role in alveolar ion transport and fluid balance, innate host defense, and, very importantly, as a progenitor population for lung repair, being capable of both proliferative expansion (self-renewal) and of transdifferentiating to AT1 cells (1, 12, 41, 47, 87).
Metrics to Determine “AT2ness”
To avoid “irrational exuberance” regarding the interpretation of current and future results in engineering stem cells for lung biology, it is essential that each new protocol be held accountable for its ability to generate cells that faithfully recapitulate the intended differentiated cell phenotype found in vivo. In the case of the distal lung epithelium, “AT2ness” can best be determined by applying a set of standard criteria based on the already well characterized molecular, morphologic, biochemical, and functional markers that have emerged over the past 50 years. These “metrics” could and should include either quantitative comparisons of each criterion present in engineered stem cell populations to those of AT2 cells using freshly isolated, species- and age-matched (fetal, adult) AT2 cells, or that significantly overlap with comparable signals/readouts/endpoints with AT2 cells evaluated in situ using well characterized lung specimens.
In addition to incorporating the wealth of data present in the lung biology literature, in assembling this Perspective, we also reached out informally to many experts in the field and have incorporated their viewpoints into a “consensus” list of potential phenotypic endpoints aimed at capturing the quintessential features of what makes an AT2 cell an AT2 cell. These features, summarized in Table 1, can be more broadly grouped thematically as follows:
Table 1.
Proposed Criteria for Assessment of an Alveolar Type 2 Cell Phenotype
| Category | Phenotypic Metric | Methodologies | Reference(s) |
|---|---|---|---|
| Molecular | |||
| Expression and maintenance of key surfactant-related AT2 mRNAs, including SFTPC, SFTPB, SFTPA, and ABCA3 | qRT-PCR | 43, 44, 48, 49, 57, 99 | |
| Expression of other abundant AT2 marker genes (HTII-280; progastricin) | IHC; WB | 46, 73 | |
| Morphometric | |||
| Presence of morphologically well formed lamellar bodies in numbers comparable to those found in native lung | TEM | 9, 112 | |
| Quantitative morphometry | |||
| Spatial fidelity of expression of proSP-B, proSP-C, ABCA3 (e.g., a cytosolic vesicular pattern) consistent with correct biosynthetic routing to lamellar bodies | IHC | 74, 75, 78, 89, 91, 93, 98 | |
| Immunogold EM | |||
| Biochemical | |||
| Evidence of biosynthesis and complete post-translational processing of proSP-B and pro SP-C with resultant production of mature protein isoforms | WB for proSP-C/SP-B | 72, 74–76, 78, 89, 90, 92 | |
| Metabolic labeling/immunoprecipitation | |||
| Evidence that reprogrammed cells biosynthesize and store surfactant phospholipids (notably DPPC) in a lamellar body compartment | Metabolic precursor labeling | 49, 60, 106–108 | |
| Lipidomic profiling of cells | |||
| Functional | |||
| Demonstration of regulated exocytosis of surfactant lipid and hydrophobic surfactant proteins in response to known secretagogues comparable to AT2 | Western blotting | 49, 107, 109 | |
| Lipidomic profiling of recovered surfactant | |||
| Ability to transdifferentiate to an AT1 cell phenotype | Expression of AT1 markers: AQP5, T1-α | 41, 55, 56 | |
| In vivo lineage trace | |||
| Ability to form tight junctions and a resistive monolayer in culture | IHC for junctional proteins | 41, 55, 56 | |
| TER | |||
| “Progenitorness” | |||
| Exhibition of capacity for self-renewal and pluripotency* | Organoid culture; in vivo lineage trace | 47, 122 | |
| Demonstration of ability to repopulate lung scaffolds or engraft injured lungs | Seeding on decellularized scaffolds | ||
| in vivo transfer | 34, 35 | ||
| “Omic” | |||
| Transcriptomic profile and PCA metric similar to isolated AT2 cell or derived from known single-cell RNA samples | Microarray/qRT-PCR | e.g., LungMAP† | |
| RNAseq | |||
| Bioinformatics/PCA | |||
| Protein expression profiles with high degrees of similarity to isolated AT2 | LC/MS | e.g., LungMAP† | |
| Bioinformatics/PCA | |||
| Intracellular and/or secreted lipid profile similar to freshly isolated cells from the same species | Lipidomics | 49, 60, 107 |
Definition of abbreviations: ABCA3, ATP-binding cassette class A3; AQP5, aquaporin-5; AT, alveolar type; DPPC, dipalmitoylphosphatidylcholine; IHC, immunohistochemistry; LC/MS, liquid chromatography/mass spectrometry; PCA, principal component analysis; qRT-PCR, quantitative RT-PCR; SFTP, surfactant protein gene; SP, surfactant protein; TEM, transmission electron microscopy; TER, transepithelial resistance; WB, Western blot.
Capacity for self-renewal (may be limited to a subset of AT2 cells; see main text).
LungMAP, available from http://www.lungmap.net/about/lungmap-team/nhlbi.
1. Evidence of a Significantly Mature Surfactant System
-
•
Expression and maintenance of mRNA for key AT2 genes, SFTPB (SP-B) and SFTPC (SP-C), plus ATP-binding cassette class A3 (ABCA3; and potentially SFTPA [SP-A]), comparable to AT2 cells from same species.
-
•
Demonstration of biosynthesis and complete post-translational processing of proSP-B and proSP-C proteins using Western blotting or metabolic labeling. The presence of detectable levels of low–molecular weight proSP-C intermediates, as well as mature SP-B, is supportive (Figures 1D and 1E).
-
•
Documentation that the resultant cell population can biosynthesize and store surfactant phospholipids (notably, DPPC).
-
•
Demonstration that surfactant phospholipids and hydrophobic proteins SP-B and SP-C are secreted via regulated exocytosis.
-
•
Expression of additional important AT2-related mRNAs/proteins (e.g., progastricin C, napsin A) and exclusion of known AT1 (AQP5, T1α) and some proximal airway (p63) markers.
Rationale and justification.
The expression of three major protein components of the surfactant system and the ability to synthesize, store, and release surfactant phospholipid species and proteins have been strongly associated with an AT2 cell phenotype.
SP-C is the only AT2 epithelial cell–specific protein, and is the most commonly used marker for identification of AT2 cells (Figure 1C). In addition, at least for the mouse, it has been reported that a subset of SP-C+ AT2 cells was also found to express low levels of the Clara/Club cell gene, SCGB1A/CC10, with these double-positive cells capable of giving rise to AT1 cells (88). Thus, the presence of coexistent CC10 expression does not absolutely preclude identification as an AT2 cell.
There are several important points to consider when evaluating for the expression of SP-C protein. First, the alveolar form of SP-C protein, which appears in surfactant, is actually synthesized as a 191–197 amino acid precursor (proSP-C) that undergoes post-translational proteolytic processing that ultimately results in generation of the 3.7-kD mature product. Importantly the proSP-C primary translation product (21 kD) and its major processing intermediates (6–10 kD) can be readily detected by Western blotting of cell lysates using most available “(pro)SP-C” antibodies (Figure 1D). Second, the 6-kD proSP-C isoform is observed to be enriched in LB subcellular fractions by Western blotting and detectable by IHC (76, 78, 89–93). The observed IHC pattern should reveal a cytosolic, vesicular pattern (see Figure 1C) that colocalizes with other LB markers, such as CD63, ABCA3, or lysosomal associated membrane protein (LAMP)3. This level of analysis has been lacking in many reports, where only a single 21-kD proSP-C band and/or low-power IHC are presented as evidence of complete SP-C biosynthesis and trafficking. Finally, it should also be recognized that the use of commercially available proSP-C antibodies cannot detect the fully processed mature (∼4 kD) isoform; there is, in fact, only one polyclonal “mature” SP-C reagent (WRAB-MSPC; Seven Hills Bioreagents, Cincinnati, OH) produced over 20 years ago, which has limited availability and sensitivity (M.F.B., unpublished data).
SP-B is an 8-kD hydrophobic protein also generated from a larger 42-kD proprotein precursor (73, 74, 77, 94). Given the essential requirement for the presence of intracellular mature SP-B for normal LB genesis (as evidenced by the lack of LB in the lungs of SP-B–deficient mice and humans) (95, 96), identification of this mature isoform via Western blotting of cell lysates is highly supportive of an AT2 phenotype (see Figure 1E). It should be noted that the well known immortalized human Club/Clara cell line, H441, expresses SP-B mRNA and the proSP-B primary translation product, but does not generate mature SP-B or contain LBs (97).
The lipid transporter, ABCA3, plays a critical role in the biogenesis of AT2 cell LBs (98, 99). Studies suggest that ABCA3 functions as an intracellular transporter of cholesterol and phospholipids, including PC, phosphatidylglycerol, phosphatidylserine, and sphingomyelin (100). Although mRNA expression has been found in other tissues, such as pancreas, ABCA3 protein can be clearly identified on the limiting membrane of LB by IHC (101). In addition, as for SP-B, both the Abca3 knockout mouse models and human ABCA3-null patients die of surfactant-deficient respiratory failure and fail to demonstrate normal LB on TEM, supporting a role for this transporter as one of the critical regulators of LB biogenesis (102).
SP-A, the first SP isolated (103, 104), is the major protein component of pulmonary surfactant. Functions of SP-A in the alveolus include the facilitation of surface tension–lowering properties of surfactant phospholipids, regulation of surfactant phospholipid synthesis, secretion, and recycling, and participation in the innate host response to microbes and particulates in the distal lung (103, 105).
Surfactant phospholipids are synthesized and assembled for secretion into the alveolus exclusively by AT2 cells. A wealth of data in the literature has been generated using metabolic labeling strategies (typically choline or palmitic acid incorporation) (106), and, more recently, lipidomic profiling of primary AT2 isolates in culture (107) to characterize the biosynthetic pathways, enzymes, and adjunctive factors required for the generation of large intracellular pools of surfactant for release into the alveolar space (reviewed in Refs. 60, 108).
Functionally, AT2 cells have been shown that release both surfactant lipids and the hydrophobic SPs (SP-B, SP-C) in a quantile fashion in response to a variety of secratagogues (calcium, ATP, protein kinase C agonists, protein kinase A agonists) via regulated exocytosis (81, 109–111)
2. Evidence of a Comparable AT2 Cell Organellar Ultrastructure
-
•
Ultrastructural evidence by TEM of LBs and quantitative morphometrics showing that the engineered cell line does achieve this metric at a magnitude equal to AT2 cells from the native lung.
-
•
Evidence for a lysosome-like organelle compartment, which is acidic, rich in phospholipid, and contains mature SP-B/proSP-C proteins.
Rationale and justification.
The presence of well formed LBs with dense core bodies (Figure 1B) implies sufficient expression of SP-B/-C, ABCA3, and DPPC, as well as key endoplasmic reticulum chaperones, adapter proteins, and processing enzymes required for their normal biogenesis. Given the biological heterogeneity of isolated and enriched stem cell–derived, differentiated epithelial populations, rigorous and statistically powerful techniques (in lieu of presenting single micrographs) should be employed. These metrics are easily derived from standard ultrastructural methodologies developed by Weibel, Hyde, Ochs, and others in concert with consensus guidelines for lung morphometry as published by the ATS/ERS (60, 112).
As LBs represent a highly specialized subpopulation of lysosome-related organelles, strong supporting evidence of a successfully reprogrammed AT2 cell population would include the presence of acidic, lipid-rich organelles (lysotracker+; nile red+, or phosphine 3R+ cytosolic vesicles), which also concentrate hydrophobic SPs (Figures 1A and 1C). This is critical, as “SP-C–positive” or “SP-B–positive” IHC should demonstrate the presence of labeled cytosolic vesicles.
3. Evidence of Participation in Maintenance or Repair of Barrier Function
Rationale and justification.
Because of their role in barrier function, both as primary constituents of the heterotypic alveolar epithelial monolayer (juxtaposed with AT1) and as potential precursors to AT1, the ability of putative re-engineered AT2 populations to transport sodium, develop tight junctions, and generate high transepithelial resistance in vitro and/or express markers of AT1 cells in vivo provides additional strong supporting evidence (41, 55, 113–115).
4. Evidence of Progenitor Cell Function
-
•
Evidence that the re-engineered population can exhibit self-renewal and pluripotency.
-
•
Evidence that the re-engineered cells can repopulate lung scaffolds, or engraft injured lungs with reasonable efficiency in vivo.
Rationale and justification.
Historical data from primate and rat models suggested that SP-C+ AT2 cells function as progenitor cells in the alveoli and proliferate and possibly transdifferentiate into AT1 cells (116, 117). Recent genetic lineage–tracing studies in the mouse have provided more definitive evidence by demonstrating that SP-C+ AT2 cells, as an unfractionated population, proliferate in vivo and give rise to AT1 cells (47). These AT2 cells placed into three-dimensional culture were shown to be able to generate self-renewing lung organoids or “alveolospheres,” which contained both AT2 cells and cells expressing multiple AT1 markers, including Hopx, a new marker for AT1 cells (56). The potential for this AT2 population to undergo clonal expansion (i.e., multiple rounds of symmetric cell division) as well as asymmetric expansion to form AT1 cells has also been shown (118).
Additional studies suggest that this property of self-renewal may be limited to only a portion of the AT2 pool. A recent preliminary report by Zacharias and colleagues (119) has identified a subpopulation of lung epithelial cells in mice (Axin2+/SP-C+) that are capable of self-renewal and of generating alveospheres composed of both AT2 and AT1 cells in organoid culture. By lineage tracing, this same population can give rise to AT1 in injured lungs in vivo. These results extend older published data that suggested that at least two populations of AT2 cells could be isolated from injured lungs based on E-cadherin expression (120). E-cadherin–negative AT2 cells were damage resistant, proliferative, and exhibited high levels of telomerase activity, suggesting that they might represent a transiently amplifying progenitor subpopulation of AT2 cells that could contribute to repopulation and repair of damaged alveolar epithelium.
Although not unique to fully differentiated AT2, the ability to home to and or engraft in areas of local injury may be advantageous. Prior studies have described the engraftment of lineage-negative or distal lung progenitor populations in injured rodent lungs (25, 26). Similarly, the instillation of whole populations of isolated primary AT2 cells has been shown to limit bleomycin-induced fibrosis in rats, although the exact degree of engraftment was difficult to interpret (121). iPSC-derived distal lung epithelial clones with some phenotypic features of AT2 cells have also been shown to be capable of engraftment on to decellularized lung scaffolds (34).
Taken together, it seems prudent to recommend that the iPSC-generated “AT2” population not only demonstrates traditional molecular, biochemical, morphological, and functional markers of the AT2 phenotype (Table1), but the derived cell population should also show a capacity to contribute to lung repair/regeneration through self-renewal and/or differentiation (which has been shown for AT2 cells). However, the application of strict criteria linking the minimal number of cell divisions and degree of pluripotency awaits additional studies.
5. Broader Quantitative Phenotypic Overlap: Harnessing the Power of “omics”
-
•
Transcriptomic profile and principal component analysis (PCA) metrics similar to that recovered from isolated AT2 cells or determined from known single-cell RNA analyses.
-
•
Protein expression profiles with comparable degrees of similarity.
-
•
Intracellular and/or secreted lipid profiles similar to cells from isolated the same species.
Rationale and justification.
To address the contribution of “nonsurfactant functions” in the overall phenotype of stem cell–generated AT2 cells, but for which candidate gene or pathway criteria are currently less developed, the emerging powerful and compelling profiling techniques coupled with bioinformatics analyses should be considered. Using this “big data” approach with bioinformatics tools, comparisons of data from freshly isolated AT2 cells and the derived candidate AT2 cells can be made. PCA of well controlled and adequately powered surveys of AT2 markers at the level of mRNA expression (microarray, single-cell RNAseq), protein expression (e.g., tandem mass spectrometry), or metabolomics/lipidomics between putative engineered AT2 cells and reference populations, is perhaps the most powerful metric. Reference resources that may provide opportunity for such comparisons could include the NHLBI-funded LUNGMap project (see http://www.lungmap.net/about/lungmap-team/nhlbi). This consortium now has sorted and single-cell RNA profiles and will ultimately have detailed lipidomics and proteomics data from which to make such comparisons (J. Whitsett, personal communication).
Final Thoughts
Despite the power of emerging stem cell technologies, a major limitation for the pulmonary biology community currently is the ability to derive mature, functional epithelial populations of the distal lung. Although the platform offers unparalleled opportunities to model in vitro human lung development and/or genetic pulmonary disease, as well as to create potential cell therapeutics for a whole host of acute and chronic lung diseases, the mainstream use of iPSCs for lung biology is squarely at a critical crossroads. To maintain consistency and reproducibility, and to interpret findings in the proper context, it is incumbent upon investigators in the field to confront the alveolar epithelial cell biology of the past by thoughtfully validating the newly engineered cell lineages to ensure that the pluripotent stem cell they begin with ends up as the bona fide AT2 cell they are seeking.
Acknowledgments
Acknowledgments
The authors acknowledge the work of the “lung epithelial biology pioneers” and the “trailblazers of lung regenerative medicine” cited herein, and apologize to the many other contributors to both fields whose work could not be included solely for the lack of space. They particularly thank Phillip Ballard (University of California–San Francisco, CA, emeritus), Zea Borok (University of Southern California, Los Angeles, CA), Edward Crandall (University of Southern California, Los Angeles, CA), Aron B. Fisher (University of Pennsylvania, Philadelphia, PA), Susan H. Guttentag (Vanderbilt University, Nashville, TN), Darrell Kotton (Boston University, Boston, MA), Robert Mason (University of Colorado, Denver, CO, emeritus), Edward Morrisey (University of Pennsylvania, Philadelphia, PA), and Jeffrey Whitsett (Cincinnati Children’s Medical Center, Cincinnati, OH) for helpful insights and provocative discussions.
Footnotes
This work was supported by Veterans Administration Merit Review 1I01BX001176 (M.F.B.) and National Institutes of Health grant RO1 HL119436 (M.F.B.).
Author Contributions: Conception and design—M.F.B. and Y.M.; drafting of manuscript—M.F.B.; editing of manuscript—M.F.B. and Y.M.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0426PS on March 22, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol. 2014;306:C987–C996. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BLM, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA. 2015;112:5099–5104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM. [DOI] [PubMed] [Google Scholar]
- 7.Steele MP, Schwartz DA. Molecular mechanisms in progressive idiopathic pulmonary fibrosis. Annu Rev Med. 2013;64:265–276. doi: 10.1146/annurev-med-042711-142004. [DOI] [PubMed] [Google Scholar]
- 8.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med. 2014;189:1161–1172. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 9.Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am Rev Respir Dis. 1983;128:S42–S46. doi: 10.1164/arrd.1983.128.2P2.S42. [DOI] [PubMed] [Google Scholar]
- 10.Kikkawa Y, Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974;30:76–84. [PubMed] [Google Scholar]
- 11.Mason RJ, Williams MC. Type II alveolar cell: defender of the alveolus. Am Rev Respir Dis. 1977;115:81–91. doi: 10.1164/arrd.1977.115.S.81. [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, Navarro S, Kikuchi A, Driscoll B. Targeted type 2 alveolar cell depletion: a dynamic functional model for lung injury repair. Am J Respir Cell Mol Biol. 2016;54:319–330. doi: 10.1165/rcmb.2014-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Reports. 2015;12:286–299. doi: 10.1016/j.celrep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2012;186:1014–1024. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 19.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow–derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 20.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow–derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 21.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rejman J, Colombo C, Conese M. Engraftment of bone marrow–derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther. 2009;17:1257–1265. doi: 10.1038/mt.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial–like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 32.Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, Jean JC, Omari A, Sampson KJ, Kotton DN, et al. Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol. 2013;141:61–72. doi: 10.1085/jgp.201210899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell–derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang SXL, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates SR, Fisher AB. Degradation of surfactant protein B by alveolar type II cells. Am J Physiol. 1993;265:L448–L455. doi: 10.1152/ajplung.1993.265.5.L448. [DOI] [PubMed] [Google Scholar]
- 37.Bates SR, Beers MF, Fisher AB. Binding and uptake of surfactant protein B by alveolar type II cells. Am J Physiol. 1992;263:L333–L341. doi: 10.1152/ajplung.1992.263.3.L333. [DOI] [PubMed] [Google Scholar]
- 38.Yan Q, Quan Y, Sun H, Peng X, Zou Z, Alcorn JL, Wetsel RA, Wang D. A site-specific genetic modification for induction of pluripotency and subsequent isolation of derived lung alveolar epithelial type II cells. Stem Cells. 2014;32:402–413. doi: 10.1002/stem.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 40.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 41.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol. 1995;12:497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- 42.Chinoy MR, Dodia C, Fisher AB. Increased surfactant internalization by rat type II cells cultured on microporous membranes. Am J Physiol. 1993;264:L300–L307. doi: 10.1152/ajplung.1993.264.3.L300. [DOI] [PubMed] [Google Scholar]
- 43.Shannon JM, Emrie PA, Fisher JH, Kuroki Y, Jennings SD, Mason RJ. Effect of a reconstituted basement membrane on expression of surfactant apoproteins in cultured adult rat alveolar type II cells. Am J Respir Cell Mol Biol. 1990;2:183–192. doi: 10.1165/ajrcmb/2.2.183. [DOI] [PubMed] [Google Scholar]
- 44.Mason RJ, Williams MC, Greenleaf RD, Clements JA. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977;115:1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1045–L1055. doi: 10.1152/ajplung.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J Histochem Cytochem. 2010;58:891–901. doi: 10.1369/jhc.2010.956433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzales LW, Angampalli S, Guttentag SH, Beers MF, Feinstein SI, Matlapudi A, Ballard PL. Maintenance of differentiated function of the surfactant system in human fetal lung type II epithelial cells cultured on plastic. Pediatr Pathol Mol Med. 2001;20:387–412. doi: 10.1080/15513810109168622. [DOI] [PubMed] [Google Scholar]
- 49.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–L951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 50.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–L354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 51.Demaio L, Tseng W, Balverde Z, Alvarez JR, Kim KJ, Kelley DG, Senior RM, Crandall ED, Borok Z. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1051–L1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mason RJ, Dobbs LG. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem. 1980;255:5101–5107. [PubMed] [Google Scholar]
- 53.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;18:554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Hubmayr RD. Type I alveolar epithelial phenotype in primary culture. Am J Respir Cell Mol Biol. 2011;44:692–699. doi: 10.1165/rcmb.2009-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol. 1995;12:50–55. doi: 10.1165/ajrcmb.12.1.7811470. [DOI] [PubMed] [Google Scholar]
- 56.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, Padmanabhan A, Manderfield LJ, Gupta M, Li D, et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugahara K, Rubin JS, Mason RJ, Aronsen EL, Shannon JM. Keratinocyte growth factor increases mRNAs for SP-A and SP-B in adult rat alveolar type II cells in culture. Am J Physiol. 1995;269:L344–L350. doi: 10.1152/ajplung.1995.269.3.L344. [DOI] [PubMed] [Google Scholar]
- 58.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283:L256–L264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 59.Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Goss V, Hunt AN, Postle AD. Regulation of lung surfactant phospholipid synthesis and metabolism. Biochim Biophys Acta. 2013;1831:448–458. doi: 10.1016/j.bbalip.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 61.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 62.Mulugeta S, Nureki S, Beers MF. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2015;309:L507–L525. doi: 10.1152/ajplung.00139.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- 64.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 65.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 66.Warr RG, Hawgood S, Buckley DI, Crisp TM, Schilling J, Benson BJ, Ballard PL, Clements JA, White RT. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci USA. 1987;84:7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glasser SW, Korfhagen TR, Weaver T, Pilot-Matias T, Fox JL, Whitsett JA. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe) Proc Natl Acad Sci USA. 1987;84:4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, Hawgood S. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50:993–996. doi: 10.1177/002215540205000713. [DOI] [PubMed] [Google Scholar]
- 69.Crouch E, Parghi D, Kuan SF, Persson A.Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells Am J Physiol Lung Cell Mol Physiol 19922631 pt 1):L60–L66. [DOI] [PubMed] [Google Scholar]
- 70.Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. FASEB J. 2001;15:59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- 71.Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408:173–179. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 72.Ueno T, Linder S, Na CL, Rice WR, Johansson J, Weaver TE. Processing of pulmonary surfactant protein B by napsin and cathepsin H. J Biol Chem. 2004;279:16178–16184. doi: 10.1074/jbc.M312029200. [DOI] [PubMed] [Google Scholar]
- 73.Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem. 2008;283:10330–10338. doi: 10.1074/jbc.M707516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guttentag S, Robinson L, Zhang P, Brasch F, Bühling F, Beers M. Cysteine protease activity is required for surfactant protein B processing and lamellar body genesis. Am J Respir Cell Mol Biol. 2003;28:69–79. doi: 10.1165/rcmb.2002-0111OC. [DOI] [PubMed] [Google Scholar]
- 75.Brasch F, Ochs M, Kahne T, Guttentag S, Schauer-Vukasinovic V, Derrick M, Johnen G, Kapp N, Muller KM, Richter J, et al. Involvement of napsin A in the C- and N-terminal processing of surfactant protein B in type-II pneumocytes of the human lung. J Biol Chem. 2003;278:49006–49014. doi: 10.1074/jbc.M306844200. [DOI] [PubMed] [Google Scholar]
- 76.Vorbroker DK, Voorhout WF, Weaver TE, Whitsett JA. Posttranslational processing of surfactant protein C in rat type II cells. Am J Physiol. 1995;269:L727–L733. doi: 10.1152/ajplung.1995.269.6.L727. [DOI] [PubMed] [Google Scholar]
- 77.Brasch F, Johnen G, Winn-Brasch A, Guttentag SH, Schmiedl A, Kapp N, Suzuki Y, Müller KM, Richter J, Hawgood S, et al. Surfactant protein B in type II pneumocytes and intra-alveolar surfactant forms of human lungs. Am J Respir Cell Mol Biol. 2004;30:449–458. doi: 10.1165/rcmb.2003-0262OC. [DOI] [PubMed] [Google Scholar]
- 78.Beers MF, Lomax C. Synthesis and processing of hydrophobic surfactant protein C by isolated rat type II cells. Am J Physiol. 1995;269:L744–L753. doi: 10.1152/ajplung.1995.269.6.L744. [DOI] [PubMed] [Google Scholar]
- 79.Baritussio AG, Magoon MW, Goerke J, Clements JA. Precursor–product relationship between rabbit type II cell lamellar bodies and alveolar surface-active material: surfactant turnover time. Biochim Biophys Acta. 1981;666:382–393. doi: 10.1016/0005-2760(81)90297-6. [DOI] [PubMed] [Google Scholar]
- 80.Oosterlaken-Dijksterhuis MA, van Eijk M, van Buel BLM, van Golde LMG, Haagsman HP. Surfactant protein composition of lamellar bodies isolated from rat lung. Biochem J. 1991;274:115–119. doi: 10.1042/bj2740115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rooney SA.Regulation of surfactant secretion Rooney SA.editor. Lung surfactant:cellular and molecular mechanisms. Austin, TX: R. G. Landes; 1998139–163. [Google Scholar]
- 82.Young SL, Wright JR, Clements JA. Cellular uptake and processing of surfactant lipids and apoprotein SP-A by rat lung. J Appl Physiol (1985) 1989;66:1336–1342. doi: 10.1152/jappl.1989.66.3.1336. [DOI] [PubMed] [Google Scholar]
- 83.Kalina M, McCormack FX, Crowley H, Voelker DR, Mason RJ. Internalization of surfactant protein A (SP-A) into lamellar bodies of rat alveolar type II cells in vitro. J Histochem Cytochem. 1993;41:57–70. doi: 10.1177/41.1.8417113. [DOI] [PubMed] [Google Scholar]
- 84.Kalina M, Socher R. Internalization of pulmonary surfactant into lamellar bodies of cultured rat pulmonary type II cells. J Histochem Cytochem. 1990;38:483–492. doi: 10.1177/38.4.2156921. [DOI] [PubMed] [Google Scholar]
- 85.Muller WJ, Zen K, Fisher AB, Shuman H. Pathways for uptake of fluorescently labeled liposomes by alveolar type II cells in culture. Am J Physiol. 1995;269:L11–L19. doi: 10.1152/ajplung.1995.269.1.L11. [DOI] [PubMed] [Google Scholar]
- 86.Wissel H, Lehfeldt A, Klein P, Müller T, Stevens PA. Endocytosed SP-A and surfactant lipids are sorted to different organelles in rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281:L345–L360. doi: 10.1152/ajplung.2001.281.2.L345. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Frank DB, Morley MP, Zhou S, Wang X, Lu MM, Lazar MA, Morrisey EE. HDAC3-dependent epigenetic pathway controls lung alveolar epithelial cell remodeling and spreading via miR-17-92 and TGF-β signaling regulation. Dev Cell. 2016;36:303–315. doi: 10.1016/j.devcel.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BLM. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brasch F, Ten Brinke A, Johnen G, Ochs M, Kapp N, Müller KM, Beers MF, Fehrenbach H, Richter J, Batenburg JJ, et al. Involvement of cathepsin H in the processing of the hydrophobic surfactant-associated protein C in type II pneumocytes. Am J Respir Cell Mol Biol. 2002;26:659–670. doi: 10.1165/ajrcmb.26.6.4744. [DOI] [PubMed] [Google Scholar]
- 90.Beers MF, Lomax CA, Russo SJ. Synthetic processing of surfactant protein C by alevolar epithelial cells: the COOH terminus of proSP-C is required for post-translational targeting and proteolysis. J Biol Chem. 1998;273:15287–15293. doi: 10.1074/jbc.273.24.15287. [DOI] [PubMed] [Google Scholar]
- 91.Beers MF. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J Biol Chem. 1996;271:14361–14370. doi: 10.1074/jbc.271.24.14361. [DOI] [PubMed] [Google Scholar]
- 92.Beers MF, Kim CY, Dodia C, Fisher AB. Localization, synthesis, and processing of surfactant protein SP-C in rat lung analyzed by epitope-specific antipeptide antibodies. J Biol Chem. 1994;269:20318–20328. [PubMed] [Google Scholar]
- 93.Beers MF, Wali A, Eckenhoff MF, Feinstein SI, Fisher JH, Fisher AB. An antibody with specificity for surfactant protein C precursors: identification of pro–SP-C in rat lung. Am J Respir Cell Mol Biol. 1992;7:368–378. doi: 10.1165/ajrcmb/7.4.368. [DOI] [PubMed] [Google Scholar]
- 94.Weaver TE, Whitsett JA. Processing of hydrophobic pulmonary surfactant protein B in rat type II cells. Am J Physiol. 1989;257:L100–L108. doi: 10.1152/ajplung.1989.257.2.L100. [DOI] [PubMed] [Google Scholar]
- 95.deMello DE, Heyman S, Phelps DS, Hamvas A, Nogee L, Cole S, Colten HR. Ultrastructure of lung in surfactant protein B deficiency. Am J Respir Cell Mol Biol. 1994;11:230–239. doi: 10.1165/ajrcmb.11.2.8049084. [DOI] [PubMed] [Google Scholar]
- 96.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pryhuber GS, O’Reilly MA, Clark JC, Hull WM, Fink I, Whitsett JA. Phorbol ester inhibits surfactant protein SP-A and SP-B expression. J Biol Chem. 1990;265:20822–20828. [PubMed] [Google Scholar]
- 98.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 99.Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, Morohoshi T, Ogawa J, Shioda S, Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 100.Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 101.Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am J Physiol. 1998;275:L172–L183. doi: 10.1152/ajplung.1998.275.1.L172. [DOI] [PubMed] [Google Scholar]
- 102.Besnard V, Matsuzaki Y, Clark J, Xu Y, Wert SE, Ikegami M, Stahlman MT, Weaver TE, Hunt AN, Postle AD, et al. Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L646–L659. doi: 10.1152/ajplung.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KBM, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 104.Floros J, Steinbrink R, Jacobs K, Phelps D, Kriz R, Recny M, Sultzman L, Jones S, Taeusch HW, Frank HA, et al. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986;261:9029–9033. [PubMed] [Google Scholar]
- 105.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 106.Kikkawa Y, Yoneda K, Smith F, Packard B, Suzuki K. The type II epithelial cells of the lung. II. Chemical composition and phospholipid synthesis. Lab Invest. 1975;32:295–302. [PubMed] [Google Scholar]
- 107.Postle AD, Gonzales LW, Bernhard W, Clark GT, Godinez MH, Godinez RI, Ballard PL. Lipidomics of cellular and secreted phospholipids from differentiated human fetal type II alveolar epithelial cells. J Lipid Res. 2006;47:1322–1331. doi: 10.1194/jlr.M600054-JLR200. [DOI] [PubMed] [Google Scholar]
- 108.King RJ, Clements JA.Lipid Synthesis and Surfactant Turnover in the Lungs. Compr Physiol 2011;Supplement 10: Handbook of Physiology, The Respiratory System, Circulation and Nonrespiratory Functions: 309–336. First published in print 1985. doi: 10.1002/cphy.cp030108 [Google Scholar]
- 109.Gobran LI, Rooney SA. Regulation of SP-B and SP-C secretion in rat type II cells in primary culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1413–L1419. doi: 10.1152/ajplung.2001.281.6.L1413. [DOI] [PubMed] [Google Scholar]
- 110.Chander A, Fisher AB. Regulation of lung surfactant secretion. Am J Physiol. 1990;258:L241–L253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 111.Dobbs LG, Mason RJ, Williams MC, Benson BJ, Sueishi K. Secretion of surfactant by primary cultures of alveolar type II cells isolated from rats. Biochim Biophys Acta. 1982;713:118–127. doi: 10.1016/0005-2760(82)90174-6. [DOI] [PubMed] [Google Scholar]
- 112.Hsia CCW, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 114.Foster CD, Varghese LS, Skalina RB, Gonzales LW, Guttentag SH. In vitro transdifferentiation of human fetal type II cells toward a type I–like cell. Pediatr Res. 2007;61:404–409. doi: 10.1203/pdr.0b013e3180332c6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borok Z. Alveolar epithelium: beyond the barrier. Am J Respir Cell Mol Biol. 2014;50:853–856. doi: 10.1165/rcmb.2014-0089PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70:175–198. [PMC free article] [PubMed] [Google Scholar]
- 117.Kapanci Y, Weibel ER, Kaplan HP, Robinson FR. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969;20:101–118. [PubMed] [Google Scholar]
- 118.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zacharias WJ, Frank D, Zepp J, Morley MP, Morrisey EE. Towards therapeutic alveolar regeneration—characterization of a novel Wnt-responsive subpopulation of type II pneumocyte which exhibits increased progenitor cell function in the adult mammalian lung [abstract] Am J Respir Crit Care Med. 2016;193:A5887. [Google Scholar]
- 120.Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L658–L667. doi: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- 121.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 122.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BLM. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]