MUC5B is the principal secreted airway mucin, present in airway mucus at a concentration ∼10-fold higher than the other secreted airway mucin, MUC5AC (1, 2). The report in 2011 that a polymorphism upstream of the MUC5B gene is a risk factor for idiopathic pulmonary fibrosis (IPF) was surprising and illuminating for several reasons (3). First, a rare disease was found to be associated with a common allele (present in 20% of Caucasians), suggesting that genetic susceptibility might be a major contributor to disease pathogenesis even in sporadic IPF. Second, a disease that centers morphologically on the alveolar region of the lung appeared to be associated with an allele of a gene expressed in the conducting airways. Third, the mutation was found to cause a gain of function resulting in increased MUC5B expression. Since Muc5b is the mucin that is primarily responsible for particle clearance in the airways of mice (4), a loss of function could have been expected to result in interstitial lung disease simply by reducing clearance of inhaled particles. This possibility was particularly appealing because there is a dose dependency of mucociliary clearance on Muc5b expression, with a 50% reduction in expression resulting in a 50% reduction in clearance, and a progressive loss of expression with aging in mice (5). A loss-of-function mutation would have placed IPF on a continuum with pneumoconioses, in which the inhalation of large amounts of inorganic particles overwhelms normal clearance mechanisms, with both disorders resulting from an imbalance between particle exposure and clearance. However, the polymorphism associated with IPF results in 10- to 20-fold overexpression of MUC5B (3).
Further discoveries from a variety of sources have extended the implications of MUC5B’s association with IPF. Multiple additional IPF susceptibility genes have been identified, and the most common are those involved in telomerase maintenance, together accounting for ∼30% of the risk for IPF (6, 7). Their involvement suggests that a key pathway in IPF pathogenesis is lung epithelial progenitor cell depletion. Recent work using virus injury models indicates that alveolar regions can be repopulated by the migration of epithelial progenitors from distal conducting airways (8–10). The abundance of goblet, basal, and ciliated cells (normally characteristic of conducting airway epithelium) in remodeled lung parenchyma in IPF (11, 12) is consistent with this notion, bringing us back to the airway protein MUC5B.
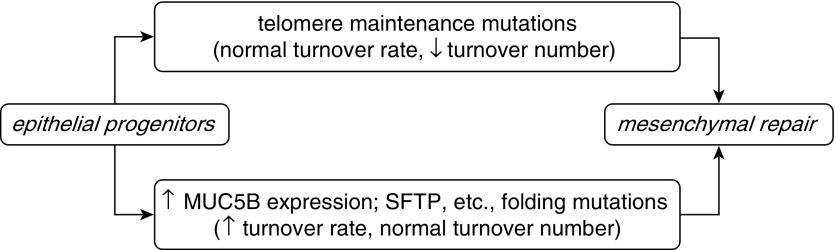
MUC5B/Muc5b is normally expressed in submucosal glands and surface epithelial secretory cells down to, but not including, the level of terminal bronchioles (1, 13, 14). Even with strong stimulation of mucin gene expression by inflammatory mediators, neither Muc5b nor Muc5ac is normally expressed in terminal bronchioles. Secreted mucins are among the largest molecules encoded in mammalian genomes, and their expression induces and requires an endoplasmic reticulum (ER) stress response (15). In IPF, MUC5B is expressed in terminal bronchioles (12), and it is possible (though not proven) that the overexpression of MUC5B in distal airway cells leads to increased cell turnover. Mutations in other genes associated with IPF (e.g., SFTPC, SFTPA2, and ABCA3) also cause ER stress (6, 7), and increased ER stress and apoptosis have been found in IPF that was not genotyped (16). Thus, a common mechanism of IPF pathogenesis between telomere maintenance and MUC5B mutations might be airway epithelial progenitor depletion leading to a repair process involving mesenchymal cell proliferation and fibrosis, and in turn to aberrant differentiation of the remaining epithelial cells in the abnormal microenvironment (Figure 1).
Figure 1.
Hypothetical model of a mechanism of idiopathic pulmonary fibrosis resulting from epithelial progenitor depletion leading to mesenchymal cell repair with fibrosis. MUC5B, mucin 5B; SFTP, surfactant protein.
In this issue of the Journal, Helling and colleagues (pp. 91–99) provide convincing evidence that a common polymorphism located in the 5′-flanking region of the MUC5B gene increases the activity of a strong enhancer that increases MUC5B expression (17). The study further demonstrates that the DNA sequence in that region provides potential binding sites for a number of transcription factors, including FOXA2, which is shown to interact directly with the enhancer in chromatin-binding assays in vitro. These new data are consistent with recent findings from Guo and colleagues (18), who used CRISPR-Cas9 to identify this same region in the MUC5B gene as an active enhancer element that also binds the transcription factor SPDEF (Sam-Pointed Domain Ets-like Factor), a gene known to regulate goblet cell differentiation. Regulation of mucus-related genes, including MUC5AC and MUC5B, is associated with transcriptional networks that regulate goblet cell differentiation from airway progenitors such as basal and club cells. Goblet cell differentiation is highly dependent on environmental contexts, responding to allergens, toxicants, infections, and inflammation, and may be further influenced during tumorigenesis. For example, in the setting of allergen-mediated goblet cell metaplasia, SPDEF works in concert with FOXA3 to induce MUC5AC production (19, 20), whereas FOXA2 is inhibitory. Because many FOX transcription family members share DNA binding motifs and are expressed in respiratory epithelial cells (e.g., FOXA1, FOXA3, FOXC1, FOXM1, and FOXP2), the precise transcriptional complexes that are active in the MUC5B enhancer in IPF are likely to be complicated, and their interactions with cofactors may form inhibitory or stimulatory complexes on target genes. A recent single-cell RNA analysis of lung epithelial cells in IPF demonstrated extensive goblet cell differentiation in which expression of MUC5B and MUC5AC was associated with SPDEF and FOXA1 (11) (data accessible from the Lung Gene Expression Analysis website [21]). On the other hand, FOXA2 was found to be increased in IPF lung tissue and to enhance MMP7 expression in an allele-specific manner, supporting its role in the pathogenesis of IPF (22). The demonstration that the MUC5B variant allele includes an active enhancer raises interesting questions regarding its role and the role of FOX transcription factors in the pathogenesis of IPF. Does increased MUC5B expression influence airway clearance, or create biophysical strain or inflammation that causes epithelial cell injury and tissue remodeling? Alternatively, does the increase in MUC5B expression itself provide an epithelial-cell–autonomous stress that causes epithelial injury and inflammation, activating progenitor cells that contribute to the increased numbers of goblet, basal, and ciliated cells in the IPF lung, as hypothesized above?
What might have driven the very high prevalence of the MUC5B-overexpressing allele in the Caucasian population, comparable to the prevalence of the sickle hemoglobin allele in areas of hyperendemic malaria? It is reasonable to surmise that if a reduction in Muc5b expression results in reduced mucociliary clearance and increased microbial infection (4, 5), then increased expression might protect against inhaled pathogens or toxicants. A Muc5b-overexpressing transgenic mouse has been generated but not yet examined in this context (4); however, transgenic overexpression of Muc5ac has been shown to protect against influenza virus infection (23). Although it remains to be studied, evidence of positive selection of the variant allele would suggest that enhanced expression of MUC5B must offer substantial protection against a respiratory pathogen early in life for it to have spread so widely in European populations (mean allele frequency = 0.11, compared with 0.02 in Hispanic, 0.008 in Asian, and 0.003 in African populations; ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=35705950).
Our understanding of IPF is evolving rapidly as a reflection of progress in understanding lung epithelial progenitor biology, the transcriptional networks that drive development and host defense, and mucus pathophysiology. Together with the remarkable progress that has been made in elucidating the molecular epidemiology of IPF, with ∼70% of IPF now known to have a genetic association (∼40% in the MUC5B enhancer and ∼30% in telomere maintenance), the field is in the midst of a change in paradigm that is likely to benefit patients and caregivers.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickey BF, Fahy JV, Kesimer M, Boucher RC, Evans CM, Thornton D. Measuring airway mucin 2 in patients with severe chronic obstructive pulmonary disease with bacterial colonization. Ann Am Thorac Soc. 2016;13:2103–2104. doi: 10.1513/AnnalsATS.201607-532LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016;310:L860–L867. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathai SK, Newton CA, Schwartz DA, Garcia CK. Pulmonary fibrosis in the era of stratified medicine. Thorax. 2016;71:1154–1160. doi: 10.1136/thoraxjnl-2016-209172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merck SJ, Armanios M. Shall we call them “telomere-mediated”? Renaming the idiopathic after the cause is found. Eur Respir J. 2016;48:1556–1558. doi: 10.1183/13993003.02115-2016. [DOI] [PubMed] [Google Scholar]
- 8.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee M, Domm W, Gelein R, Bentley KL, Kottmann RM, Sime PJ, Lawrence BP, O’Reilly MA.Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells Am J Respir Cell Mol Biol 201756453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, Schwarz MI, Schwartz DA, Reynolds SD. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O’Neal WK, Ribeiro CM. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helling BA, Gerber AN, Kadiyala V, Sasse SK, Pedersen BS, Sparks L, Nakano Y, Okamoto T, Evans CM, Yang IV, et al. Regulation of MUC5B expression in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;57:91–99. doi: 10.1165/rcmb.2017-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, Karns R, Warth A, Fink-Baldauf IM, Nagayasu T, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med. 2017;9:462–481. doi: 10.15252/emmm.201606711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X, Woodruff PG, Fahy JV, Erle DJ. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am J Respir Crit Care Med. 2009;180:603–610. doi: 10.1164/rccm.200811-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, et al. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax. 2017;72:481–484. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards TJ, Park C, Chen Y, Gibson KF, Peter Di Y, Pardo A, Watkins SC, Choi AM, Selman M, Pilewski J, et al. Allele-specific transactivation of matrix metalloproteinase 7 by FOXA2 and correlation with plasma levels in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L746–L754. doi: 10.1152/ajplung.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci USA. 2012;109:16528–16533. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]