Abstract
Obliterative bronchiolitis (OB), characterized by fibrous obliteration of the small airways, is a major impediment to long-term survival in lung allograft recipients. We found previously that IL-17A is produced primarily by CD4+ T cells and γδ T cells after lung transplant in a mouse model of orthotopic lung transplant. The absence of either subset of T cells was compensated for by expansion of the other subset, which suggested that systemic blockade of IL-17A was necessary. To determine the specific role of IL-17A in the development of OB, we treated lung allograft recipients with an IL-17A antagonistic antibody. After IL-17A blockade, the incidence of OB was significantly reduced in lung allografts. IL-17A blockade also significantly attenuated the severity of acute rejection and overall lung fibrosis. The decreased OB incidence was associated with reduced lymphocyte recruitment, particularly CD8+ T cells and other IFN-γ–producing lymphocytes, to the lung allograft. Interestingly, IL-17A blockade led to an increase in the frequency of IL-17A–producing T-helper cell type 17 cells and γδ T cells in lung allografts, suggesting that IL-17A is a negative regulator of these T cells. Our data suggest that blocking IL-17A after lung transplant reduces the overall IFN-γ–mediated lymphocyte response and decreases the development of OB.
Keywords: lung transplant, IL-17, obliterative bronchiolitis
Clinical Relevance
Chronic rejection manifested as obliterative bronchiolitis is a major impediment to long-term survival in lung allograft recipients, and novel therapies are needed. We have found that blocking IL-17A after lung transplant in a mouse model reduces the cellular IFN-γ response, the severity of vascular rejection, and the development of obliterative airway disease. Targeting IL-17A may prevent the development of obliterative bronchiolitis after lung transplant.
The development of bronchiolitis obliterans syndrome (BOS), a phenotype of chronic lung allograft dysfunction, limits the long-term survival of patients undergoing lung transplant (1). The progressive reduction of airflow in BOS correlates with chronic fibrous obliteration of the small airways, a histopathologic finding known as obliterative bronchiolitis (OB) (2). Both alloimmune and nonalloimmune components are thought to participate in BOS/OB development, but the exact mechanisms are not understood completely, which has led to ineffective therapies for BOS/OB treatment (1, 3). Studies in human lung allograft recipients have found an association between IL-17A and the development of BOS and its risk factors, such as acute rejection and lymphocytic bronchiolitis (4–6). Furthermore, IL-17A+ cells can be found in endobronchial biopsy specimens early after lung transplant in humans, possibly in response to ischemia-reperfusion injury or infection, which are risk factors for BOS (7). IL-17A is a pleiotropic cytokine, known to promote recruitment of neutrophils and other inflammatory cells and to induce a local fibrotic response (8–12). Previously, it was shown that blockade of multiple IL-17 family members, such as IL-17A, IL-17F, IL-17E, and IL-17C, prevented OB in a mouse lung transplant model (13). Therapies targeting IL-17A indirectly have also been associated with attenuation of acute and chronic rejection in other mouse models of lung transplant (14–16). These studies suggest that IL-17A may play a key role in the pathophysiology of OB, but whether IL-17A is an absolute requirement for the development of OB is not clear.
New biological therapies to target IL-17A and IL-17RA have shown very promising results for the treatment of autoimmune disorders such as psoriasis and ankylosing spondylitis (17–20). When these biological agents become more widely available, they will be attractive therapies to use for other diseases and the complications related to lung transplant. Because IL-17A and its related family members are important mediators of host defense, it is necessary to determine the precise contribution of each to lung transplant rejection and the cell types involved in producing the cytokines (21). We have found previously that CD4+ Th17 cells and γδ T cells are the major source of IL-17A in a minor mismatch mouse model of lung transplant (22). However, their contributions to OB development were redundant, because the absence of either Th17 cells or γδ T cells did not prevent the development of OB and they appeared to compensate for each other in the production of IL-17A (22). To investigate further the role of IL-17A in lung transplant rejection and airway fibrosis, we used a monoclonal antagonistic antibody to block IL-17A in our model. We found that blocking IL-17A decreased the incidence of OB and reduced the lung cellular inflammatory response, particularly the IFN-γ–mediated immune response. Our data suggest that IL-17A is an important mediator of lung fibrosis after transplant and may be a potential target for preventing chronic rejection and fibrosis in human lung allograft recipients. Some of the results have been reported previously in the form of an abstract at the American Thoracic Society International Conference 2016 (Abstract A4880).
Materials and Methods
Animals and Orthotopic Left Lung Transplant
Male C57BL/6N (H-2b) and C57BL/10 (H-2b) mice were purchased from Harlan Laboratories (Indianapolis, IN). Left lungs from C57BL/10 mice (allograft) were transplanted orthotopically into C57BL/6 mice as described previously (13, 22). Mice between 24 and 30 g were used as lung donors and recipients. Lungs and thoracic lymph nodes (LN) (left and right cranial mediastinal and tracheobronchial) were harvested from recipients on Day 21 after transplant (23). The University of Illinois at Chicago Institutional Animal Care and Use Committee approved all animal protocols.
IL-17A Antibody Administration
Mice were injected intraperitoneally with 250 µg of IL-17A neutralizing monoclonal antibody (clone 17F3, Bio-X-cell, West Lebanon, NH) or with an isotype control antibody on Days −2 and 0 and twice a week after transplant.
Histology
Paraffin-embedded lung tissue sections were stained with hematoxylin and eosin or Masson’s trichrome stain for acute lung rejection (24) and fibrosis (25), respectively. Briefly, the scoring system consisted of four grades (from 1 to 4) on the basis of the severity of vascular lesions (“A” scores); A0 = no acute rejection, A1 = minimal acute rejection, A2 = mild acute rejection, A3 = moderate acute rejection, and A4 = severe acute rejection. For the severity of fibrosis, an arbitrary scale was used, with F1 = normal, F2 = mild fibrosis, F3 = moderate fibrosis, and F4 = severe fibrosis. Presence of obliterative airway disease pathology was determined. Histology was scored in a blinded fashion.
Flow Cytometry
Single-cell suspensions of perfused lungs were obtained by digestion with collagenase I (Life Technologies, Carlsbad, CA). LN were dissociated mechanically. Red blood cells were lysed with hypotonic lysis buffer. All cells were stained with Live/Dead Fixable Aqua Dead Cell Stain (Invitrogen, Eugene, OR) before phenotypic staining. For phenotyping, cells were stained with fluorochrome-conjugated antibodies for the markers CD45, CD3, CD4, CD8, TCRγδ, NK1.1, IL-17A, IFN-γ, Foxp3, CD11b, and Ly6G (Biolegend, San Diego, CA). Because of the low number of cells recovered from the left lung allografts, not all markers were tested on all samples. Cells were stimulated with phorbol myristate acetate and ionomycin for 4 hours (in the presence of Brefeldin A during the last 3 h), fixed with paraformaldehyde, and permeabilized for staining of intracellular antigens. Cells were acquired on an LSR Fortessa (BD Biosciences, San Jose, CA), and data analysis was performed with Flowjo software (Tree Star, Ashland, OR).
Statistical Analysis
All data are expressed as mean ± SEM unless stated otherwise and were analyzed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered significant. The two-tailed unpaired t test and Mann–Whitney U test were used for analysis of normally distributed and skewed data, respectively. The prevalence of OB between the different groups was compared using a two-tailed Fisher’s exact test. (For additional materials and methods, see the online supplement).
Results
IL-17A Blockade Reduces Incidence of OB
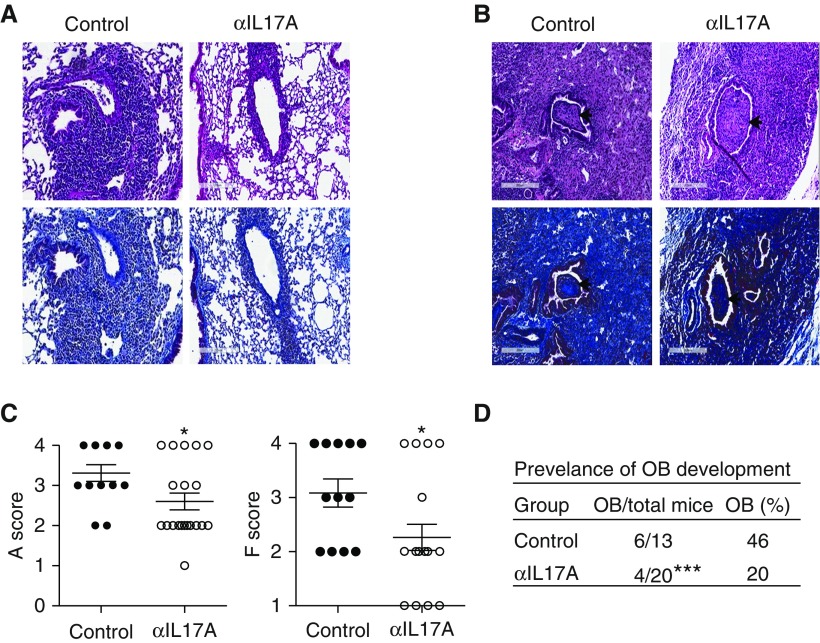
To determine the requirement for IL-17A, lung allograft recipients were treated with anti–IL-17A for 2 days before transplant and twice a week after transplant (13). The incidence of OB was reduced significantly after IL-17A blockade, with 4 of 20 mice (20%) developing OB compared with 6 of 13 control mice (46%) (Figure 1). The anti–IL-17A group is larger because some of the mice were used for preliminary investigation into the cell types infiltrating the lungs. All experimental mice are included for completeness. Anti–IL-17A also significantly decreased the severity of rejection as determined using established criteria from the International Society of Heart and Lung Transplantation for A scores (Figures 1A and 1C) (24). The majority of anti–IL-17A allografts developed mild acute rejection (grade A2), whereas the majority of control allografts developed moderate acute rejection (grade A3) (Figures 1A and 1C). In addition, anti–IL-17A–treated allografts developed less lung fibrosis, with a mean score of F2, compared with control mice with a mean score of moderate fibrosis (F3) (Figures 1A and 1C). The few anti–IL-17A–treated mice that did develop OB had pathology similar to control mice with OB, suggesting the fibrosis was not attenuated in these mice (Figure 1B). These data suggest that IL-17A promotes cellular rejection and obliterative airway fibrosis in this model.
Figure 1.
IL-17A blockade attenuates rejection, fibrosis, and the development of obliterative bronchiolitis (OB). B10 left lungs were transplanted orthotopically into B6 recipients and were treated with either anti–IL-17A (17F3) or isotype control antibody on Days −2 and 0 and twice a week after transplant. Lungs were harvested and analyzed on Day 21. (A) Hematoxylin and eosin– (top panels) and Masson’s trichrome– (bottom panels) stained lung allografts; original magnification × 20. (B) Control and anti–IL-17A–treated allografts with severe acute rejection, fibrosis, (F4), and evidence of OB (arrow); top panels are hematoxylin and eosin and bottom panels are Masson’s trichrome; original magnification × 20. Scale bars: 200 μm. (C) Acute rejection (A score) and fibrosis (F score) in the allograft. (D) Prevalence of OB in the lung allografts (n = 13 [control], n = 20 [anti–IL-17A]). Data were analyzed using unpaired t test and two-tailed Fisher’s exact test in C and D, respectively; *P < 0.05, ***P < 0.001.
IL-17A Blockade Decreases Lymphocytic and IFN-γ Response in Lung Allografts
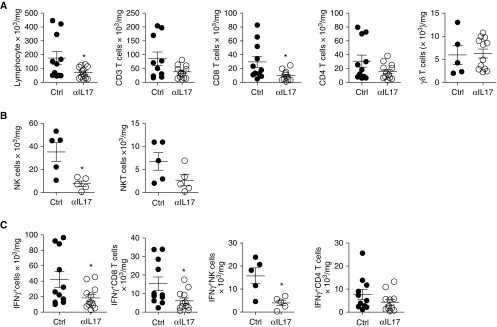
We found that the total number of lymphocytes, particularly CD8+ T cells, was significantly reduced in anti–IL-17A–treated allografts compared with control allografts. CD4+ T cells had a trend toward a decrease, whereas there was no effect on total γδ T cells (Figure 2A). In a subset of mice, we found a significant effect on natural killer (NK) cells but no significant effect on NKT cells (Figure 2B). Interestingly, the total number of IFN-γ–producing lymphocytes was decreased significantly in anti–IL-17A–treated allografts (Figure 2C). This was primarily because of a reduction in IFN-γ+ CD8 T cells and IFN-γ+ NK cells, which were the majority of IFN-γ+ lymphocytes (Figure 2C). There was a trend toward an effect on IFN-γ+ CD4+ T cells (Figure 2C). In the thoracic LN, there was no difference in the total number of lymphocytes or individual subsets of IFN-γ–producing lymphocytes, with the exception that there was a small but significant difference in NK cells between control mice and anti–IL-17A–treated recipients (see Figure E1 in the online supplement). Because IL-17A is a known modulator of neutrophils, we investigated neutrophil recruitment in lung allografts by immunohistochemistry and flow cytometry at Day 21 after transplant (26). In the lung allografts, recipients treated with anti–IL-17A had a slight decrease in neutrophils that was not statistically significant (Figures E2A and E2B). The frequency of neutrophils in the allografts was also similar to that in the native lungs in both groups. No appreciable difference in tissue neutrophils in the lung allografts was found by immunohistochemistry (Figure E2C). Overall, these results suggest that IL-17A exacerbates local lung allograft immunity by affecting both innate and adaptive lymphocytes, particularly IFN-γ–producing cells.
Figure 2.
IL-17A blockade decreases IFN-γ–producing lymphocytes. (A) Absolute number of total lymphocytes and indicated lymphocytic subsets, n = 11 (Ctrl), n = 12 (anti–IL-17A); for γδ T cells, n = 5 (Ctrl), n = 12 (anti–IL-17A), analyzed using unpaired t test or Mann–Whitney U test as appropriate. *P < 0.05. (B) Absolute number of natural killer (NK) and natural killer T (NKT) cells, n = 5 (Ctrl and anti–IL-17A), analyzed using unpaired t test. *P < 0.05. (C) Absolute number of overall IFN-γ+ cells and major IFN-γ–producing lymphocytes; n = 11 (Ctrl), n = 12 (anti–IL-17A); for IFN-γ+ NK cells, n = 5 (Ctrl and anti–IL-17A), analyzed using unpaired t test or Mann–Whitney U test. *P < 0.05. The number of cells has been normalized to the weight of the lung. Ctrl, control.
Reduced Expression of IFN-γ and Proinflammatory Cytokines and Chemokines after IL-17A Blockade
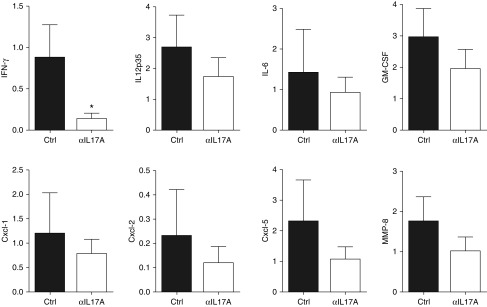
To further investigate the decrease in cellular rejection and fibrosis, we measured the expression of several profibrotic and proinflammatory genes. In agreement with the cellular data, the expression of IFN-γ was decreased significantly in anti–IL-17A–treated lung allografts compared with control allografts (Figure 3). IL-12 expression was also reduced, but this did not reach significance. We found a trend toward reduced expression of other IL-17A signature cytokines (IL-6, granulocyte/macrophage colony-stimulating factor), chemokines (C-X-C motif ligand [CXCL]1, CXCL2, CXCL5), and the tissue remodeling factor matrix metalloproteinase-8 in anti–IL-17A–treated lung allografts (Figure 3). Our data suggest that IL-17A blockade is globally decreasing the expression of known mediators of inflammation and fibrosis after minor mismatch lung transplant.
Figure 3.
IL-17A blockade reduces expression of IFN-γ– and IL-17A–associated cytokines and chemokines. As in Figure 1, on Day 21, relative mRNA expression of the indicated genes in transplanted lungs was quantified by real-time PCR. Expression is relative to one of the control allografts and normalized to GAPDH. For IFN-γ and IL-6, n = 12 (control), n = 8 (anti–IL-17A); for IL-12, C-X-C motif ligand (CXCL)1, CXCL2, CXCL5, and matrix metalloproteinase-8 (MMP-8), n = 5 (control and anti–IL-17A); unpaired t test or Mann–Whitney U test was used for comparison. *P < 0.05. GM-CSF, granulocyte/macrophage colony-stimulating factor.
Reduced OB Incidence after IL-17A Blockade Is Not Associated with an Increase in Regulatory T Cells
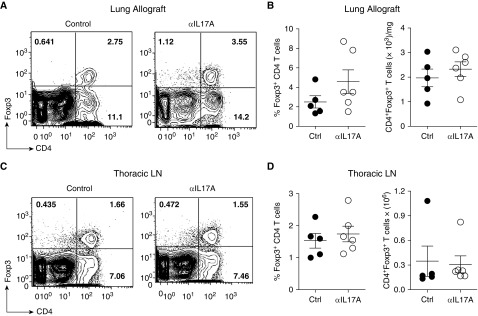
Previous work has suggested that IL-17A deficiency prolongs cardiac transplant survival by enhancing the expansion of regulatory T cells (Tregs) (27). We found that the frequency and absolute number of Foxp3+CD4+ Tregs in the lung allografts was not affected by IL-17A blockade at Day 21 and was comparable to that of control allografts (Figures 4A and 4B). Similarly, the thoracic LN showed no change in Treg population after IL-17A blockade as compared with those of control mice (Figures 4C and 4D). These data suggest that IL-17A blockade does not enhance Treg expansion or recruitment at Day 21 after lung transplant. However, given that fewer effector T cells were present in the lungs with IL-17A blockade, the ratio of Tregs to effectors is increased and the effectiveness of the Tregs may be enhanced.
Figure 4.
IL-17A blockade does not affect regulatory T cell (Treg) accumulation in lung allografts. As in Figure 1, lungs were analyzed on Day 21 after transplant. (A) Representative dot plots showing frequency of Tregs of lymphocytes in the lung allografts. (B) Frequency and absolute number of CD4+Foxp3+ Treg in the lung allografts. (C) Representative dot plots showing frequency of CD4+ Foxp3+ T cells of lymphocytes in thoracic lymph nodes (LN). (D) Frequency and absolute number of Tregs in the thoracic LN. For lungs, the absolute number of cells has been normalized to their weight; n = 5 (control), n = 6 (anti–IL-17A); unpaired t test or Mann–Whitney U test was used for comparison. Data are representative of two independent experiments.
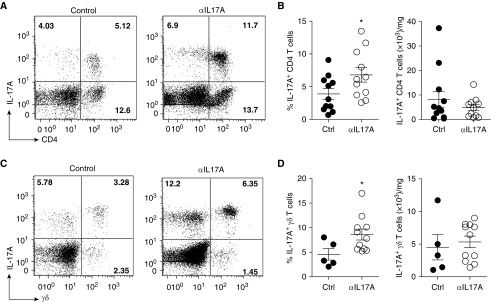
IL-17A Blockade Is Associated with a Higher Frequency of Th17 and IL-17A+ γδ T Cells in Lung Allografts
IL-17A blockade was very effective in our model but did not completely prevent acute rejection, fibrosis, or OB development. These data suggest some redundancy in effector mechanisms among the IL-17 family members. Studies have suggested that the major IL-17A source, Th17 cells, can induce graft rejection independent of IL-17A (28). We hypothesized that IL-17A+ T cells may participate in the observed rejection in anti–IL-17A–treated allografts by mechanisms other than IL-17A production. Interestingly, we found an increase in the frequency of IL-17A+ lymphocytes in anti–IL-17A–treated lung allografts. Most of these lymphocytes were either CD4+ Th17 or γδ T cells (Figure 5). Furthermore, we found that the frequency of both Th17 and IL-17A+ γδ T cells was significantly higher in the anti–IL-17A–treated lung allografts as compared with control allografts (Figures 5A–5D). These data suggest that there is some negative regulation of IL-17A+ T cells by IL-17A (29). However, there was no difference in the absolute number of Th17 and IL-17A+ γδ T cells between anti–IL-17A and control allografts (Figures 5B and 5D).
Figure 5.
IL-17A blockade is associated with enrichment of T-helper cell type 17 and IL-17A+ γδ T cells. As in Figures 1–4, lungs were analyzed on Day 21 after transplant. Untreated or isotype antibody–treated allografts were used as control allografts. (A) Dot plots with a frequency of IL-17A+ CD4+ T cells of lymphocytes in the lung allografts. (B) Frequency and absolute number of IL-17A+ CD4+ T cells in the lung allografts. (C) Dot plots showing frequency of IL-17A+γδ T cells on gated lymphocytes in the lung allografts. (D) Frequency and absolute number of IL-17A+ γδ T cells in the lung allografts. The absolute number of cells has been normalized to the weight of the lung. In A and B, n = 11 (control and anti–IL-17A); in C and D, n = 5 (control), n = 11 (anti–IL-17A); unpaired t test or Mann–Whitney U test was used for comparison; *P < 0.05. Data are representative of two independent experiments.
Discussion
In this study, we have demonstrated a role for IL-17A in the development of OB in a minor mismatch mouse lung transplant model. Blockade of IL-17A significantly reduced rejection, fibrosis, and the incidence of OB development. The attenuated pathology after IL-17A blockade was associated with a decrease in immune cell recruitment, particularly of IFN-γ–producing cells. The protective effect of IL-17A neutralization was not associated with an increase in Tregs in the allografts. Although lymphocytic infiltration was reduced significantly in the lung allografts after IL-17A blockade, Th17 and IL-17A+ γδ T cell frequencies were increased relative to control allografts. Together, our data suggest that IL-17A is a mediator of OB and that blocking IL-17A reduces the overall alloimmune response to limit OB development. However, amelioration of OB development after IL-17A blockade was not complete, and the data suggest redundant effector mechanisms for inducing fibrosis.
Previous work in the same lung transplant model using an adenovirus vector expressing IL-17RA-Fc to neutralize IL-17 completely prevented the development of OB (13). IL-17RA binds IL-17A, IL-17F, IL-17E (also called IL-25), and IL-17C (8). We have investigated the expression of IL-25 and IL-17C in lung allografts and syngeneic control allografts and have found that IL-25 and IL-17C are not expressed consistently in the lungs of mice with OB at Day 21 (data not shown). In an adoptive transfer model of skin allograft rejection, it was also found that Th17 cells could promote rejection of skin allografts independent of IL-17A production, possibly through IL-17F (28). IL-17F, which is also produced by Th17 cells, is known to have biological effects similar to those of IL-17, such as inducing neutrophilia in the lung and IL-6 production by fibroblasts (30). In a subset of mice, we preliminarily investigated the production of IL-17F concurrent with IL-17A by lymphocytes. Interestingly, although we see IL-17F produced consistently by in vitro–differentiated Th17 cells, we did not find consistent IL-17F staining ex vivo by lung IL-17A–producing T cells in our preliminary studies. The reason for this is not clear and is the subject of ongoing work. It is probable that at other time points, IL-17F is being produced, and blockade with IL-17RA-Fc was more effective because it neutralized both IL-17F and IL-17A. In addition, IL-17A and IL-17F can form heterodimers that may also escape neutralization by the IL-17A antagonistic antibody used in our study (31). It is also possible that IL-17RA-Fc delivered by adenovirus was more effective at blocking IL-17A than was a monoclonal antibody. Direct comparison of these reagents in the future may be helpful but was beyond the scope of the current investigation. Our results suggest that targeting IL-17RA instead of IL-17A alone may be more effective.
We found that by neutralizing IL-17A, the overall lymphocytic infiltration in the lungs was decreased and, most significantly, the lymphocytes producing the Th1 cytokine, IFN-γ were also decreased. In models of cardiac transplantation, IL-17A deficiency has been associated with a decrease in inflammatory cell recruitment to the allograft, and others have found an effect on the expression of IFN-γ in a kidney transplant model (27, 32–34). In our model, IFN-γ+CD8+ T cells and IFN-γ+NK cells were decreased most significantly by IL-17A blockade, although there was a trend toward a decrease in Th1 cells. Gene expression of IFN-γ was also decreased significantly in the lung, and we also found a trend toward a reduction in IL-12 expression. IL-17A has been reported to augment IFN-γ production and expression in other models. Infection with Francisella tularensis has been found to require IL-17, and IL-17 mediated IFN-γ and IL-12 production from macrophages during infection in a mouse model (35). Similarly, IL-17A was found to enhance the lung immune response to the fungal pathogen Cryptococcus neoformans by augmenting the recruitment of immune cells and IFN-γ production by T cells (36). We did not find an effect in the thoracic LN, which suggests an effect on the recruitment of these lymphocytes, rather than their development.
We found, in addition to the effect on the Th1 pathway, diminished expression of many of the chemokines and cytokines known to be affected by IL-17A, CXCL1, CXCL2, CXCL5, granulocyte/macrophage colony-stimulating factor, and the tissue remodeling factor matrix metalloproteinase-8. Surprisingly, we did not see a significant effect on tissue neutrophilia by flow cytometry of the lungs or immunohistochemistry. This may be because of the low prevalence of neutrophils in the tissue at Day 21, because the frequency was similar to the native right lungs in both treatment groups. Interestingly, although IL-17A blockade decreased the IFN-γ response, we found that blockade of IL-17A increased the frequency of IL-17A–producing γδ T cells and CD4+ T cells in the lungs compared with control lungs. Others have also found that IL-17A can negatively regulate IL-17A production and gene expression through the IL-17RA receptor on Th17 cells in vivo (29). These data suggest that if IL-17A blockade is stopped, potentially deleterious IL-17–producing T cells that have remained may cause graft damage through IL-17A. Furthermore, the other cytokines produced by Th17 cells would still be present.
The reciprocal relationship between the development of Th17 cells and Tregs is well established (37). Th17 cells can be induced in the presence of transforming growth factor-β and the inflammatory cytokine IL-6, whereas Tregs are suppressed by IL-6. Previous work in a cardiac transplant model found that a deficiency of IL-17A enhanced Treg expansion in allografts (27). Tregs have also been found to mediate the increase in allograft survival in IL-17–deficient kidney allograft recipients in a mouse model (34). This model also did not show an increase in the number of Tregs in the kidney allografts. Although IL-17A neutralization was effective in decreasing the development of obliterative airway disease, we did not find an increase in Tregs in the lungs or thoracic LN when IL-17A was blocked. However, it is probable that Tregs may function more effectively when IL-17A is neutralized and the balance of Tregs to effectors is higher (8). In the lung allografts, we saw a trend toward a decrease in IL-6 gene expression after IL-17A blockade, which may also improve Treg function. Future studies are required to determine the function of Tregs in OB development and after IL-17A neutralization.
Our previous work suggested that γδ T cells and CD4+ T cells were able to compensate for the lack of the other T-cell subset to produce IL-17A (22). Here, we have found that blocking all sources of IL-17A decreases rejection and OB in this minor mismatch model. Furthermore, our data suggest that IL-17A promotes an inflammatory cellular rejection response in the lung, leading to fibrosis, but also that it may limit the expansion of more IL-17A–producing T cells in the lung. A limitation of our study is that we focused primarily on the T cells in the lungs and their cytokine production. We did not investigate innate immune cells such as monocytes and activated dendritic cells that are influenced by IL-17A and are likely to be contributing to the pathology (34). Given the limited cells harvested from the left lung, the extent of our investigation was limited and we chose to focus on T cells, NK cells, and neutrophils. It will be interesting to determine the contributions of other cell types in the lung during IL-17A neutralization in the future and the mechanisms of IL-17A–independent rejection and fibrosis. Overall, our results support a key role for IL-17A in driving lung transplant-related fibrosis in this model and suggest that IL-17A is an important therapeutic target after lung transplant. However, breaks in therapy may lead to a resurgence of the IL-17A–mediated immune response.
Acknowledgments
Acknowledgments
The authors thank the staff of the Flow Cytometry core facility at the University of Illinois at Chicago for their technical assistance.
Footnotes
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Grant R01HL109310).
Author Contributions: Conception and design: P.K.G., Q.W., and R.A.S.; acquisition, analysis, and interpretation: P.K.G., S.R.W., Q.W., and R.A.S.; and drafting of the manuscript and review for important intellectual content: P.K.G., Q.W., and R.A.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0154OC on January 24, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Weigt SS, DerHovanessian A, Wallace WD, Lynch JP, III, Belperio JA. Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Semin Respir Crit Care Med. 2013;34:336–351. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714–1718. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, Dupont LJ, Verleden GM. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 5.Verleden SE, Vos R, Vandermeulen E, Ruttens D, Vaneylen A, Dupont LJ, Verbeken EK, Verleden GM, Van Raemdonck DE, Vanaudenaerde BM. Involvement of interleukin-17 during lymphocytic bronchiolitis in lung transplant patients. J Heart Lung Transplant. 2013;32:447–453. doi: 10.1016/j.healun.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Vanaudenaerde BM, Dupont LJ, Wuyts WA, Verbeken EK, Meyts I, Bullens DM, Dilissen E, Luyts L, Van Raemdonck DE, Verleden GM. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 7.Snell GI, Levvey BJ, Zheng L, Bailey M, Orsida B, Williams TJ, Kotsimbos TC. Interleukin-17 and airway inflammation: a longitudinal airway biopsy study after lung transplantation. J Heart Lung Transplant. 2007;26:669–674. doi: 10.1016/j.healun.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 13.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirayama S, Sato M, Loisel-Meyer S, Matsuda Y, Oishi H, Guan Z, Saito T, Yeung J, Cypel M, Hwang DM, et al. Lentivirus IL-10 gene therapy down-regulates IL-17 and attenuates mouse orthotopic lung allograft rejection. Am J Transplant. 2013;13:1586–1593. doi: 10.1111/ajt.12230. [DOI] [PubMed] [Google Scholar]
- 15.Oishi H, Martinu T, Sato M, Matsuda Y, Hirayama S, Juvet SC, Guan Z, Saito T, Cypel M, Hwang DM, et al. Halofuginone treatment reduces interleukin-17A and ameliorates features of chronic lung allograft dysfunction in a mouse orthotopic lung transplant model. J Heart Lung Transplant. 2016;35:518–527. doi: 10.1016/j.healun.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Zhou X, Gaowa S, Meng Q, Zhan Z, Liu J, Li J, Fan H, Liu Z. The critical role of induced CD4+ FoxP3+ regulatory cells in suppression of interleukin-17 production and attenuation of mouse orthotopic lung allograft rejection. Transplantation. 2015;99:1356–1364. doi: 10.1097/TP.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 17.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 18.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72:ii116–ii123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 19.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, et al. ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 20.Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22:230–241. doi: 10.1016/j.molmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Gupta PK, Suzuki H, Wagner SR, Zhang C, Cummings OW, Fan L, Kaplan MH, Wilkes DS, Shilling RA. CD4 T cells but not Th17 cells are required for mouse lung transplant obliterative bronchiolitis. Am J Transplant. 2015;15:1793–1804. doi: 10.1111/ajt.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG. Transforming growth factor-α deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol. 1999;20:924–934. doi: 10.1165/ajrcmb.20.5.3526. [DOI] [PubMed] [Google Scholar]
- 26.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO α chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 27.Itoh S, Kimura N, Axtell RC, Velotta JB, Gong Y, Wang X, Kajiwara N, Nambu A, Shimura E, Adachi H, et al. Interleukin-17 accelerates allograft rejection by suppressing regulatory T cell expansion. Circulation. 2011;124:S187–S196. doi: 10.1161/CIRCULATIONAHA.110.014852. [DOI] [PubMed] [Google Scholar]
- 28.Agorogiannis EI, Regateiro FS, Howie D, Waldmann H, Cobbold SP. Th17 cells induce a distinct graft rejection response that does not require IL-17A. Am J Transplant. 2012;12:835–845. doi: 10.1111/j.1600-6143.2011.03971.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith E, Stark MA, Zarbock A, Burcin TL, Bruce AC, Vaswani D, Foley P, Ley K. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J Immunol. 2008;181:1357–1364. doi: 10.4049/jimmunol.181.2.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46:7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CMM, Wright JF, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 32.Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, Iwakura Y, Saito H, Adachi H, Steinman L, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–240. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 33.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177:1265–1273. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan T, Chadban SJ, Ma J, Bao S, Alexander SI, Wu H. IL-17 deficiency attenuates allograft injury and prolongs survival in a murine model of fully MHC-mismatched renal allograft transplantation. Am J Transplant. 2015;15:1555–1567. doi: 10.1111/ajt.13140. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun. 2014;82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]